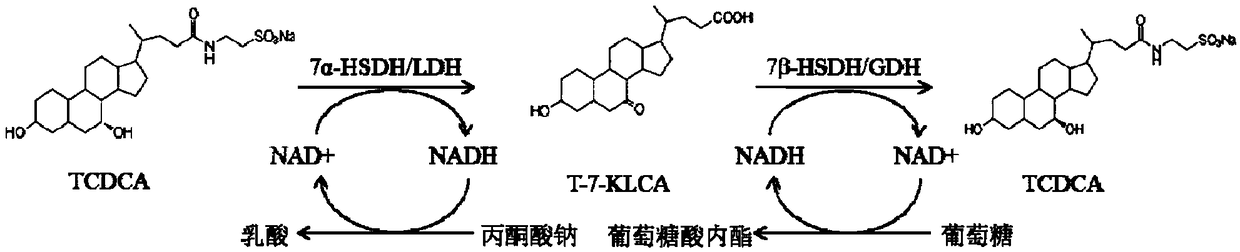
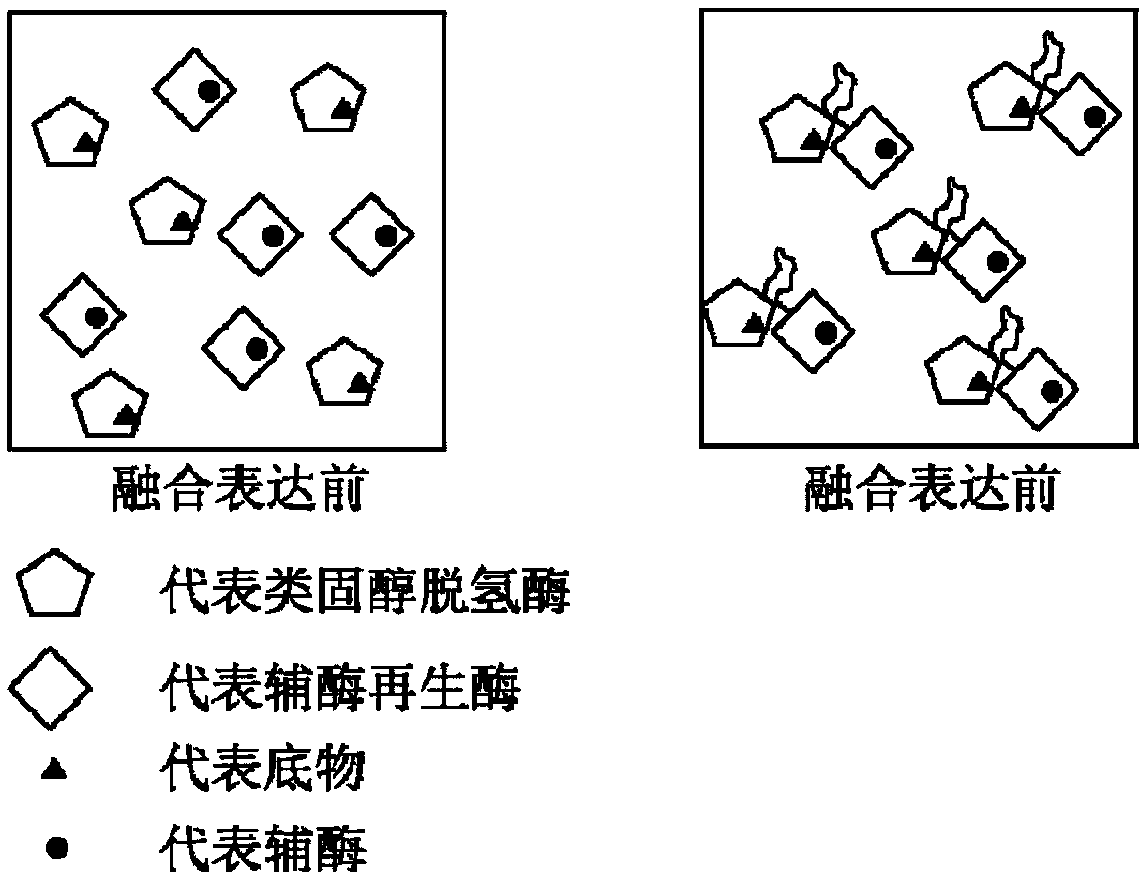
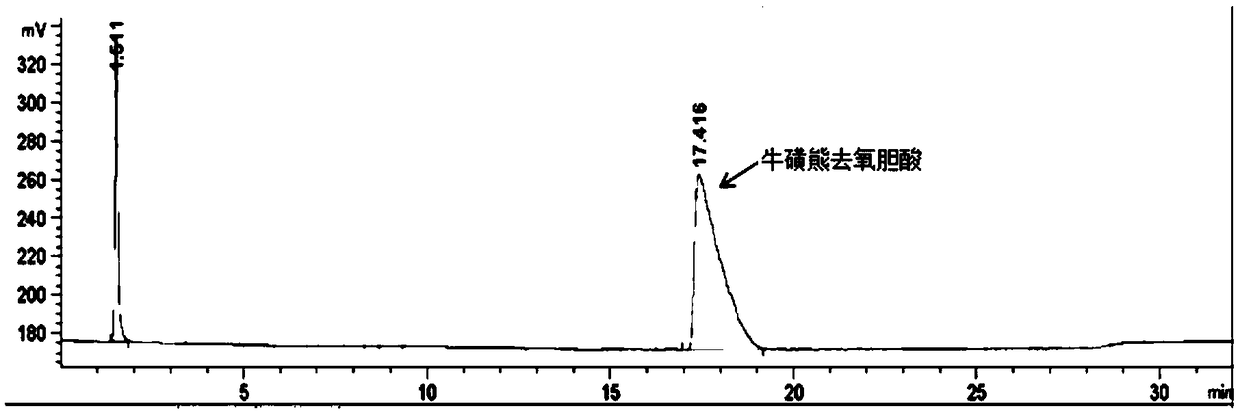
Method of preparing tauroursodeoxycholic acid by biotransformation and application of method
A technology of tauroursodeoxycholic acid and biotransformation, applied in biochemical equipment and methods, botanical equipment and methods, chemical instruments and methods, etc., can solve the problem of low substrate concentration, difficult industrial production, taurine 7 -The problem of high ketolithocholic acid content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Escherichia coli containing the recombinant plasmid fermented and expressed in the Erlenmeyer flask
[0083] Take 20 μL of Escherichia coli BL21(DE3) strain containing the recombinant plasmid, inoculate it into 200 mL of ampicillin-resistant LB medium, and culture it overnight at 37°C, 220 rpm, with an OD600 value of 2.5-4.0. Inoculate 20 mL of culture solution into 1 L of ampicillin-resistant medium, culture at 37°C and 140 rpm for 3 hours, and when the OD600 value is 1, add 0.5 mM IPTG to induce overnight expression. Bacteria were collected by centrifugation. A small amount of bacteria was resuspended in 100mM phosphate buffer, and ultrasonically disrupted to obtain crude enzyme solution. Enzyme activity was determined according to the method in the technical scheme.
Embodiment 2
[0084] Example 2 Escherichia coli containing recombinant plasmid fermented and expressed in a fermenter
[0085] Take 20 μL of Escherichia coli BL21(DE3) strain containing the recombinant plasmid, inoculate it into 200 mL of ampicillin-resistant LB medium, and culture it overnight at 37°C, 220 rpm, with an OD600 value of 2.5-4.0. Inoculate 20 mL of culture solution into 1 L of ampicillin-resistant medium, and culture overnight at 37°C and 140 rpm. Aseptically inoculate 10L of the seed solution into a fermenter equipped with 200L of Escherichia coli high-density fermentation medium, culture at 37°C with aeration and stirring for 8 hours. Escherichia coli high-density fermentation medium contains: 18g / L dipotassium hydrogen phosphate dodecahydrate, 6.8g / L potassium dihydrogen phosphate, 0.7g / L anhydrous sodium sulfate, 0.48g / L magnesium sulfate, 2.25 g / L glycerin, 2.5g / L yeast powder, 5g / L peptone. After cultivating with aeration and stirring for 8 hours, add an IPTG solution ...
Embodiment 3
[0086] Example 3 Transformation of tauroursodeoxycholic acid with single gene expression protein in 1L reaction system
[0087]Dissolve 250g taurochenodeoxycholic acid in 700mL of 100mM glycine buffer, add 0.25mM NAD + , add the sodium pyruvate of 60g / L, add the 7α-steroid dehydrogenase (containing pure enzyme about 5g) and lactate dehydrogenase (containing pure enzyme about 5g) of purified or partially purified enzyme solution or cell lysate or bacterial suspension Pure enzyme is about 2g), add 100mM glycine buffer to 1L, adjust the pH to 7.5 with 5M NaOH, and react for 6-18h at 25°C. Add 100g / L of glucose, 7β-steroid dehydrogenase (containing about 5g of pure enzyme) and glucose dehydrogenase (containing about 5g of pure enzyme) of purified or partially purified enzyme solution or cell lysate or bacterial suspension 2g) of Escherichia coli, adjust the pH to 7.5 with 5M NaOH. React at 25°C for 6-18h. The substrate conversion rate is over 98%, the finished product content is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com