Treatment with omega-3 fatty acids and ppar agonist and/or antagonist and a combination product thereof
A fatty acid, agonist technology, applied in obesity, to address problems that do not show synergistic effects of hyperlipidemia and hyperlipoproteinemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
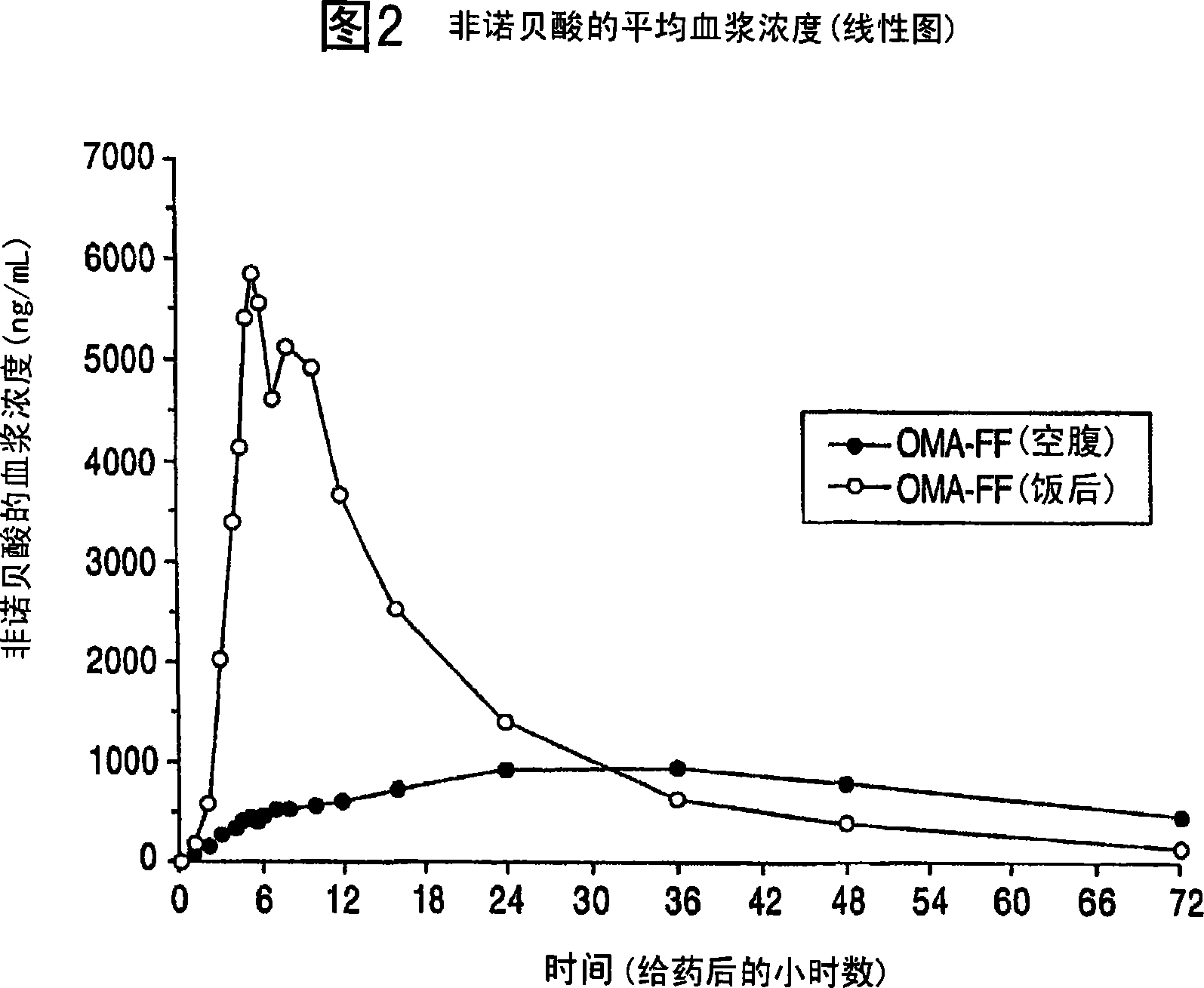
Image
Examples
Embodiment 1
[0057] The following formulations can be prepared according to the present invention:
[0058] Recipe 1
[0059] Element
Mg / capsule
K85EE
1000
32.5~100
[0060] Recipe 2
[0061] Element
Mg / capsule
K80EE
1000
Dehydrated ethanol
50
Propylene glycol monocaprylate (ester)
20
15~100
[0062] Recipe 3
[0063] Element
Mg / capsule
K85EE
1000
Glycerin
35
Polyethoxylated castor oil
25
Biglitazone
5~50
[0064] Recipe 4
[0065] Element
Embodiment 2
[0067] A 51-year-old man presented with acute pancreatitis and was diagnosed with familial hypertriglyceridemia. On a strict diet and started fenofibrate treatment (Antara 130mg QD), the pancreatitis improved and the patient was discharged. However, after about 2 weeks of fenofibrate treatment, the patient still maintained a triglyceride (TG) level of 749 mg / dL. Thereafter, the patient started Omacor Treatment (90% omega-3 acid ethyl ester, 4 g per day, QD) while continuing fenofibrate treatment. After one month of combination therapy, the patient's TG was reduced by 69% to 235 mg / dL. In addition, the patient's total cholesterol level was reduced by 46% (from 280 mg / dL to 151 mg / dL) after combination therapy. See Table 1.
[0068] Table 1
[0069] Fenofibrate only
[0070] The above results show that when Omacor Synergistic effect when administered with fenofibrate. Since the general experience is that either agent alone reduces total cholesterol by o...
Embodiment 3
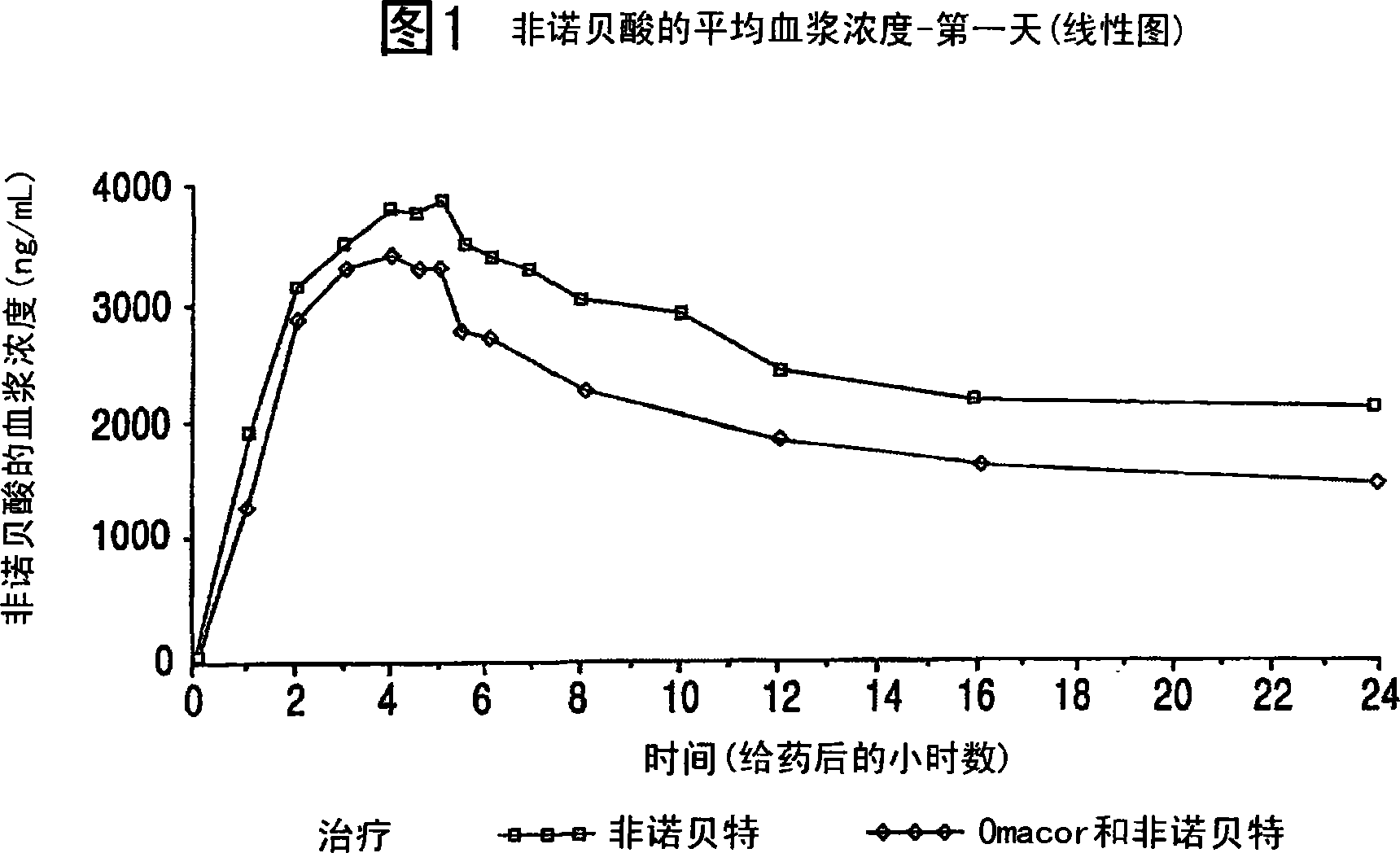
[0072] Omacor was administered to 24 patients Studies with fenofibrate. As shown in Figure 1, it was observed that Omacor Blood levels (AUC) of fenofibric acid were reduced by nearly 30% compared to fenofibrate (circle). The results in Figure 1 show that when fenofibrate and Omacor When administered together, the increased elimination constant of fenofibrate was consistent with the decreased half-life compared to when fenofibrate was administered alone.
[0073] As it is well known that the addition of fatty or oily substances (e.g. fatty meat) to fenofibrate increases blood levels (AUC) of fenofibrate, the observed results were unpredictable (see e.g. Antara Prescribing Information for Fenofibrate Capsules). However, joining Omacor , an oily substance mainly composed of fatty acid esters, has the opposite effect. Without being bound by theory, the observed decrease in systemic levels of fenofibrate may be related to enhanced metabolism of fenofibrate. Thus, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com