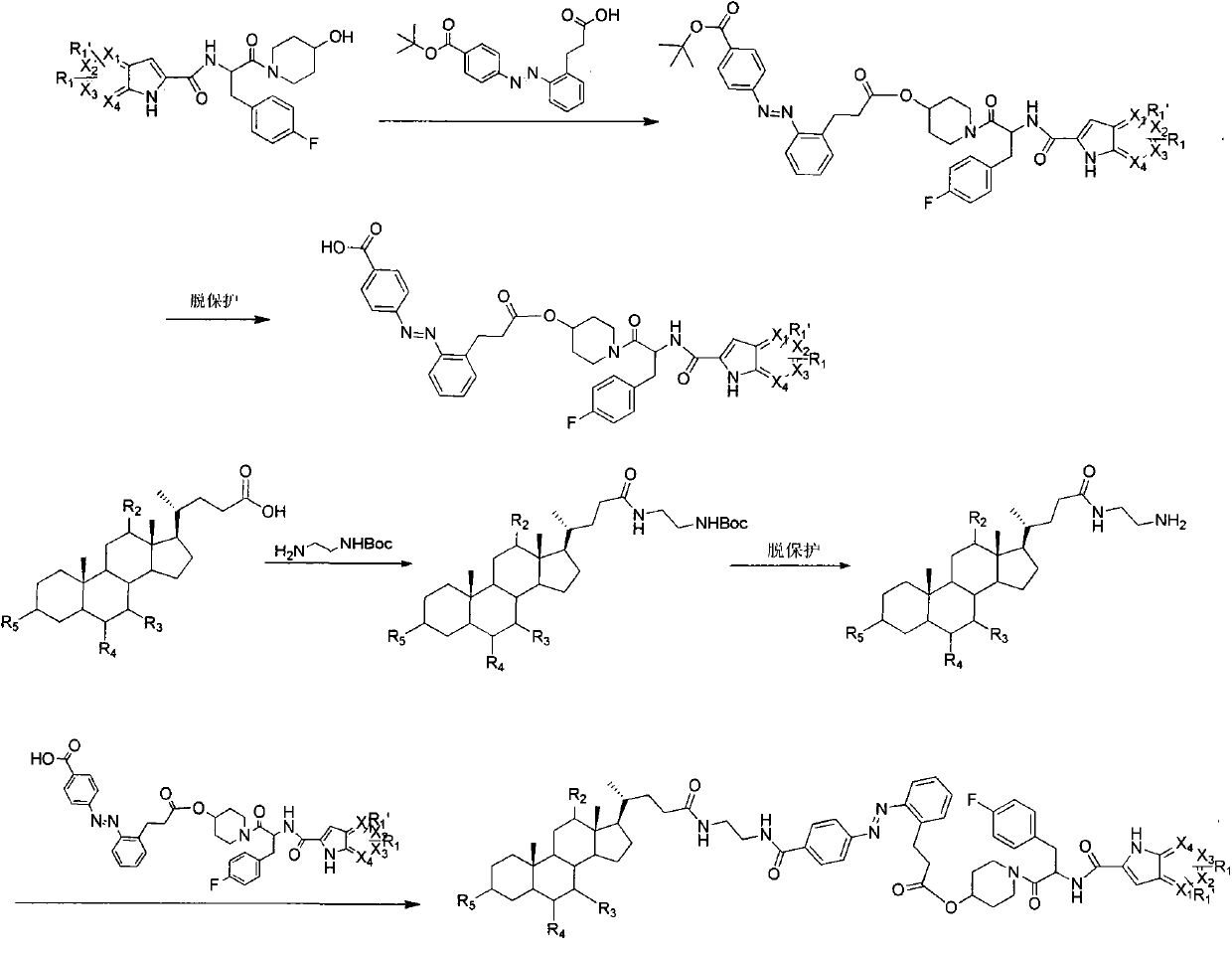
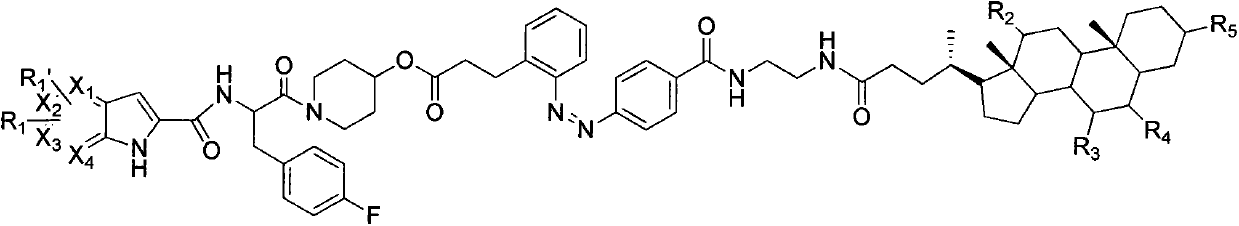
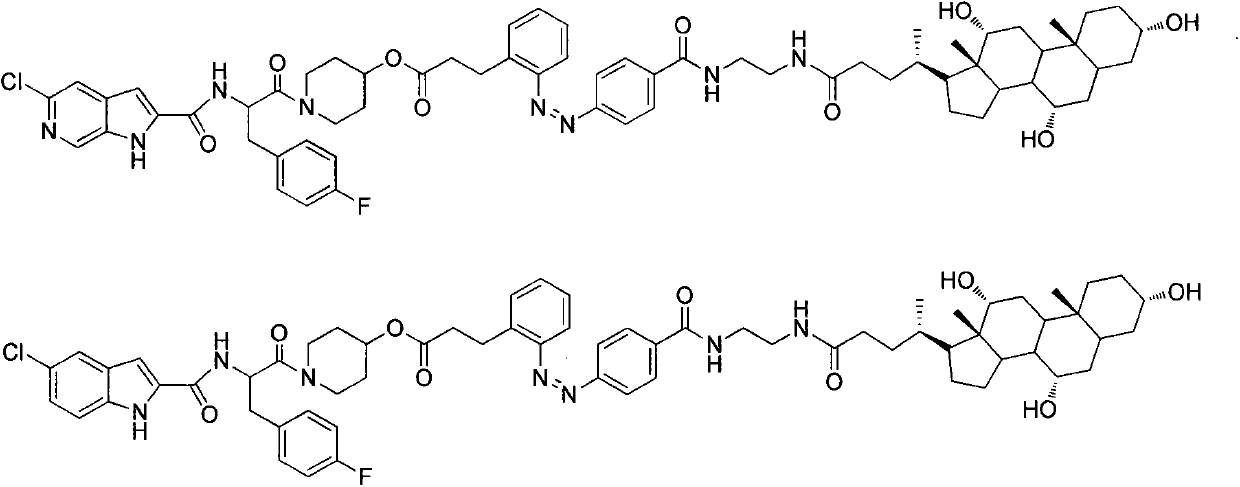
Azo bond contained glycogen phosphorylase inhibitor cholic acid derivative and preparation method and medical application thereof
A kind of azo, compound technology, applied in the field of glycogen phosphorylase inhibitor cholic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (S)-2-tert-butoxycarbonylamino-3-(4-fluorophenyl)-1-(4-hydroxypiperidin-1-yl)acetone
[0043] Dissolve BOC-4-fluoro-L-phenylalanine (15.6g, 55.1mmol) in anhydrous dichloromethane (160mL), add HATU (25g, 66.1mmol) and DIPEA (8.54g, 66.1 mmol), stirred at room temperature for 10 minutes, then added 4-hydroxypiperidine (6.7 g, 66.1 mmol), stirred at room temperature overnight. The reaction solution was washed with saturated brine, anhydrous Na 2 SO 4 It was dried, filtered and concentrated, and the residue was separated by reverse phase HPLC to give a white solid (50mg, 29.8%). Flash column chromatography (petroleum ether / ethyl acetate 1 / 1, V / V) gave a white solid (19.7 g, 98%).
[0044] ESI-MS m / z: 367.2[M+H] + .
[0045] 1 H NMR (CDCl 3 , 400MHz): 7.14-7.18(m, 2H), 6.95-7.00(m, 2H), 5.47(dd, J=8.8, 14.8Hz, 1H), 4.83(dd, J=6.0, 13.6Hz, 1H), 3.81-4.01(m, 2H), 3.46-3.62(m, 1H), 3.15-3.33(m, 1H), 2.89-3.00(m, 2H), 1.73-1.83(m, 2H), 1.42-1.52(m , 2H), 1.40(s, 9H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com