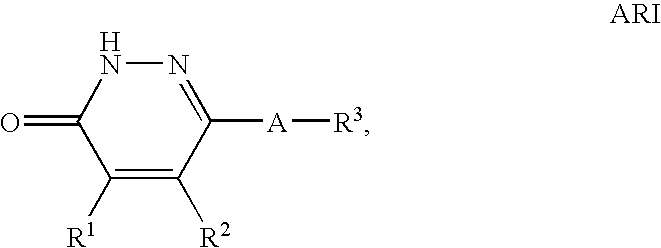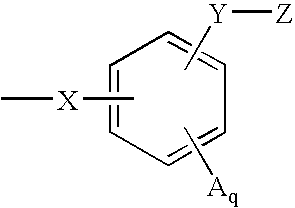Patents
Literature
42results about How to "Impaired glucose tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
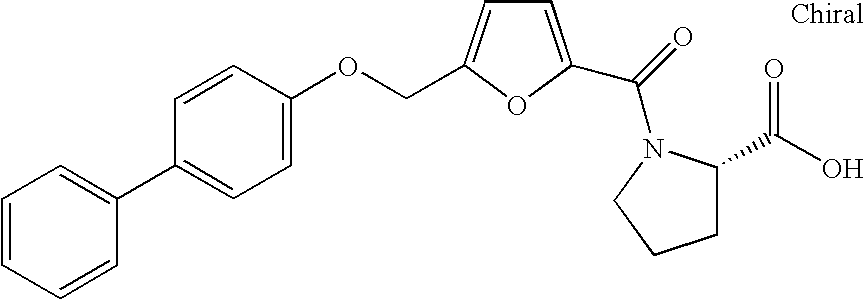
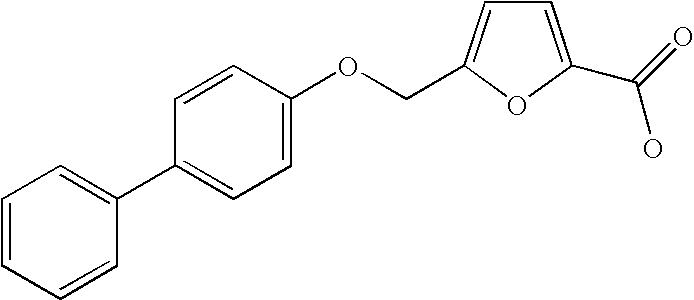
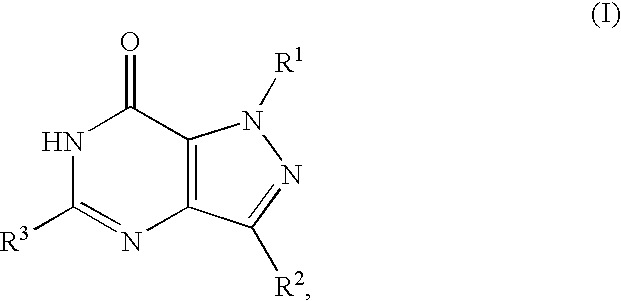
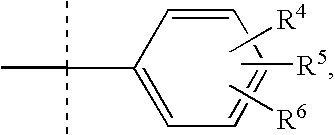
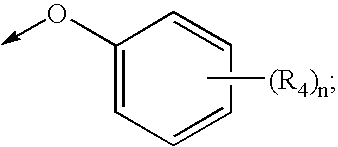
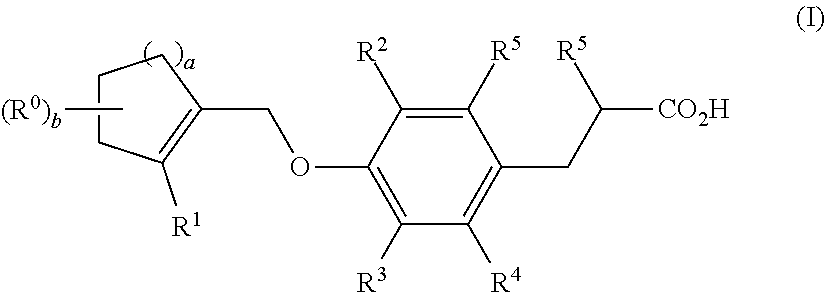
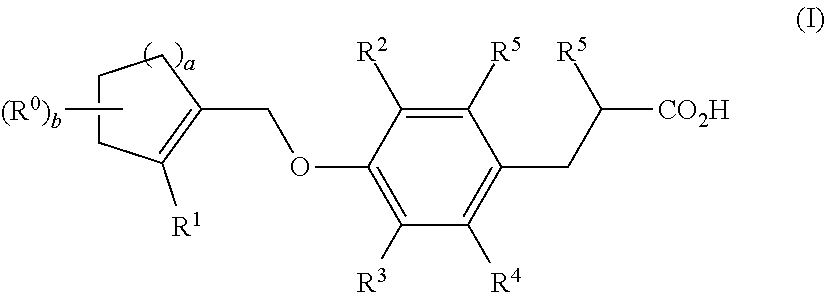
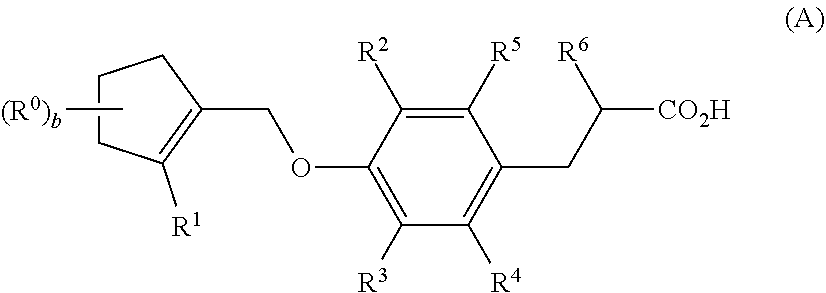
Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives
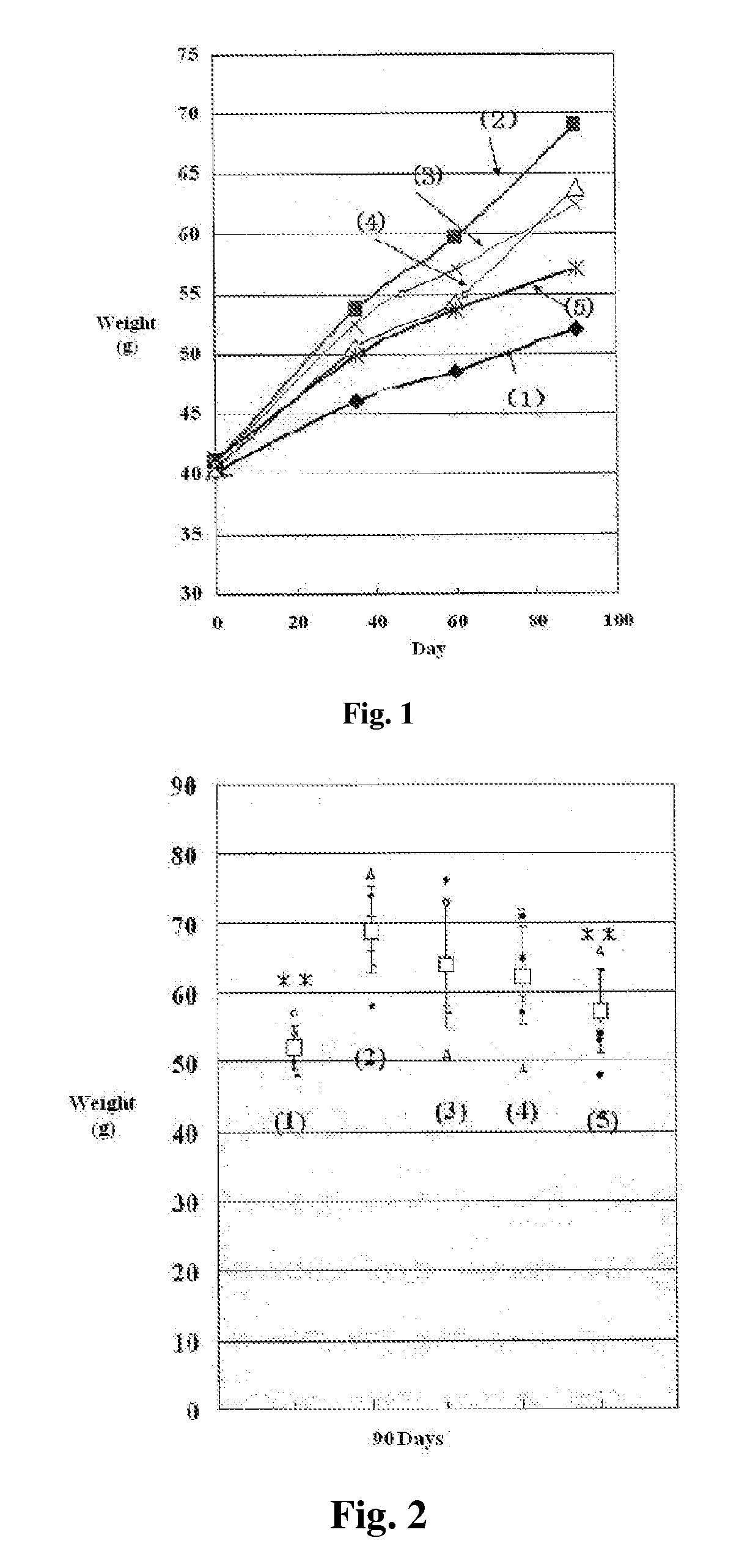
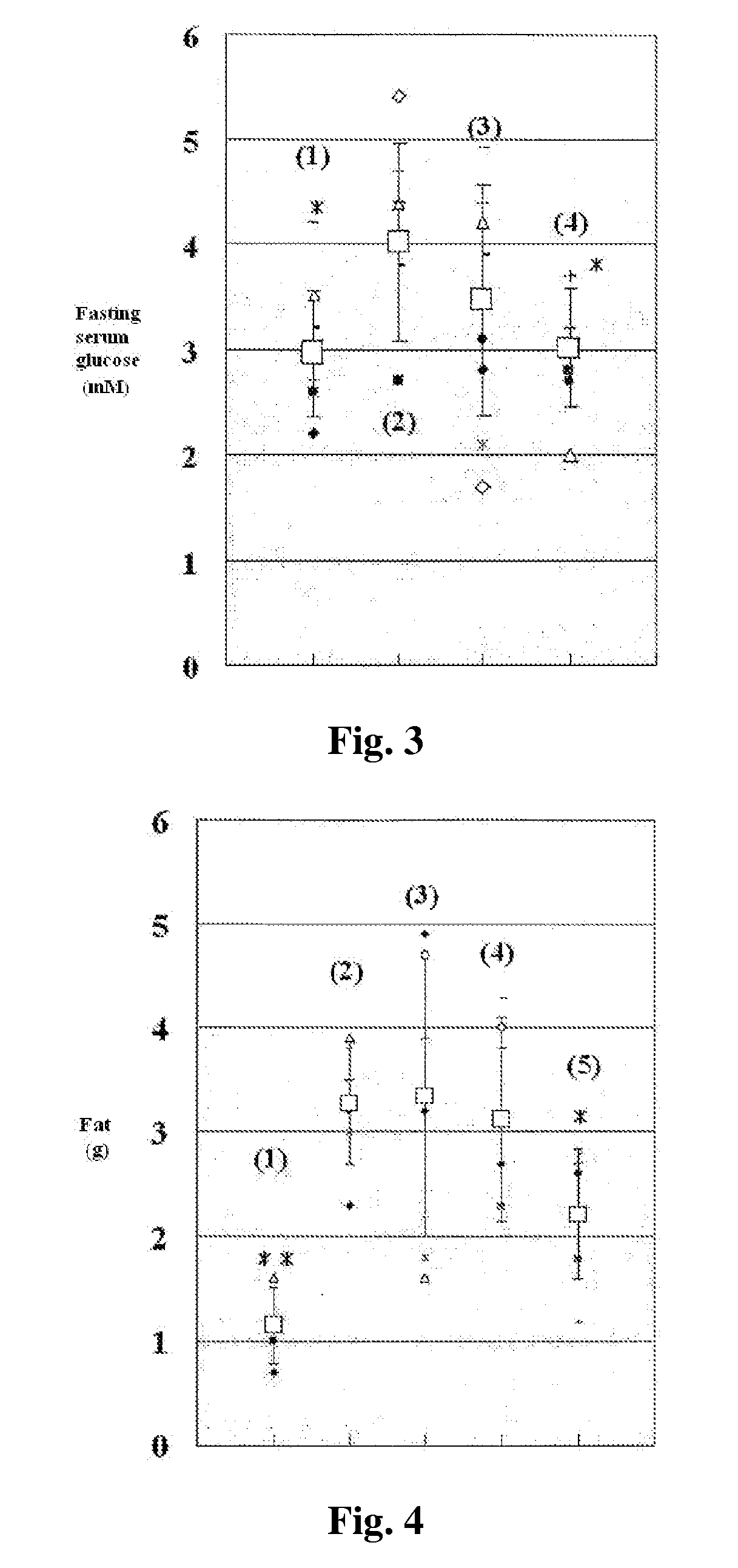
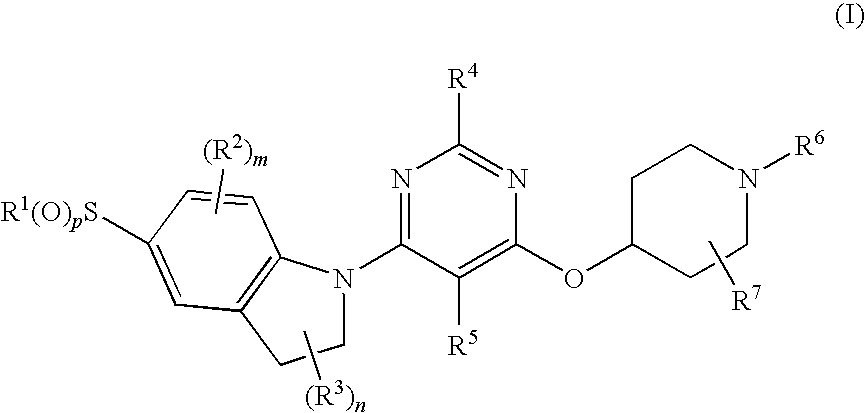
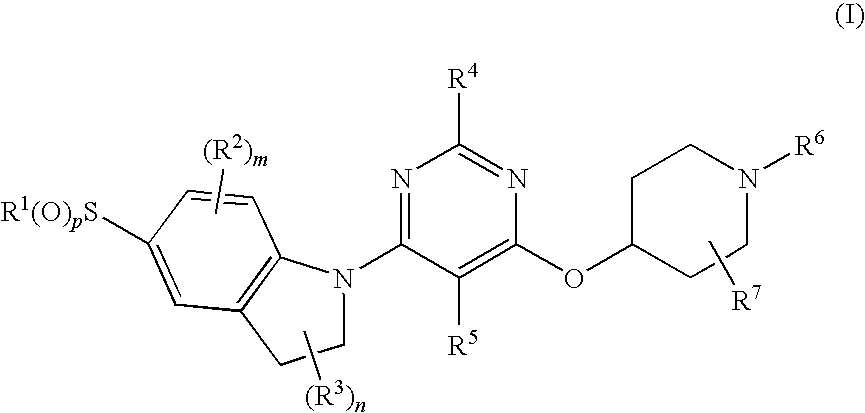
ActiveUS8080580B2Lower blood sugar levelsImpaired glucose toleranceBiocideSenses disorderDiseaseSGLT2 Inhibitor
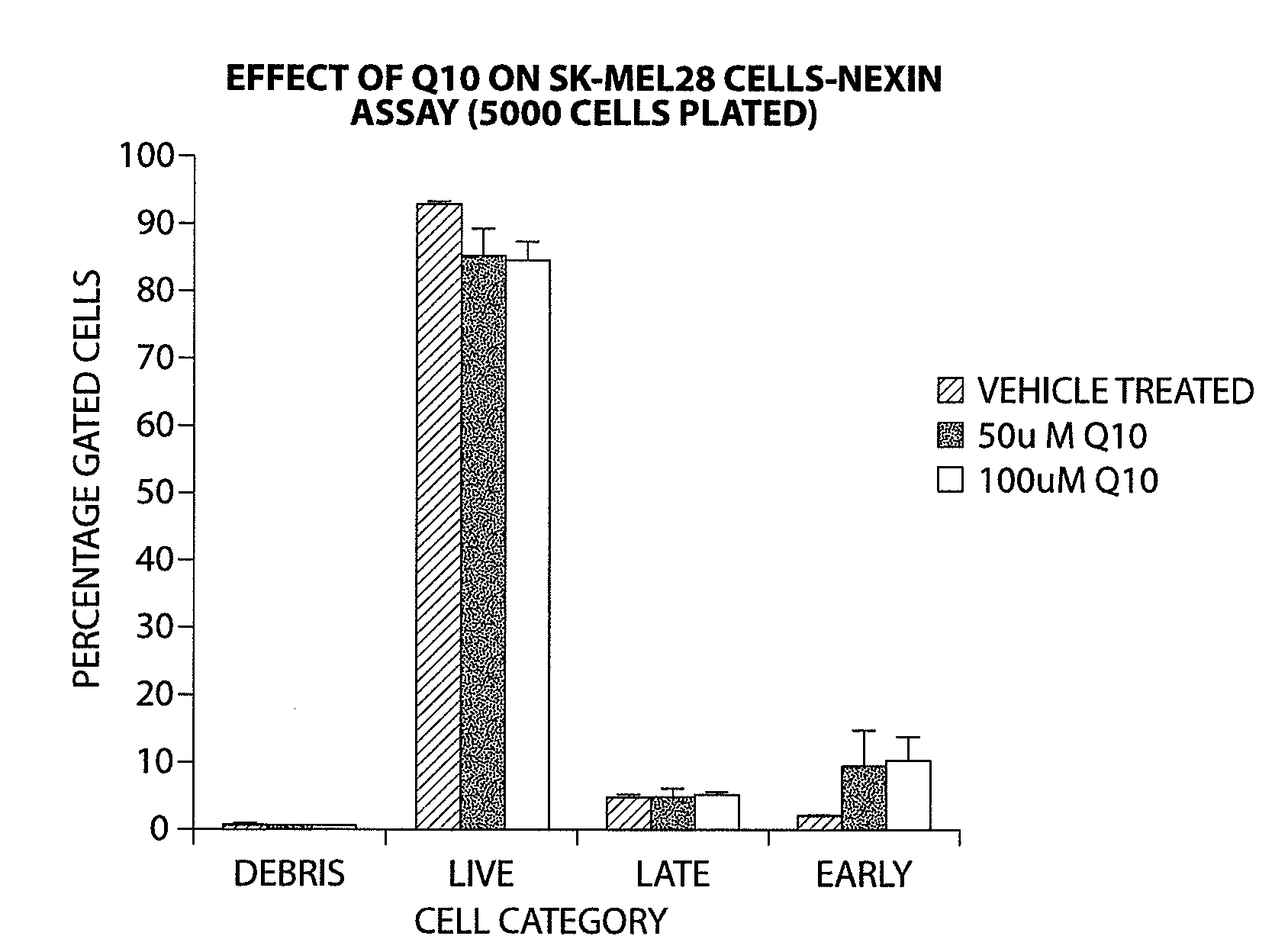
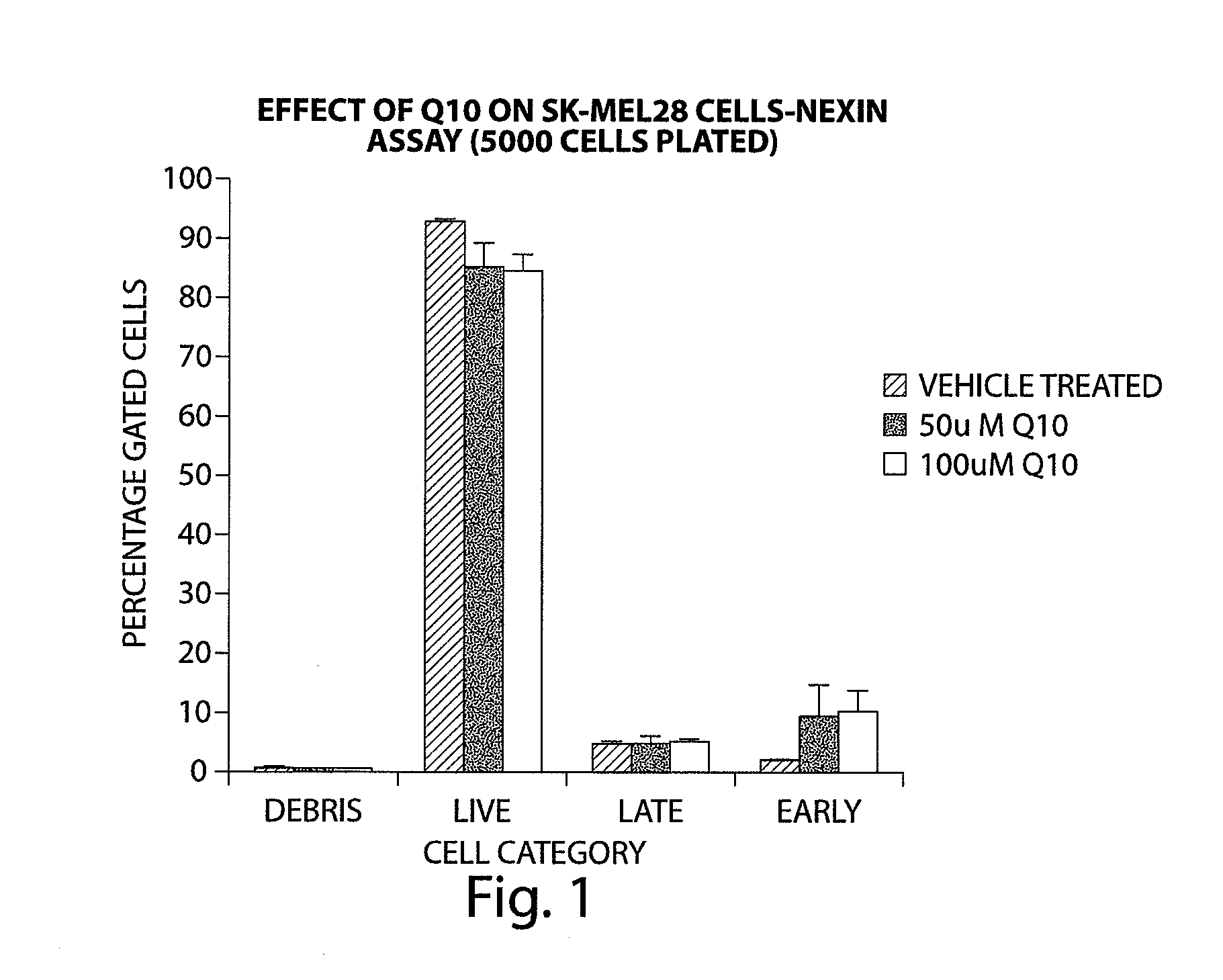
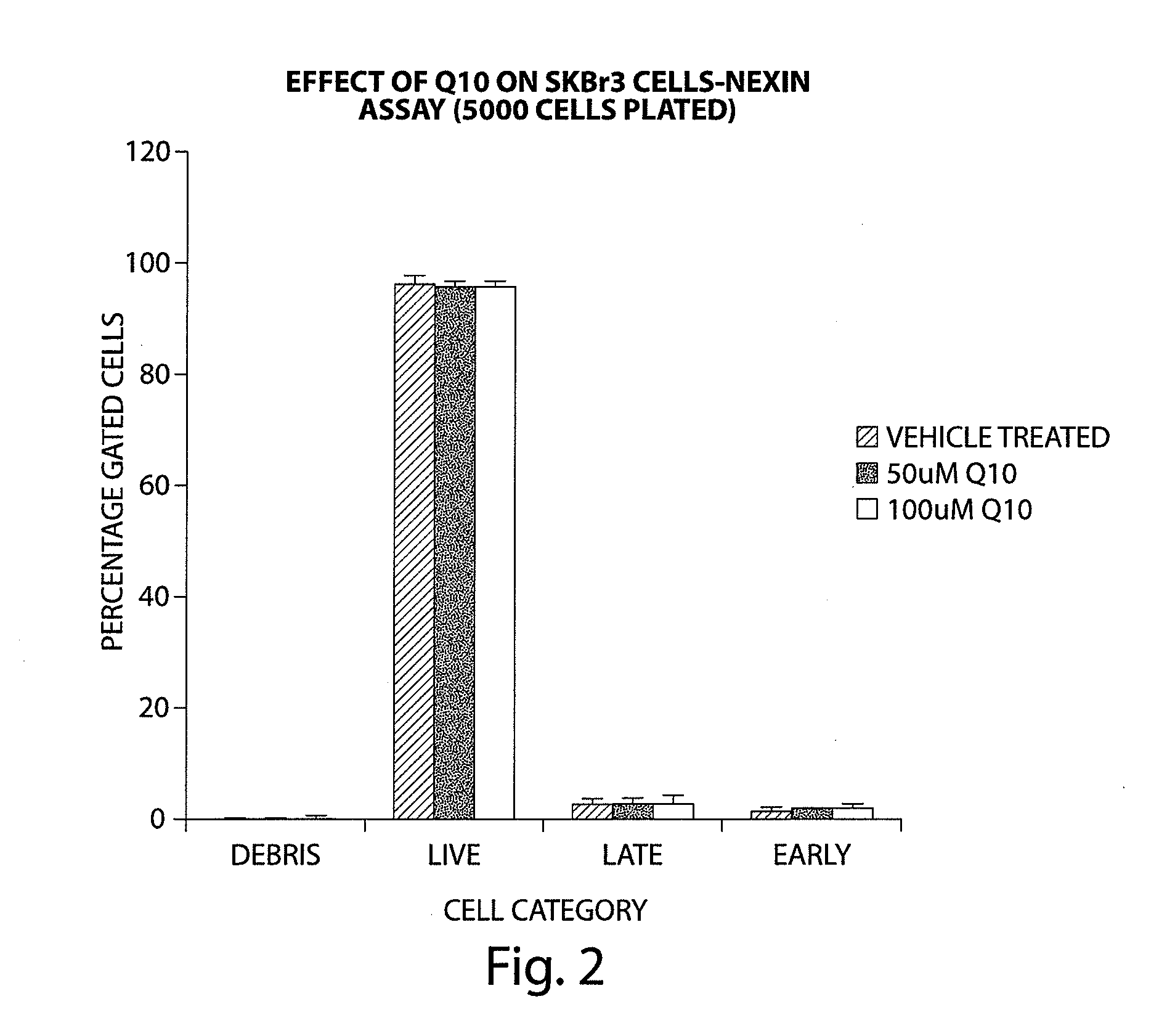
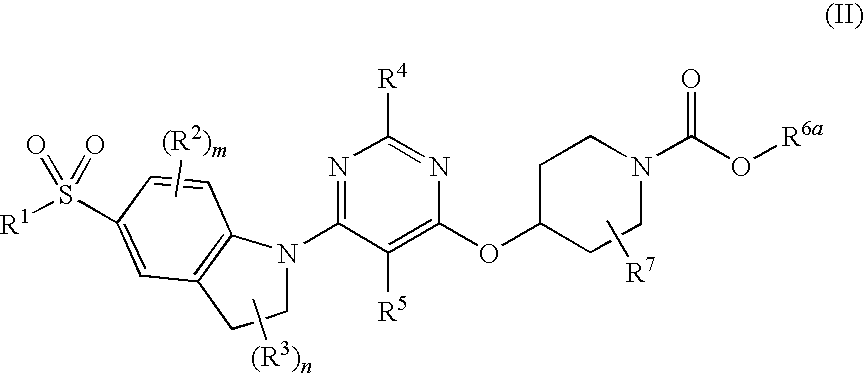
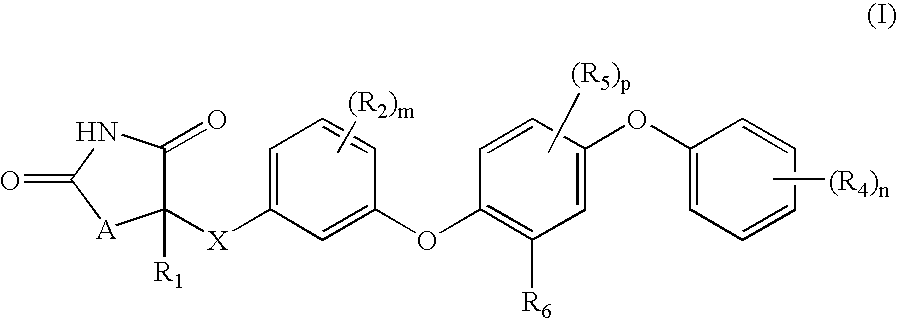
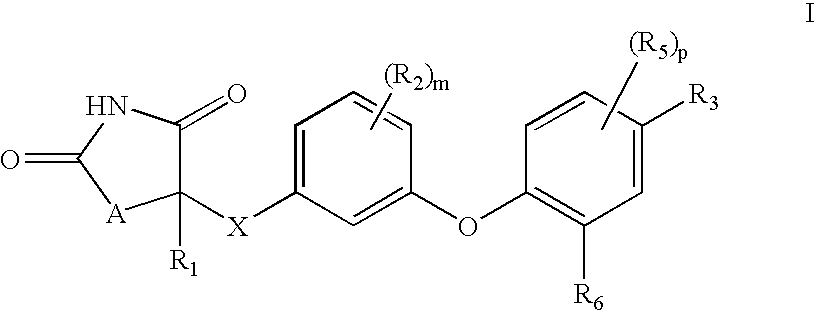
Compounds of Formula (I) are described herein and the uses thereof for the treatment of diseases, conditions and / or disorders mediated by sodium-glucose transporter inhibitors (in particular, SGLT2 inhibitors).
Owner:PFIZER INC
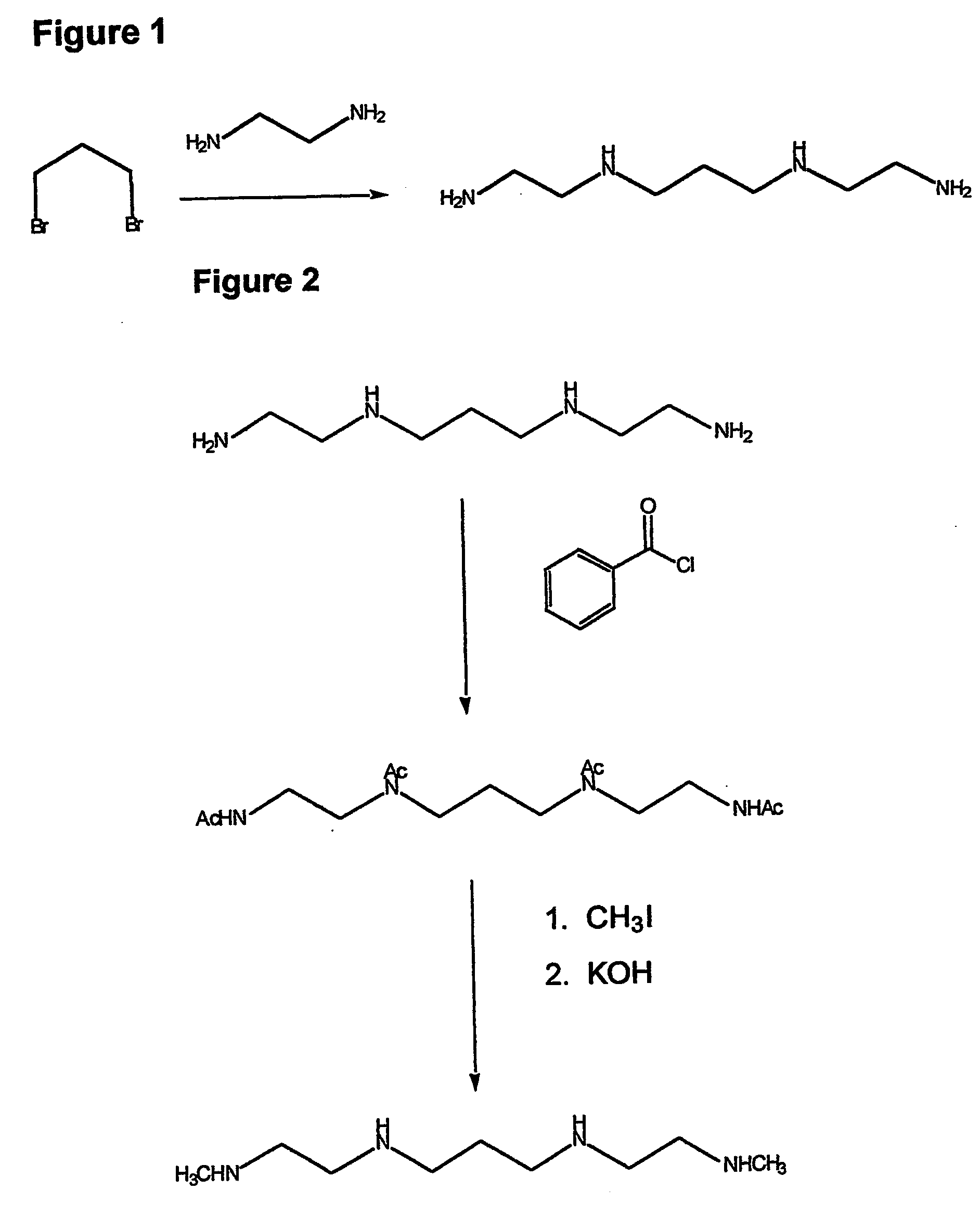
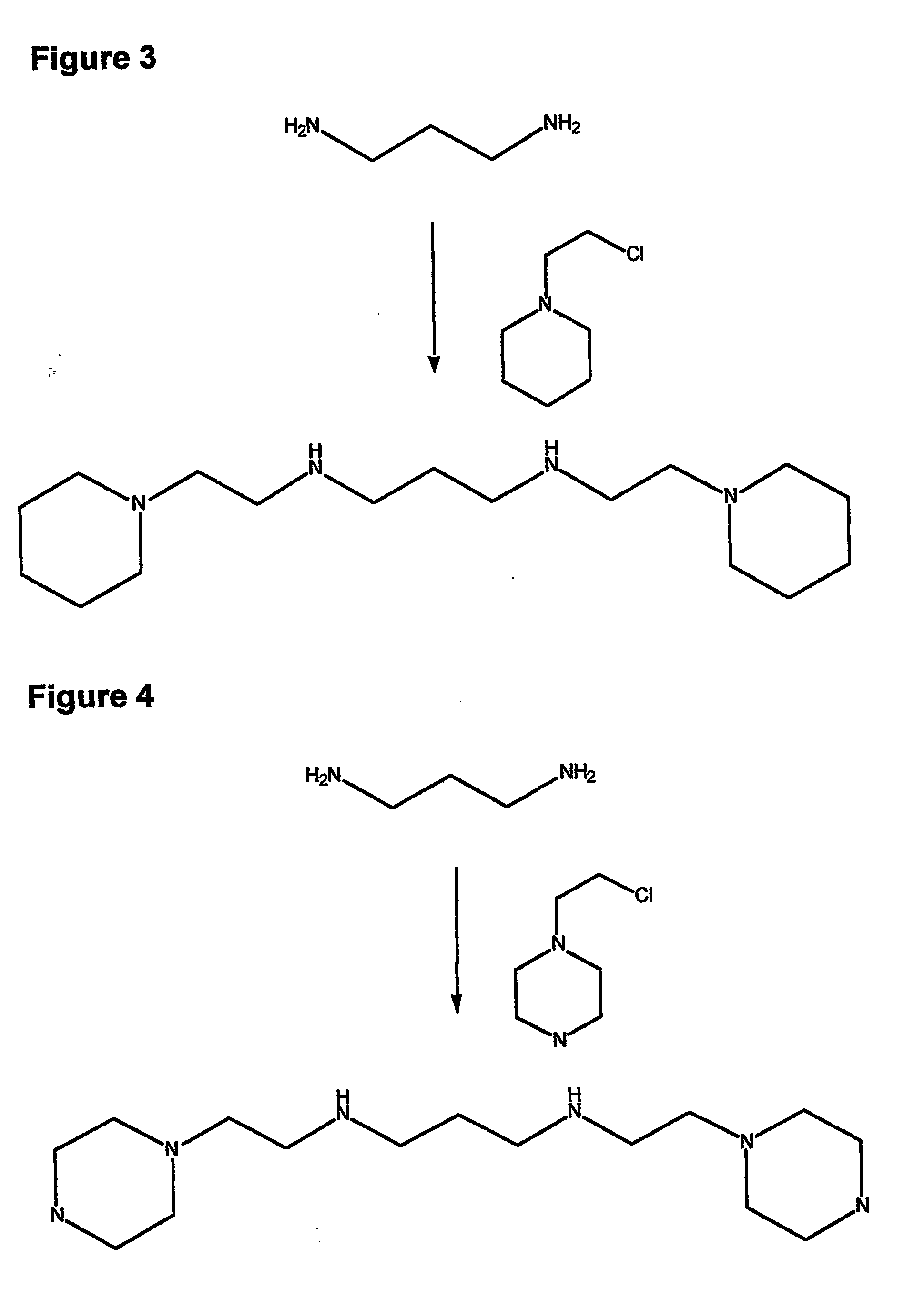
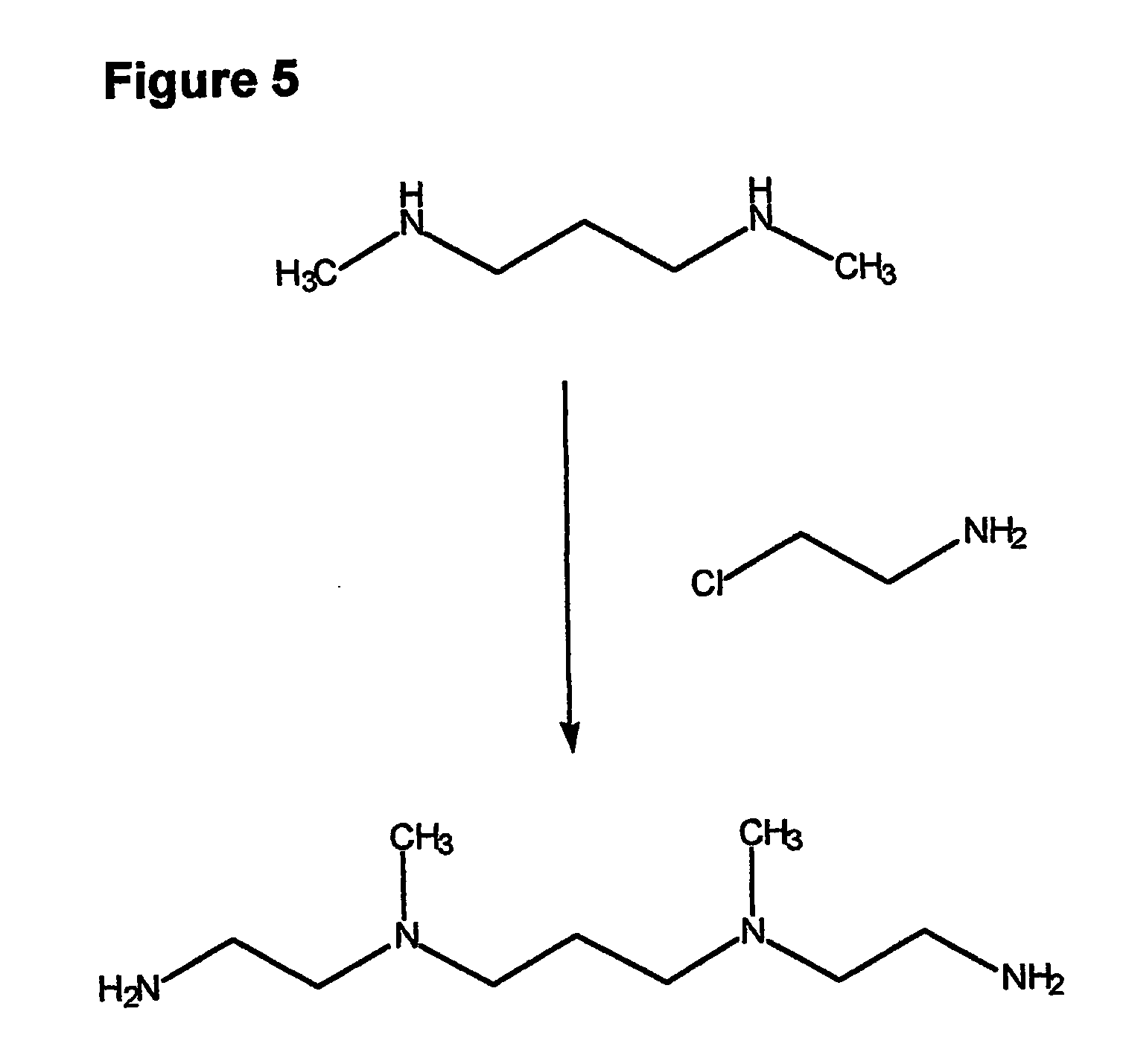
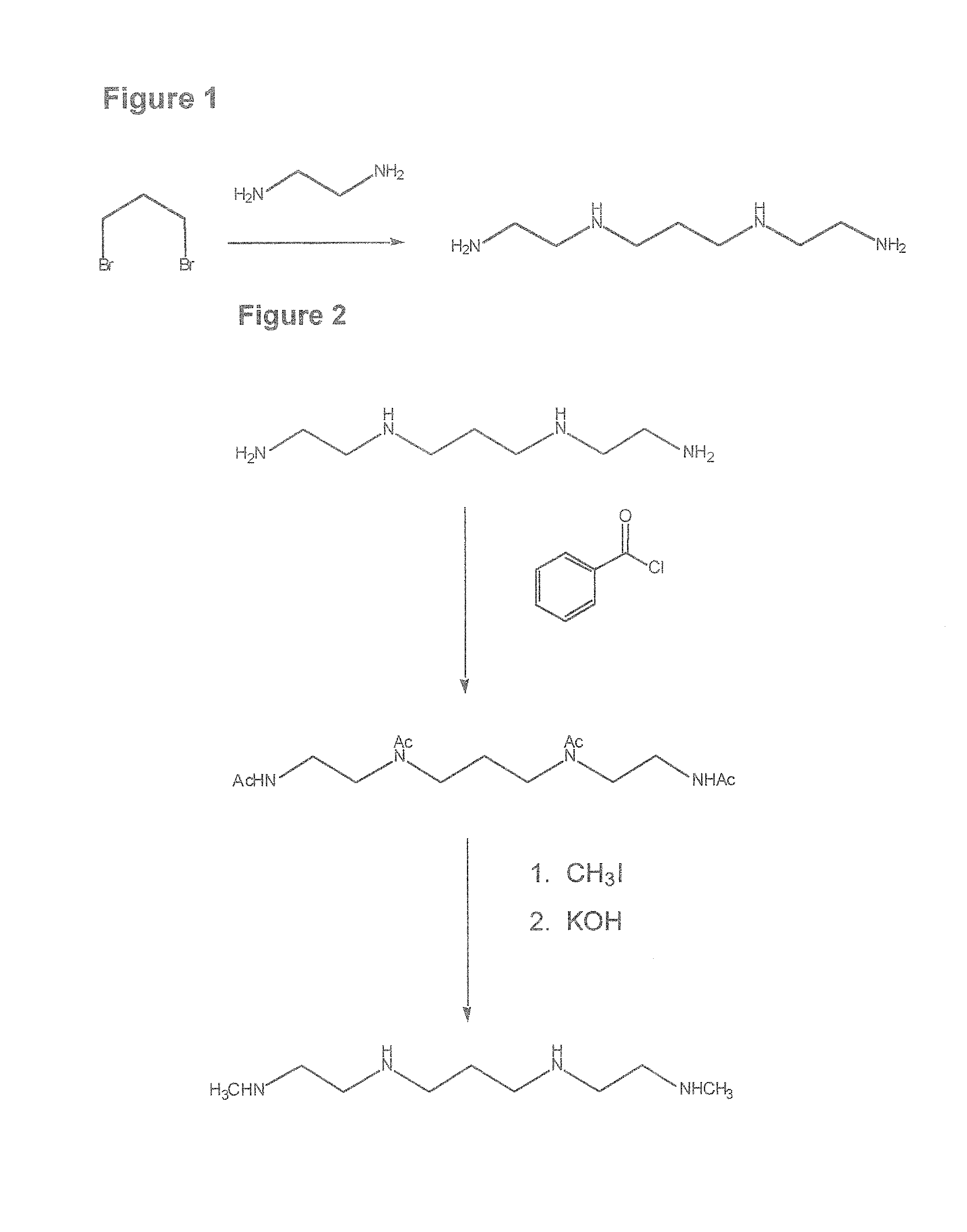
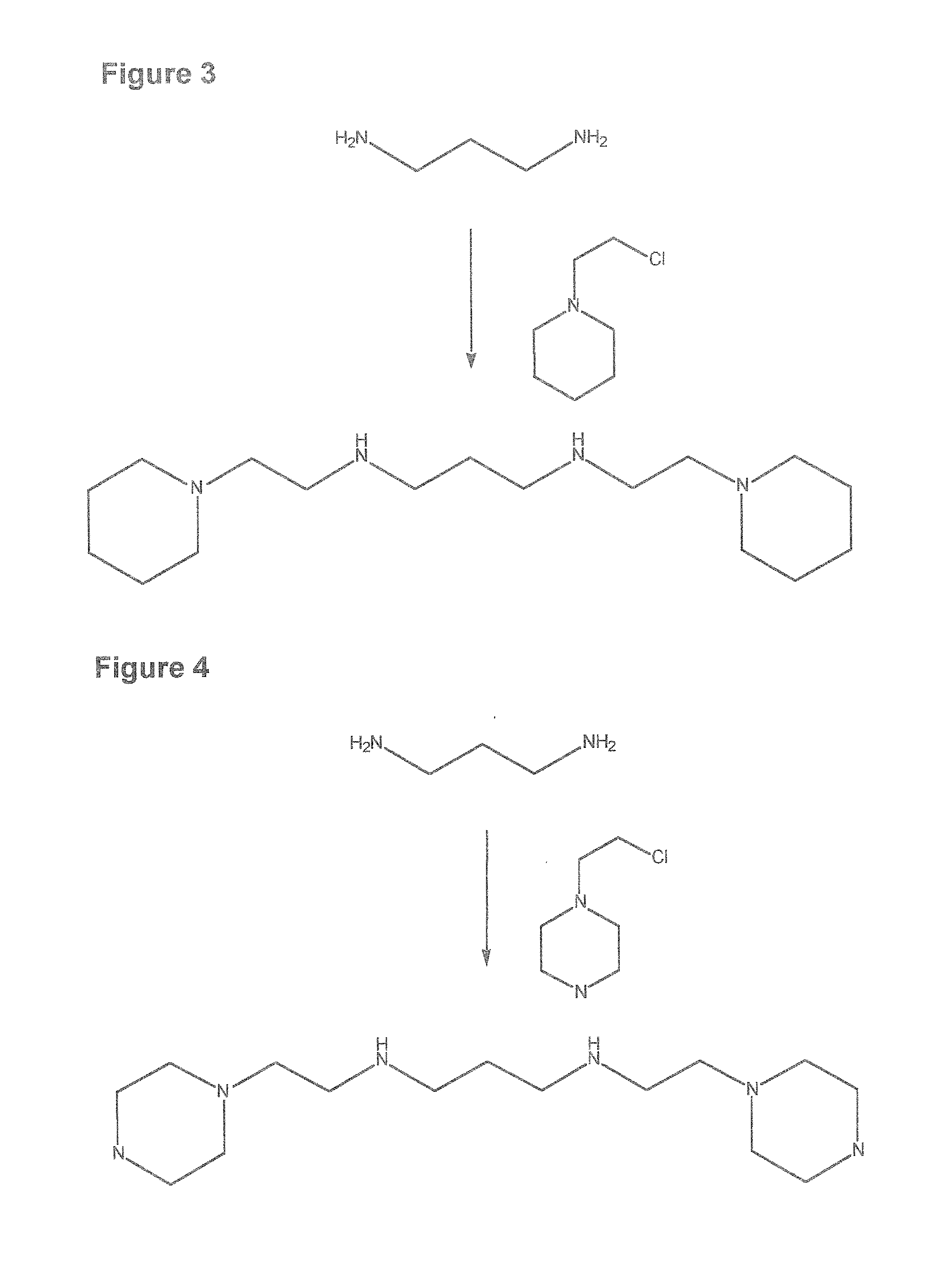
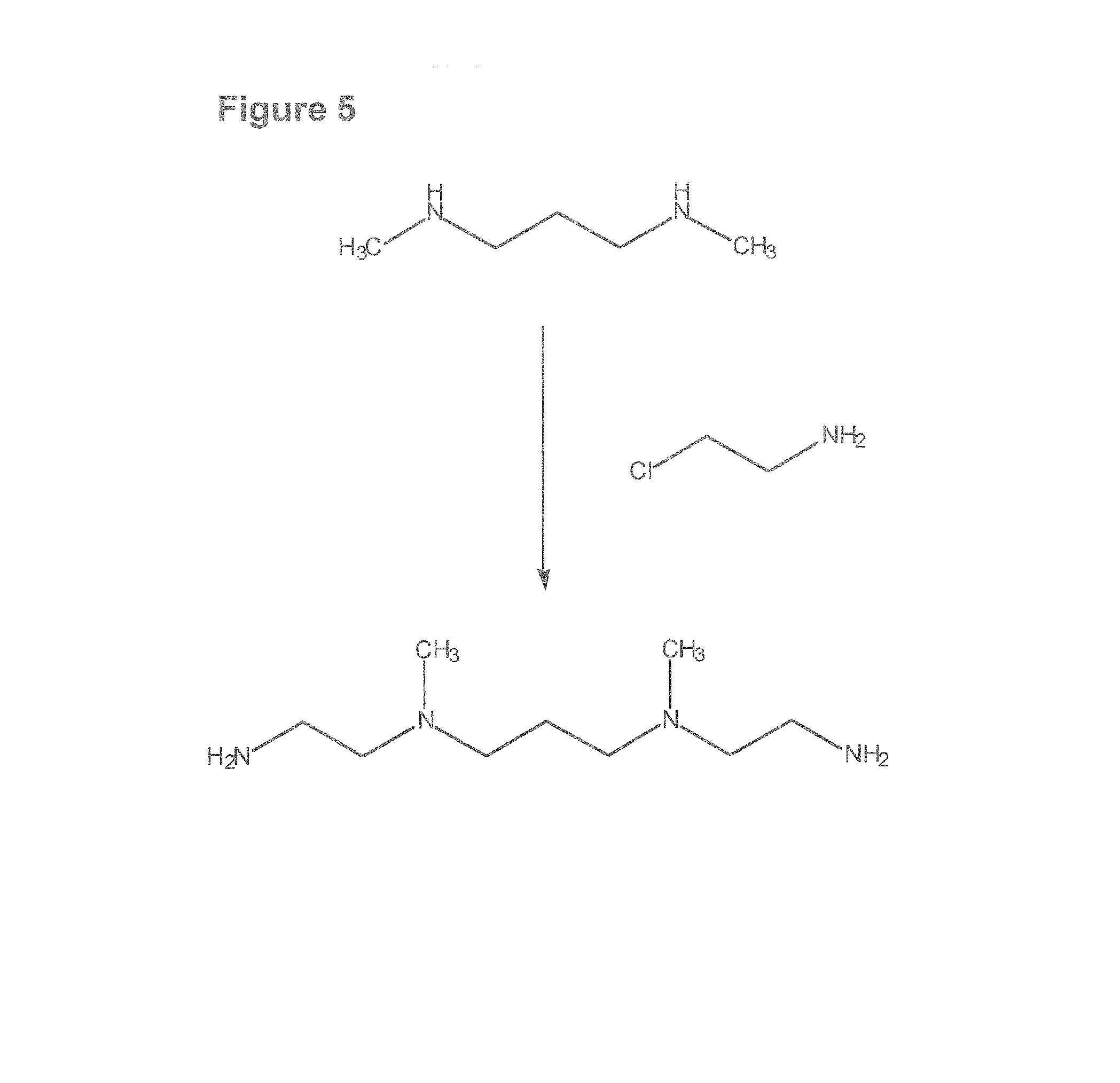
Composition, synthesis and therapeutic applications of polyamines
InactiveUS20050085555A1Chromium concentration were decreasedIncrease excretionBiocideGroup 5/15 element organic compoundsAntidoteRisk stroke
This invention relates to a process of synthesis and composition of open chain (ring), closed ring, linear branched and or substituted polyamines, polyamine derived tyrosine phosphatase inhibitors and PPAR partial agonists / partial antagonists via a series of substitution reactions and optimizing the bioavailability and biological activities of the compounds. Polyamines prevent the toxicty of neutoxins and diabetogenic toxins including paraquat, methyphenyl pyridine radical, rotenone, diazoxide, streptozotocin and alloxan. These polyamines can be to treat neurological, cardiovascular, endocrine acquired and inherited mitochondrial DNA damage diseases and other disorders in mammalian subjects, and more specifically to the therapy of Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease, Binswanger's disease, Olivopontine Cerebellar Degeneration, Lewy Body disease, Diabetes, Stroke, Atherosclerosis, Myocardial Ischemia, Cardiomyopathy, Nephropathy, Ischemia, Glaucoma, Presbycussis, Cancer, Osteoporosis, Rheumatoid Arthritis, Inflammatory Bowel Disease, Multiple Sclerosis and as Antidotes to Toxin Exposure.
Owner:MURPHY MICHAEL A
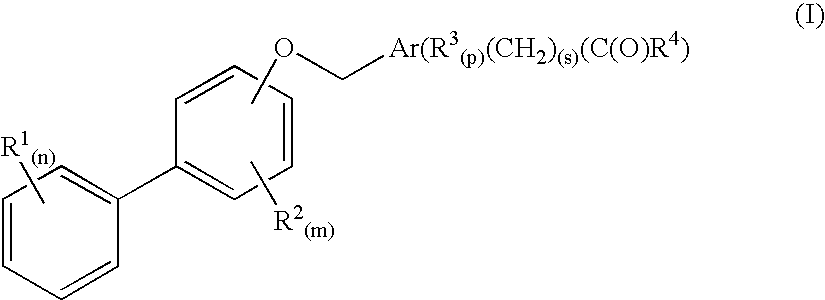
Biaryloxymethylarenecarboxylic acids as glycogen synthase activator
The present invention relates to compounds of formula (I) wherein R<1>, R<2>, R<3>, R<4>, m, n, p and s are as defined in the description and claims, and pharmaceutically acceptable salts thereof. The compounds are useful for the treatment and / or prophylaxis of diseases that are associated with the activation of the glycogen synthase enzyme, such as diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
Treatment of insulin resistance syndrome and type 2 diabetes with PDE9 inhibitors
InactiveUS20050070557A1Impaired glucose toleranceBiocideOrganic active ingredientsInsulin resistanceTreatment hypertension
This invention is directed to a method of treating insulin resistance syndrome (IRS), hypertension and / or type 2 diabetes in a mammal comprising administering to said mammal a cGMP PDE9 inhibitor or a pharmaceutical composition thereof. This invention is also directed to such methods wherein said cGMP PDE9 inhibitor is used in combination with other agents to treat IRS, hypertension and / or type 2 diabetes.
Owner:PFIZER INC
Methods for treatment of metabolic disorders using epimetabolic shifters, multidimensional intracellular molecules, or environmental influencers
InactiveUS20110020312A1Inhibit progressNormal mitochondrial functionBiocidePeptide/protein ingredientsMetabolic disorder
Methods and formulations for treating metabolic disorders in humans using epimetabolic shifters, multidimensional intracellular molecules or environmental influencers are described.
Owner:BERG
Retinoid-related receptor function regulating agent
InactiveUS6545009B1Excellent function-activating effectGood effectBiocideOrganic chemistryIGT - Impaired glucose toleranceRetinoid
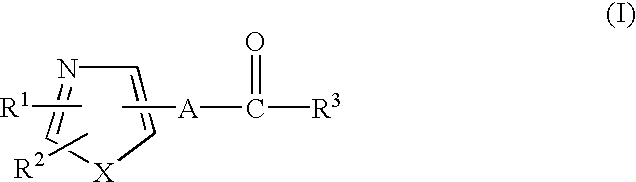
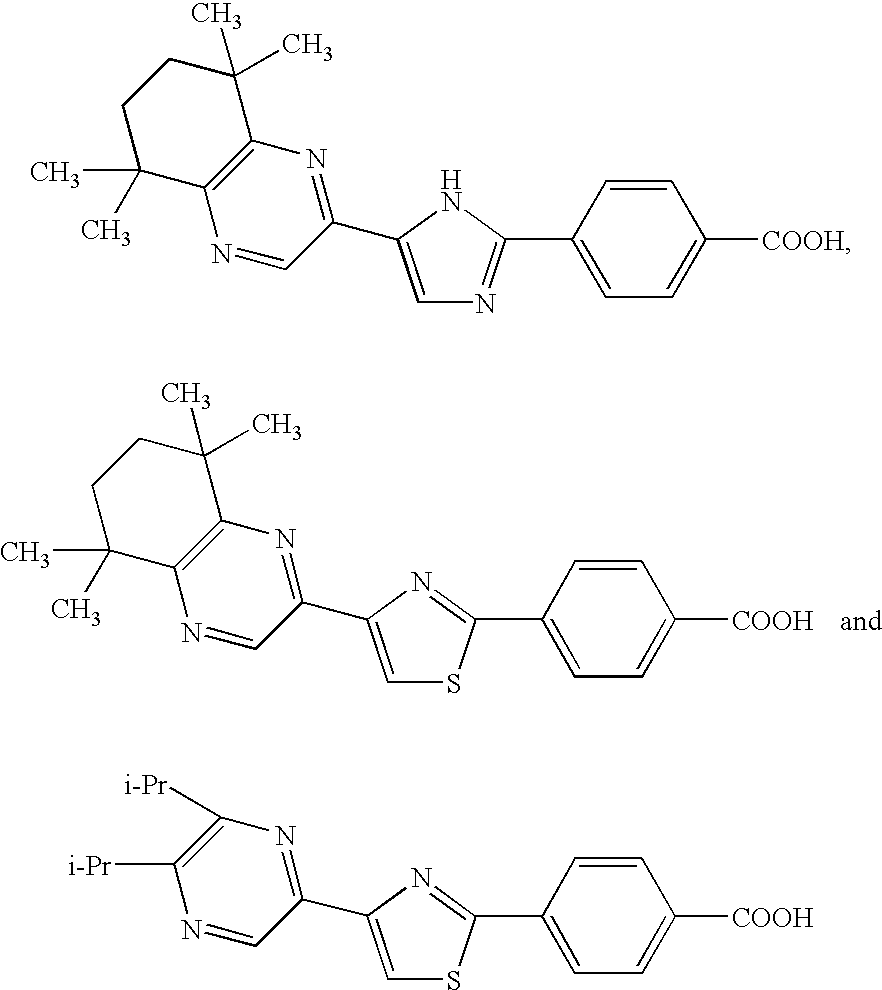
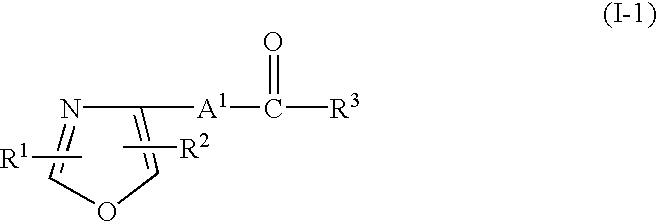
1,3-Azole derivatives, pharmaceutical compositions thereof and methods for regulating the function of retinoid-related receptors with 1,3-azole derivatives are disclosed. Such regulation may be useful for preventing or treating diabetes, preventing or treating hyperlipidemia, preventing or treating impaired glucose tolerance (IGT) or for preventing transition from impaired glucose tolerance to diabetes.
Owner:TAKEDA PHARMA CO LTD
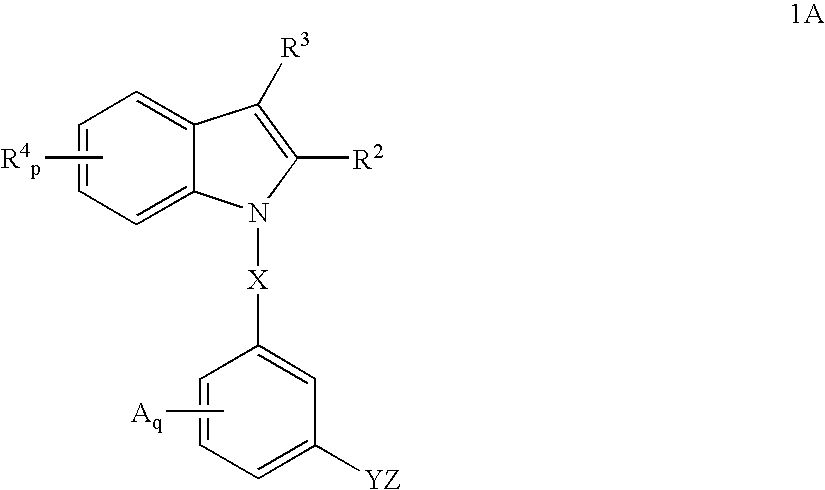
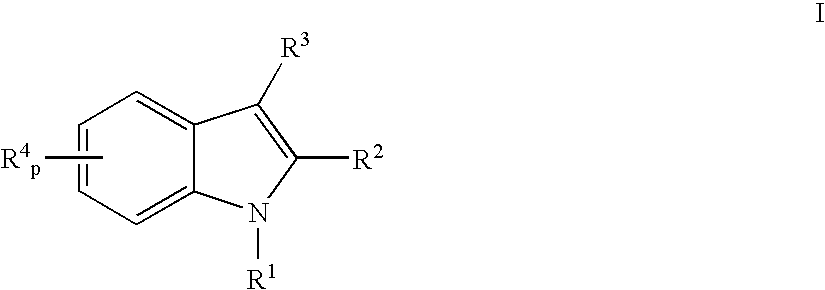
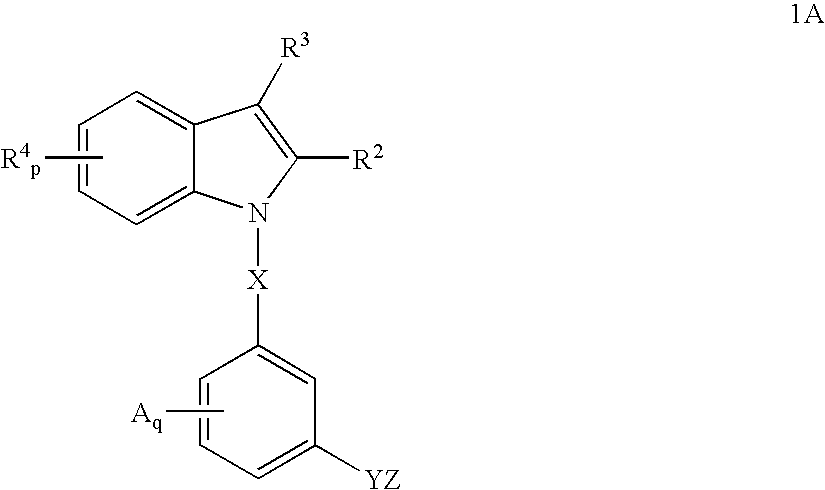
Indoles having anti-diabetic activity
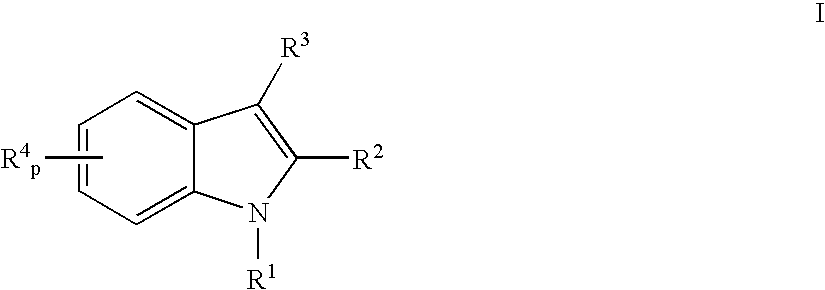
ActiveUS7186746B2Efficacious in treatmentLowering glucose, lipids, and insulinBiocideSenses disorderDyslipidemiaAcute hyperglycaemia
Indoles having aryloxyalkanoic acid substituents or arylalkanoic acid substituents are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME LLC
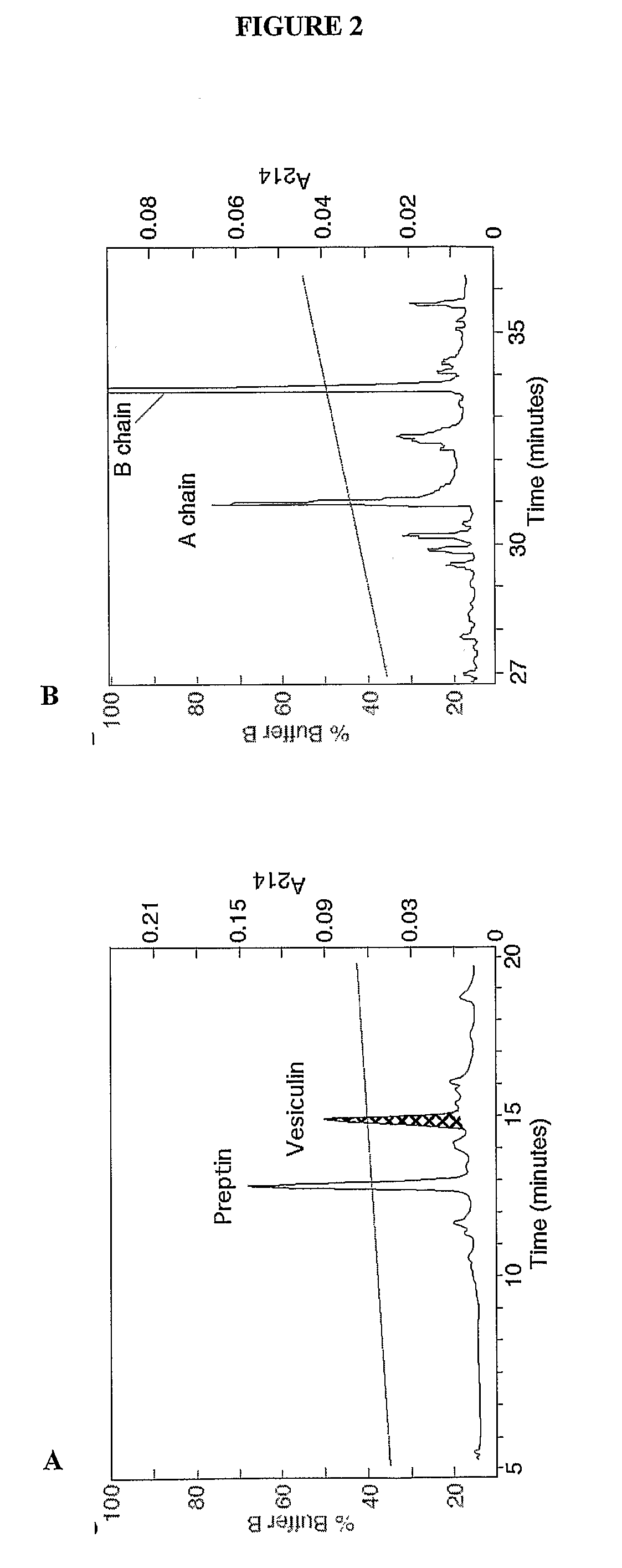
Vesiculins
InactiveUS20090054320A1Impaired fasting glucoseImpaired glucose toleranceBacteriaPeptide/protein ingredientsPeptideAntibody
The inventions relate generally to vesiculin peptides and vesiculin peptide chains, and fragments, variants and derivatives thereof, related compositions and formulations and their preparation and use, nucleic acids encoding such vesiculin peptides and vesiculin peptide chains, and fragments, variants and derivatives thereof and related vectors and host cells, hybridomas and antibodies, and methods for the prevention and treatment of conditions, diseases and disorders that would be improved, eased, or lessened by the administration of a composition of the invention, including but not limited to glucose metabolism diseases.
Owner:ORAYA THERAPEUTICS +1
Method of treatment of vascular complications
InactiveUS20120058105A1Improve abilitiesPromote repairBiocideOrganic active ingredientsIGT - Impaired glucose toleranceCarbohydrate tolerance
The present invention provides methods for the prevention or treatment of one or more vascular complication(s) in a subject at risk of developing diabetes mellitus, impaired glucose tolerance and / or hyperglycemia or a subject suffering from diabetes mellitus, impaired glucose tolerance and / or hyperglycemia, wherein an amount of a composition effective to inhibit, repress, delay or otherwise reduce expression and / or activity and / or level of TXNIP and / or an amount of a composition effective to induce, enhance or otherwise increase expression and / or activity and / or level of TRX is / are administered to a subject in need thereof. The present invention also provides methods for identifying and isolating modulators of TXNIP expression and / or activity and / or level and / or TRX expression and / or activity and / or level for use in such therapeutic and prophylactic methods.
Owner:HEART RES INST LTD
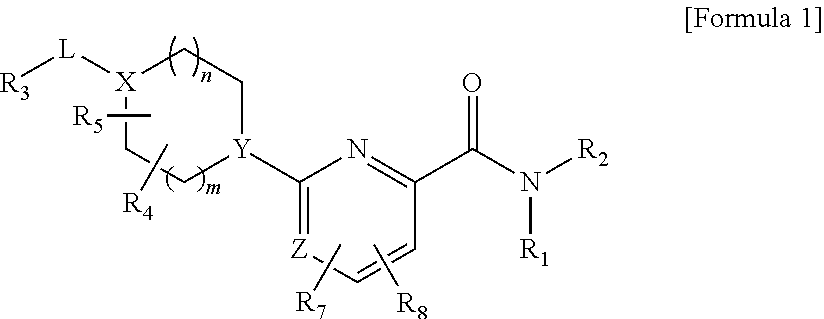
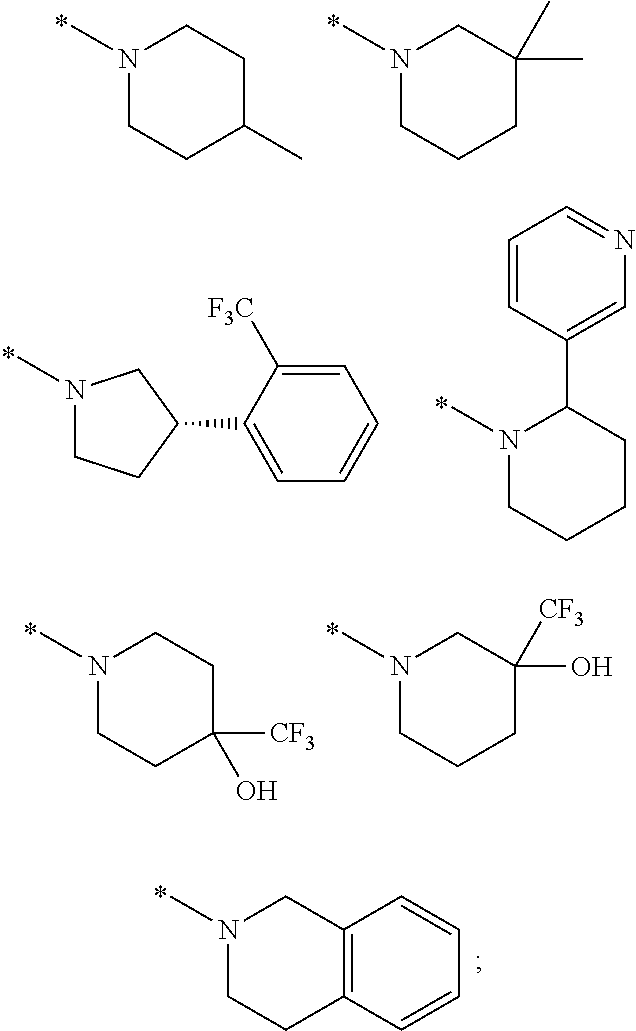
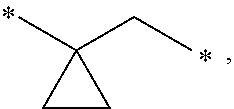
Picolinamide and pyrimidine-4-carboxamide compounds, process for preparing and phamaceutical composition comprising the same
ActiveUS20130210811A1Selective inhibitory activityImpaired glucose toleranceAntibacterial agentsBiocideDyslipidemiaGlucocorticoid
Provided are picolinamide and pyrimidine-4-carboxamide compounds, a method for preparing the same, a pharmaceutical composition containing the same, and a medical use using the compound as an agent for preventing, regulating, and treating diseases related to regulation of glucocorticoids by using selective inhibitory activity of the compound for an 11β-HSD1 enzyme. The picolinamide and pyrimidine-4-carboxamide compounds of the present invention are selective inhibitors of human-derived 11β-HSD1 enzymes, and are useful in an agent for preventing, regulating, and treating diseases related to glucocorticoid regulation in which human-derived 11β-HSD1 enzymes are involved, for example, metabolic syndromes such as, type 1 and type 2 diabetes, diabetes later complications, latent autoimmune diabetes adult (LADA), insulin tolerance syndromes, obesity, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), damaged glucose tolerance, dyslipidemia, atherosclerosis, hypertension, etc.
Owner:SK CHEM CO LTD
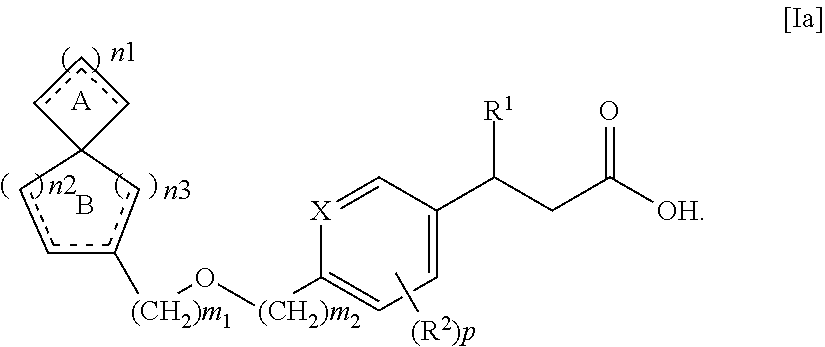
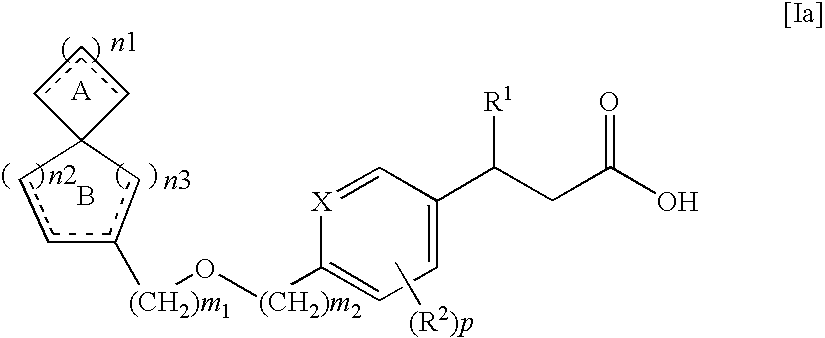
Spiro compounds and pharmaceutical use thereof
ActiveUS8299296B2Impaired glucose toleranceImpaired fasting glucoseBiocideSenses disorderSpiro compound
The spiro compound represented by the following general formula [Ia], its pharmaceutically acceptable salt or a solvate thereof
Owner:JAPAN TOBACCO INC
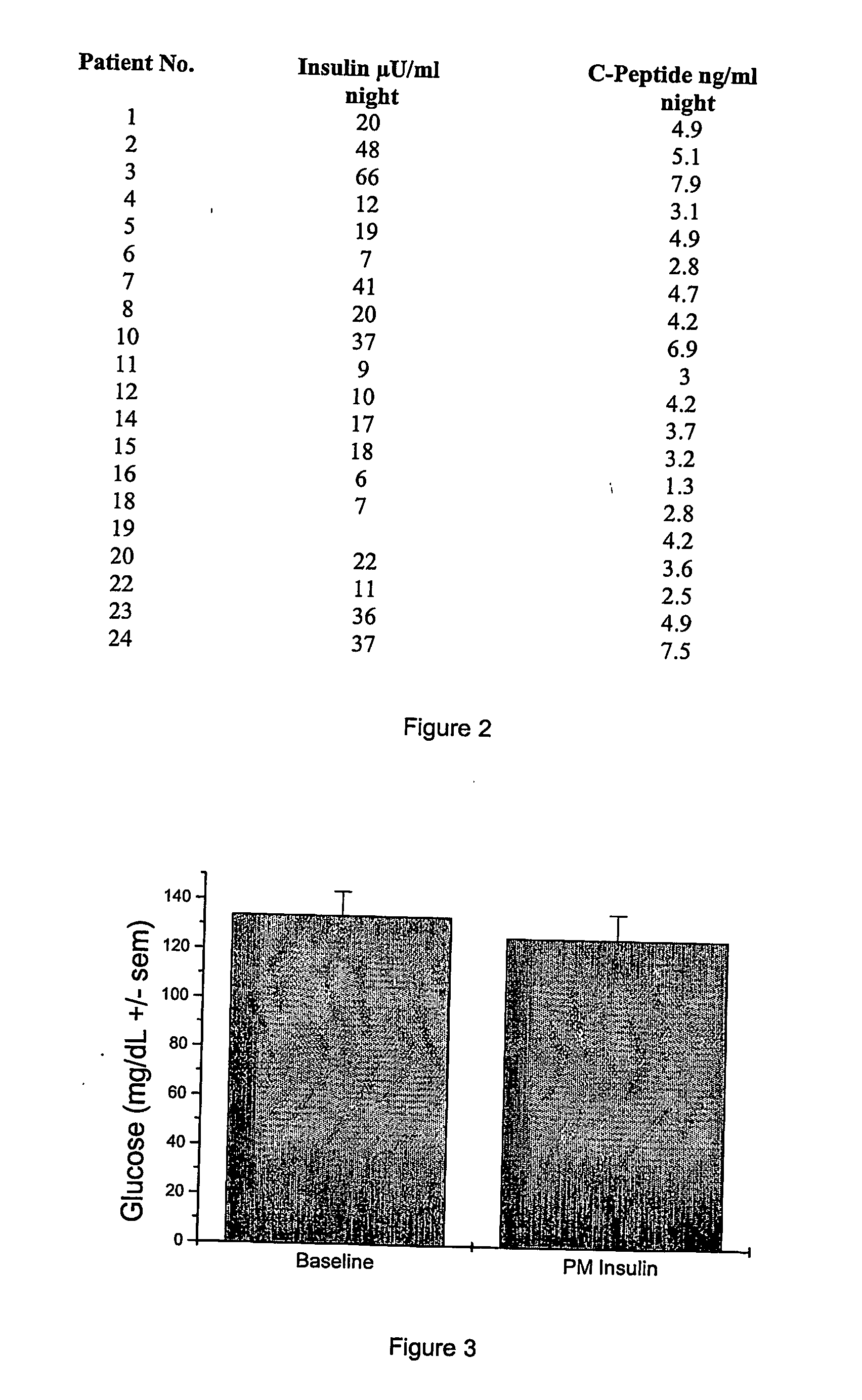
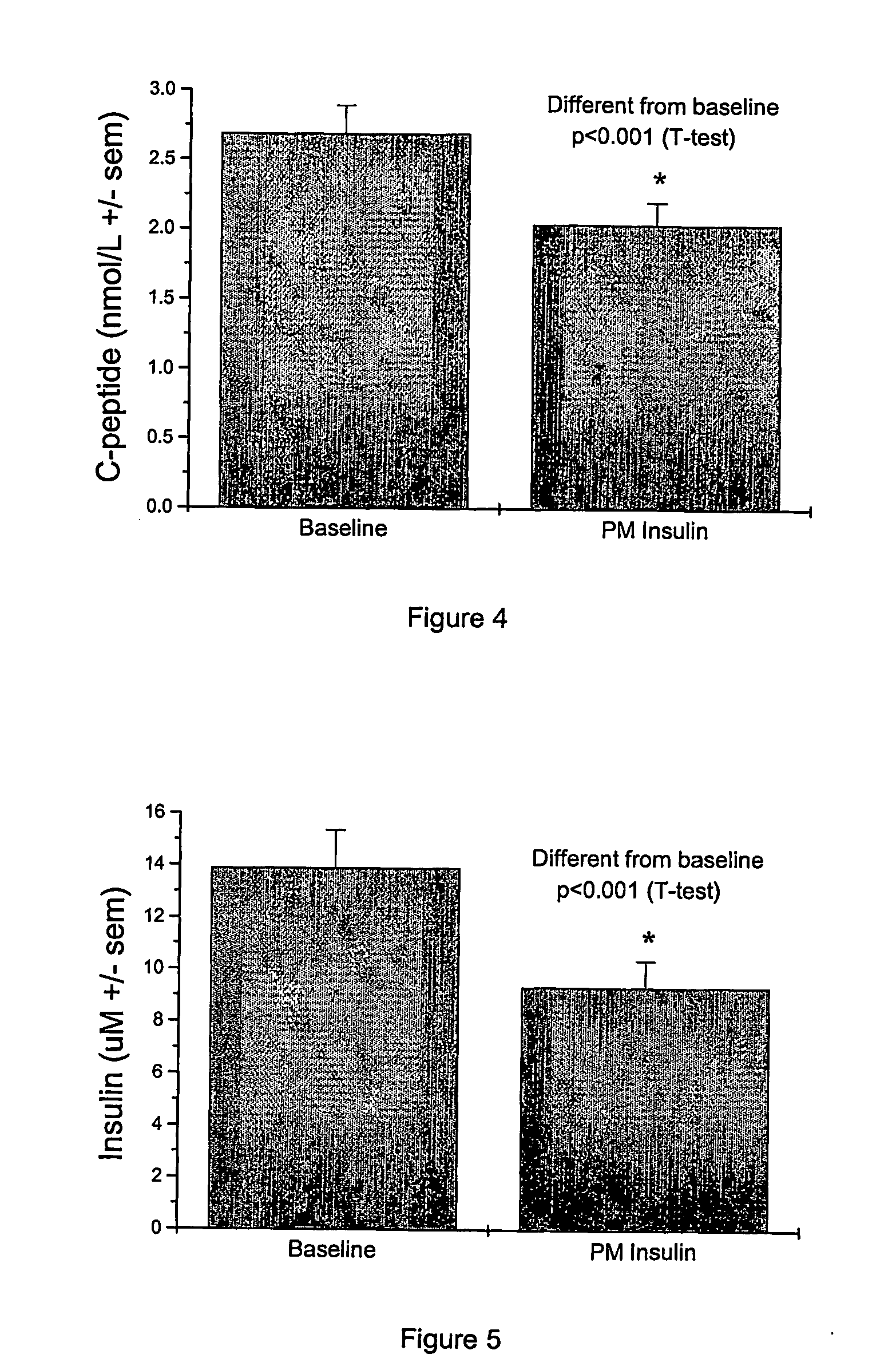
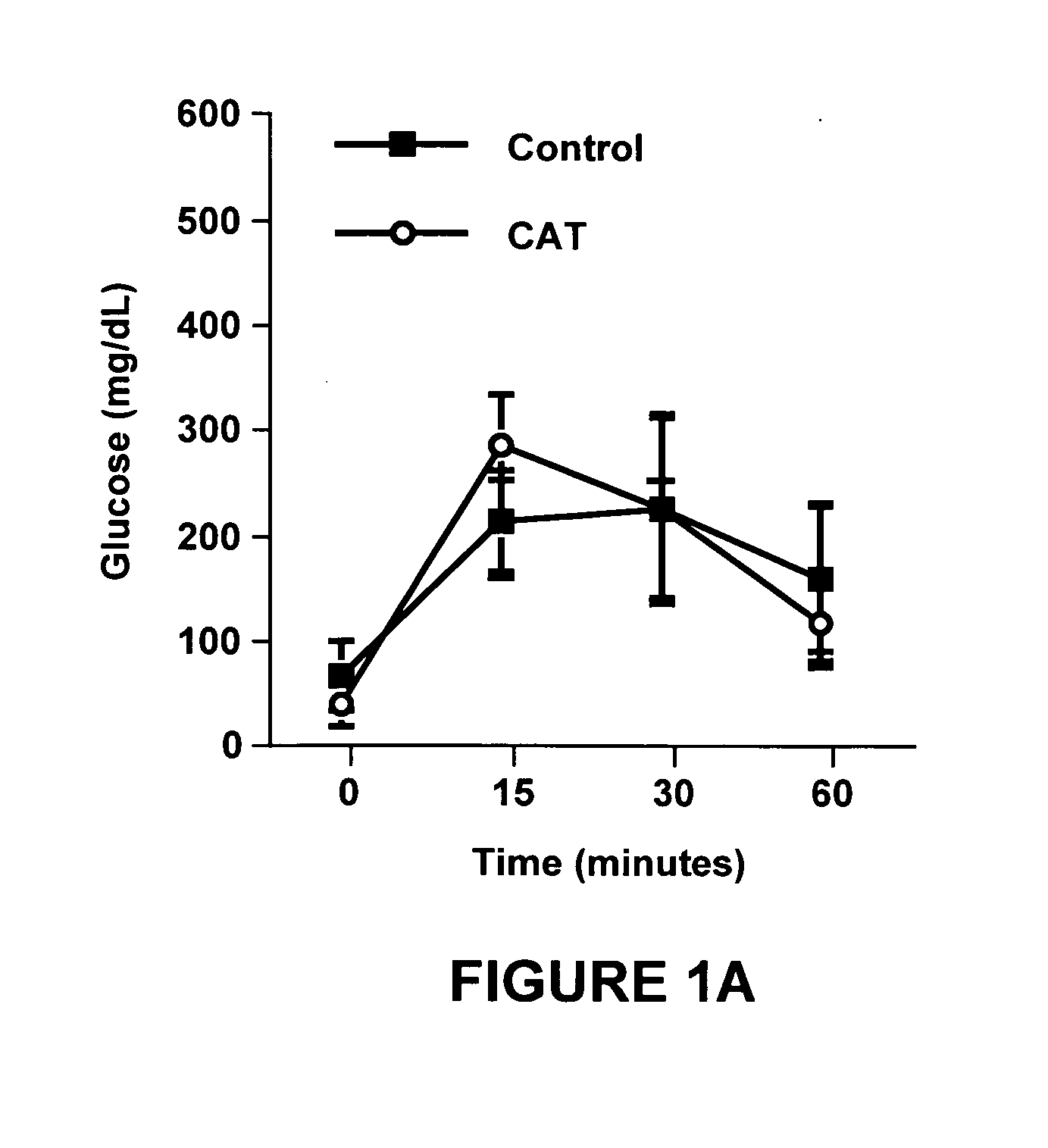
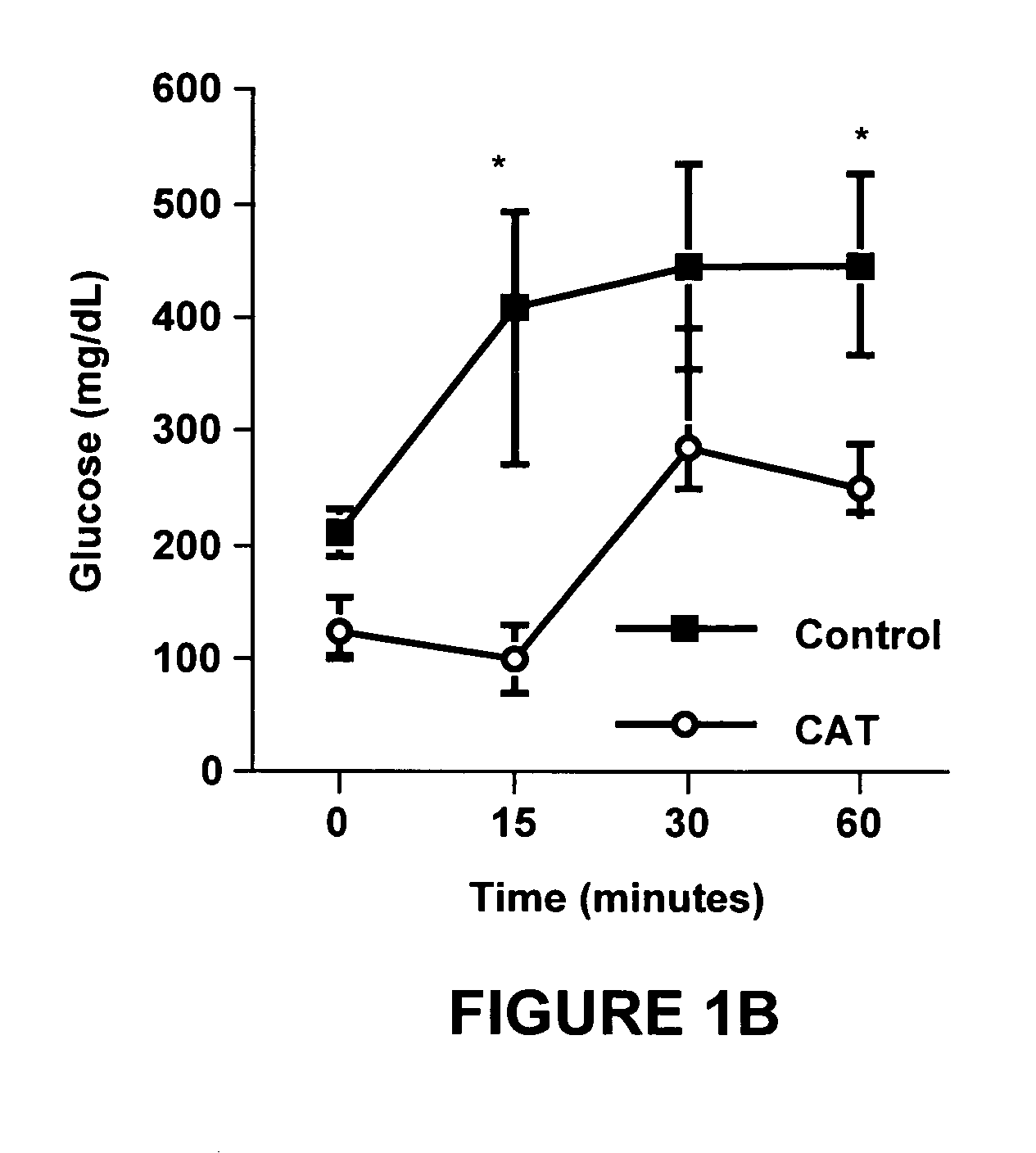
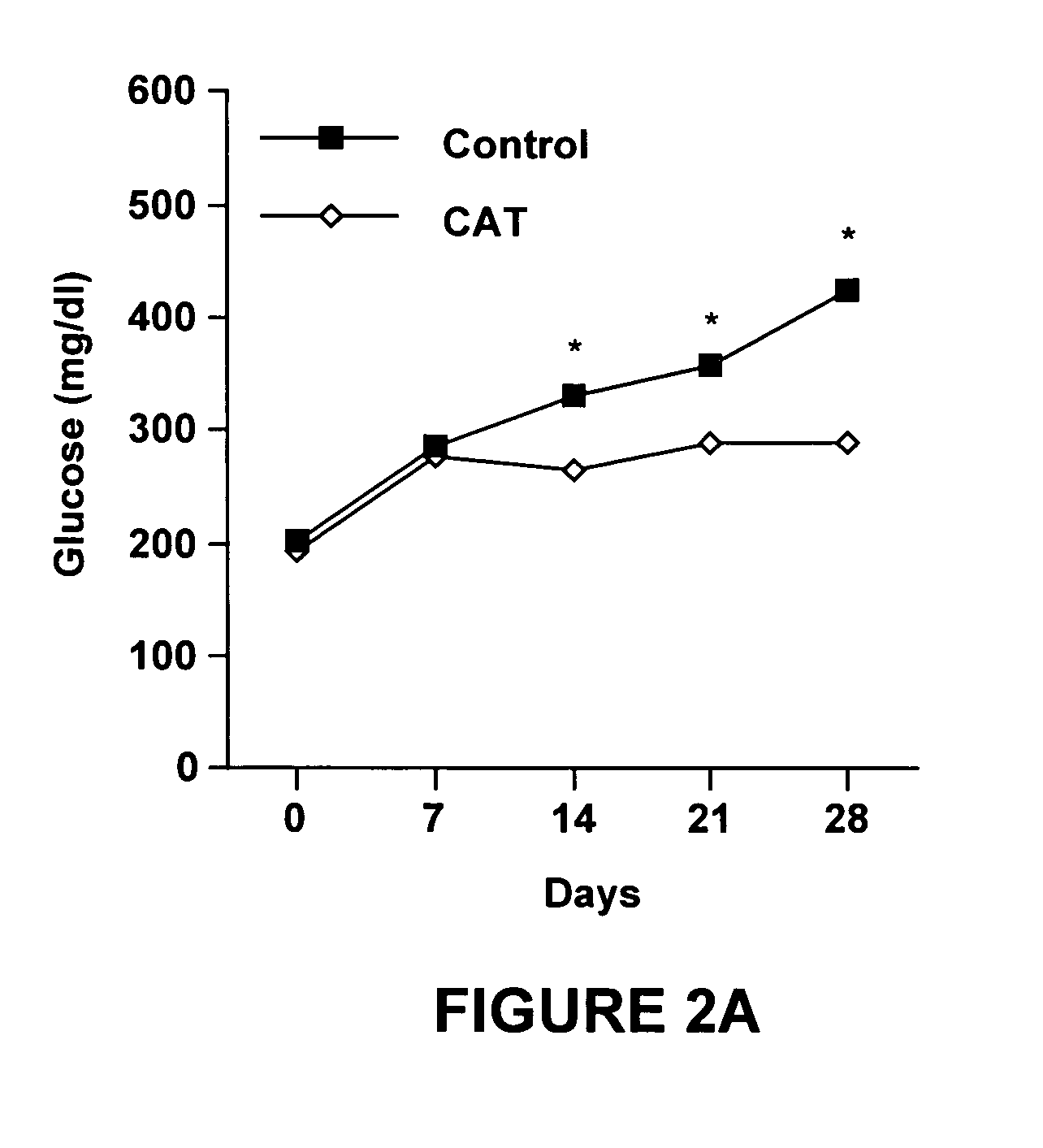
Night-time oral insulin therapy
ActiveUS20060178296A1Facilitates insulin transportPreventing beta cell deathPowder deliveryPeptide/protein ingredientsIGT - Impaired glucose toleranceInsulin dependent diabetes
A method for protection of a mammal that has impaired glucose tolerance or early stage diabetes mellitus from developing overt or insulin dependent diabetes comprises administering an orally effective dose of a pharmaceutical formulation comprising insulin at nighttime, e.g., at or shortly before bedtime.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Method of using catalpic acid to treat and prevent type 2 diabetes and associated disorders
ActiveUS20060160898A1Impaired glucose tolerancePrevent hyperglycemiaBiocideMetabolism disorderDiseaseObesity
A method of treating and preventing type 2 diabetes and obesity an animal, including mammals and humans, in which a therapeutically effective amount of catalpic acid to the animal is administered orally or parentally.
Owner:NUTRITION THERAPEUTICS
Method for detecting mild impaired glucose tolerance or insulin hyposecretion
ActiveUS20050214885A1Simplified determinationGood reproducibilityOrganic chemistryMicrobiological testing/measurementIGT - Impaired glucose toleranceDefinite period
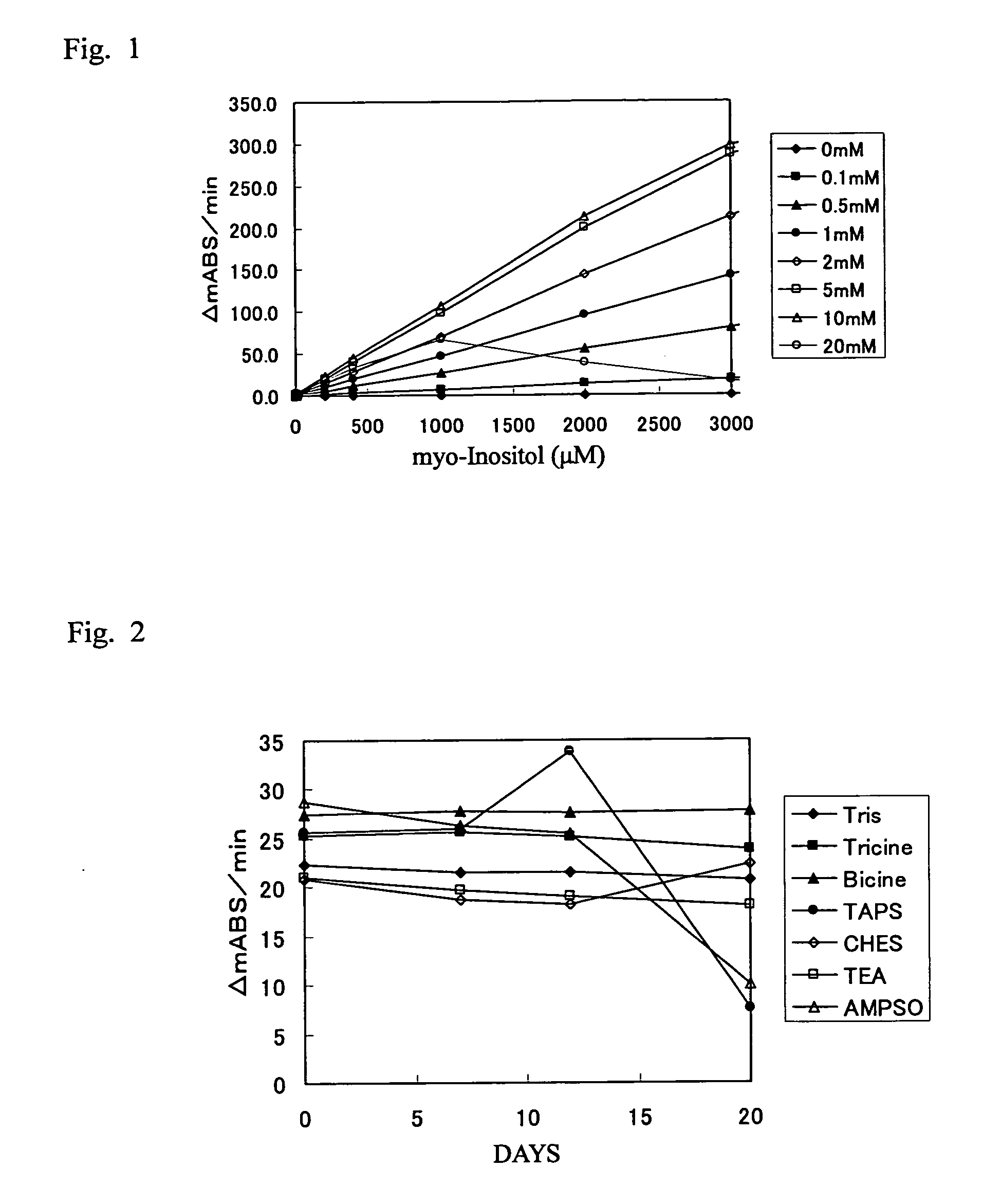
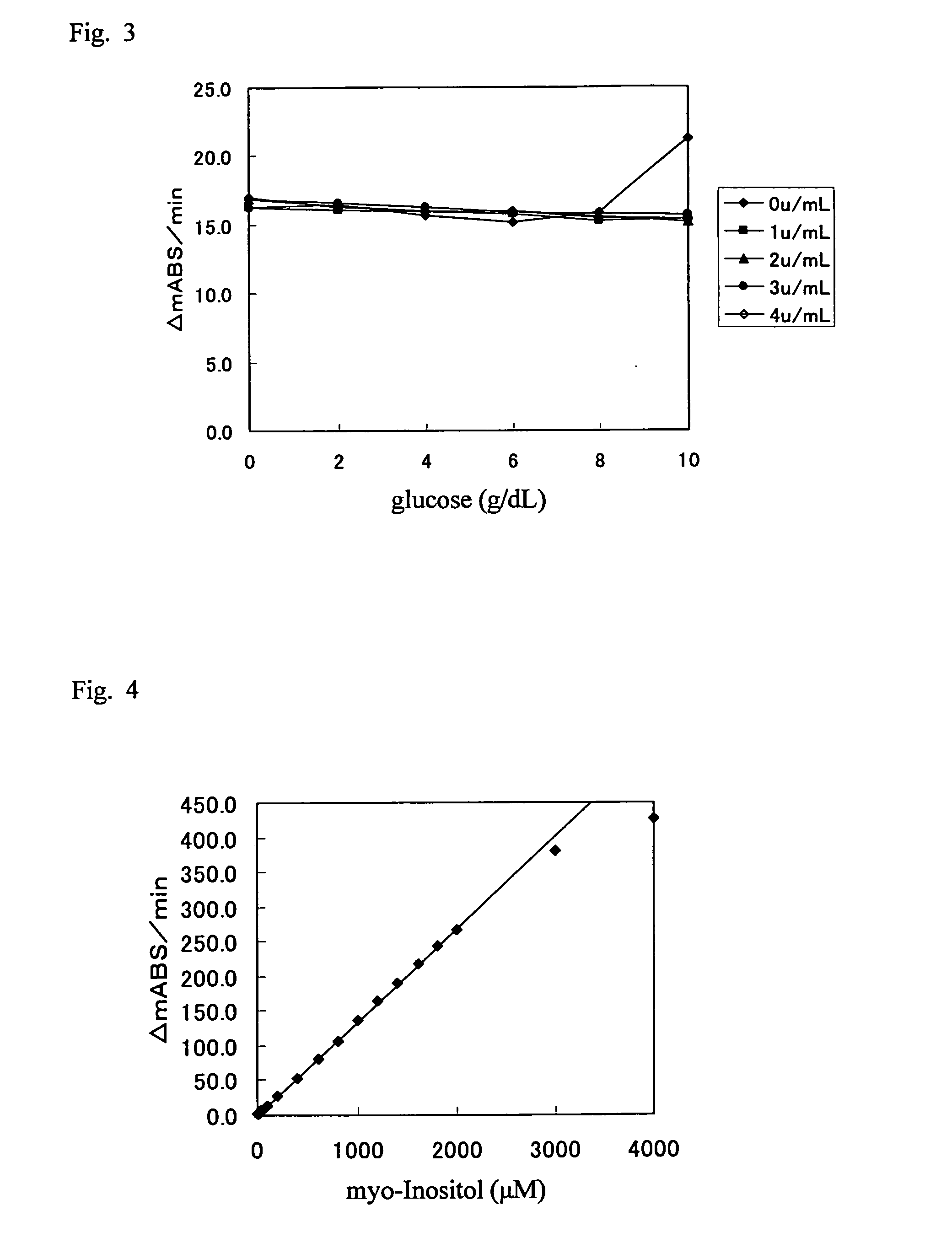
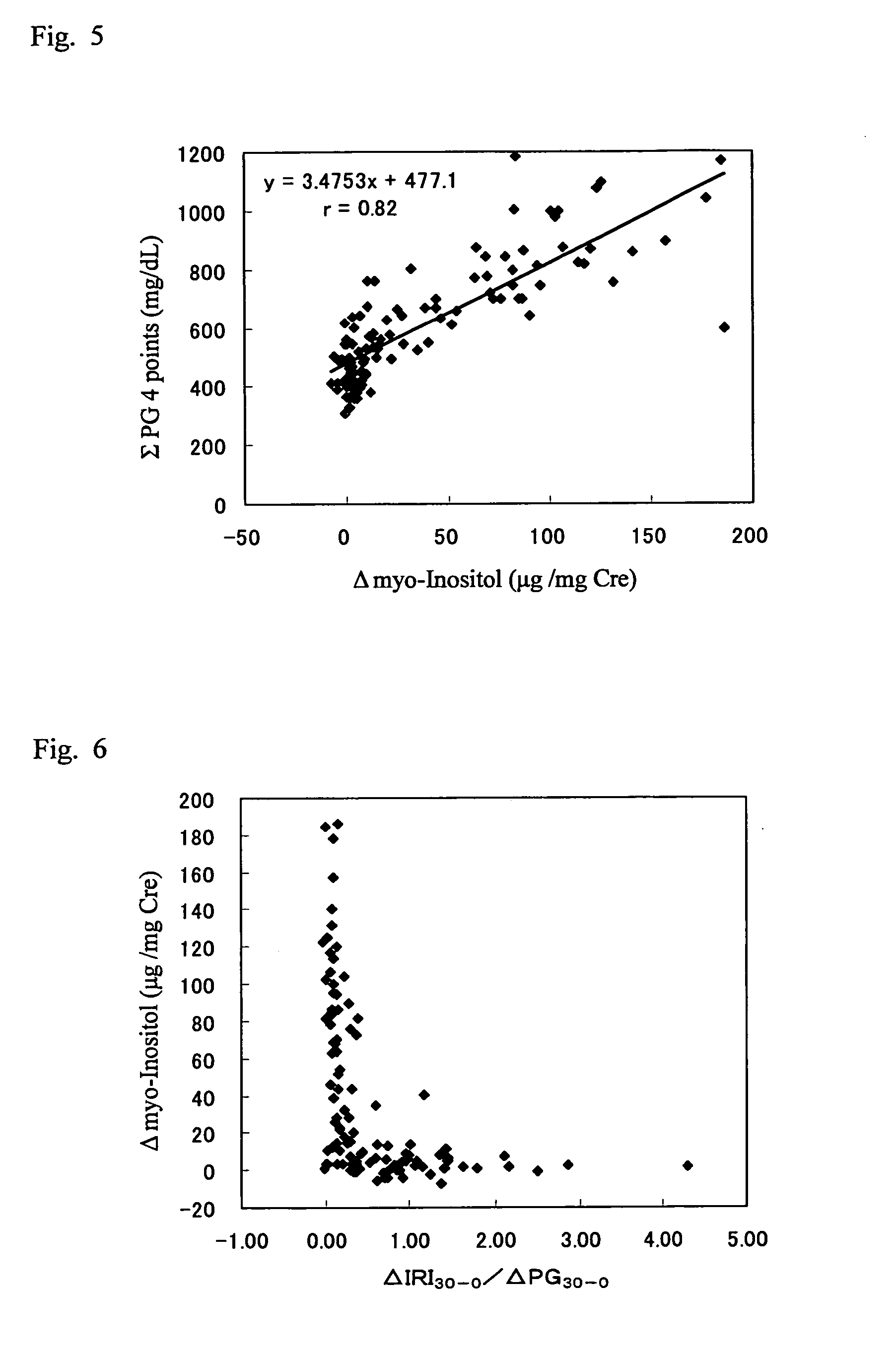
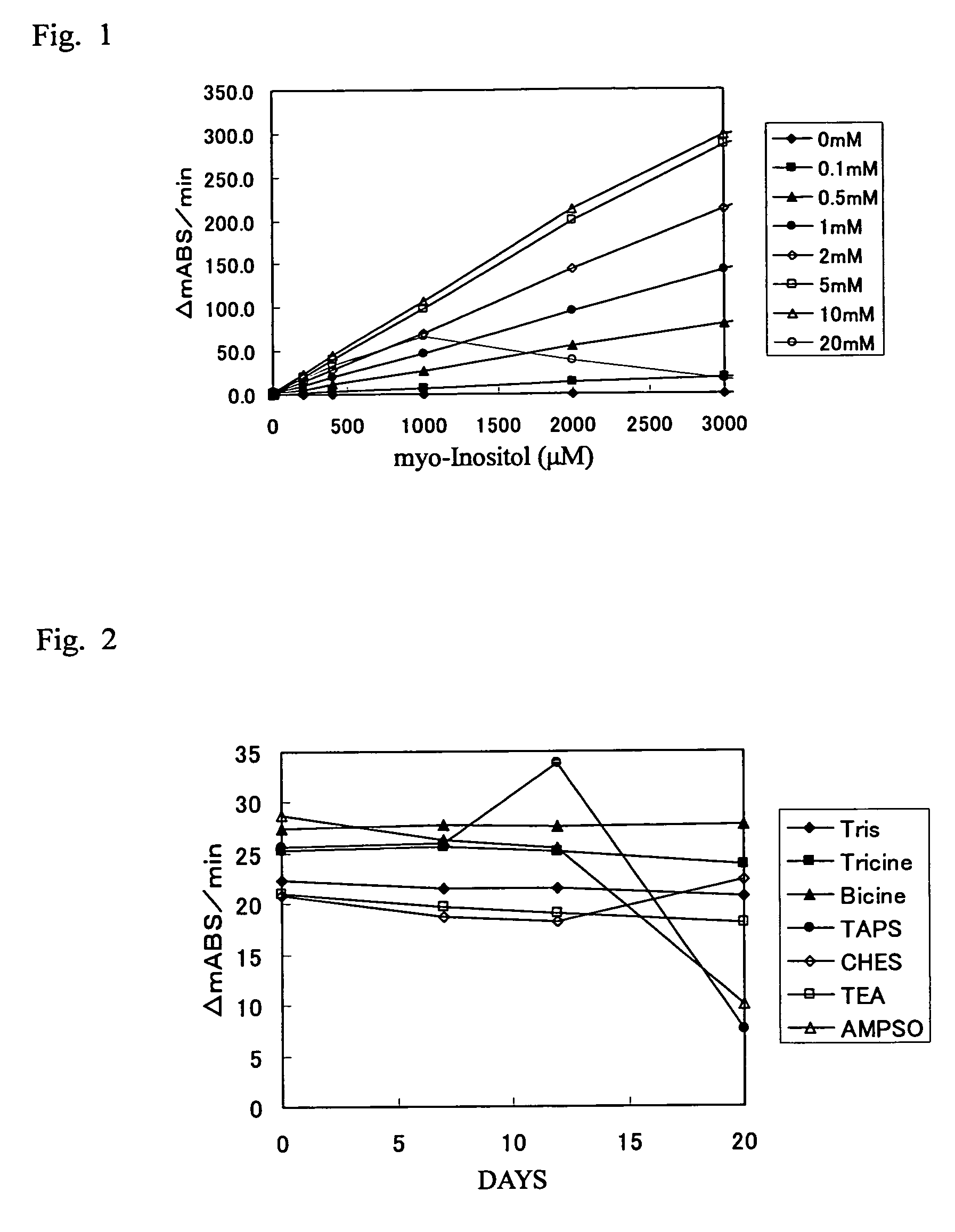
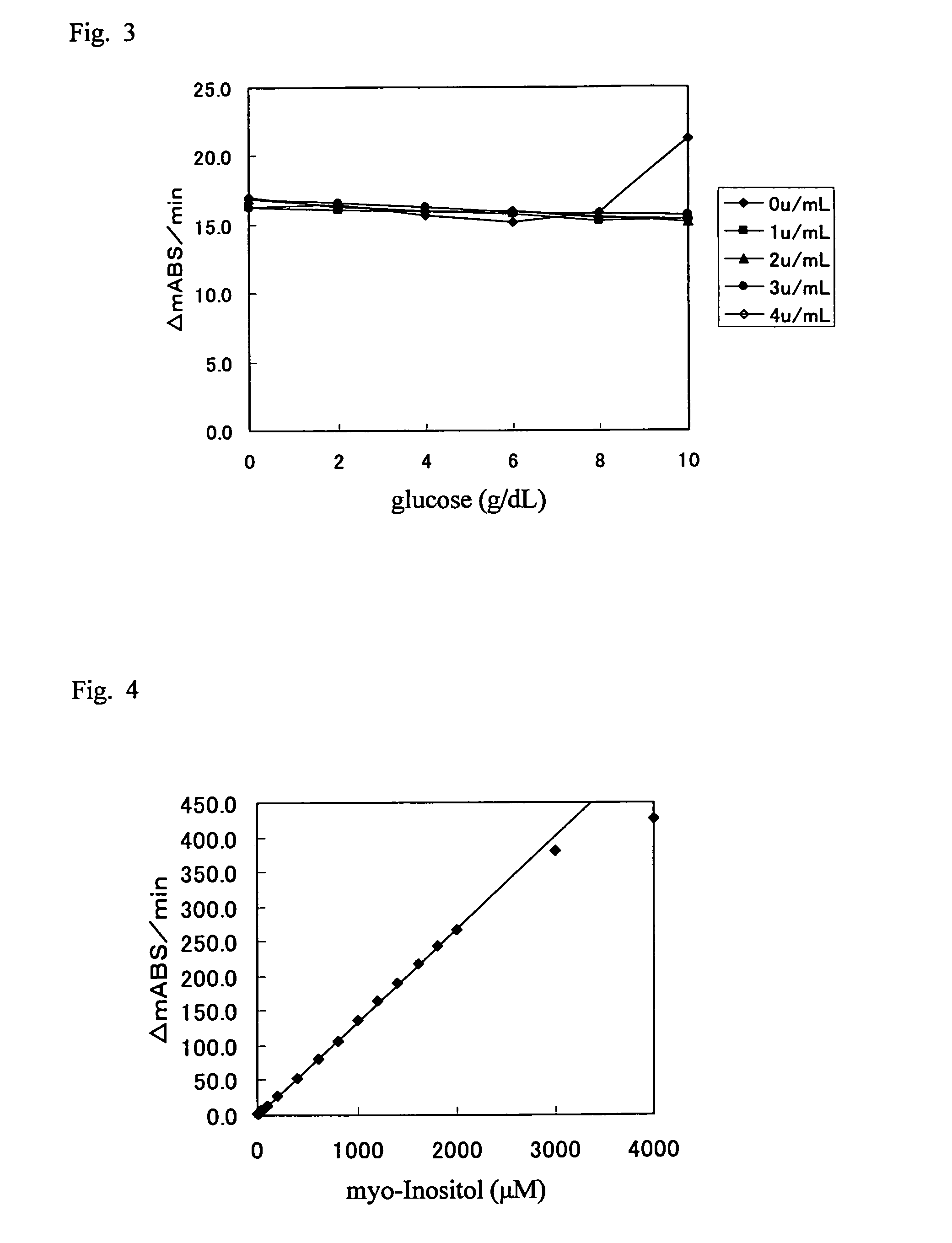
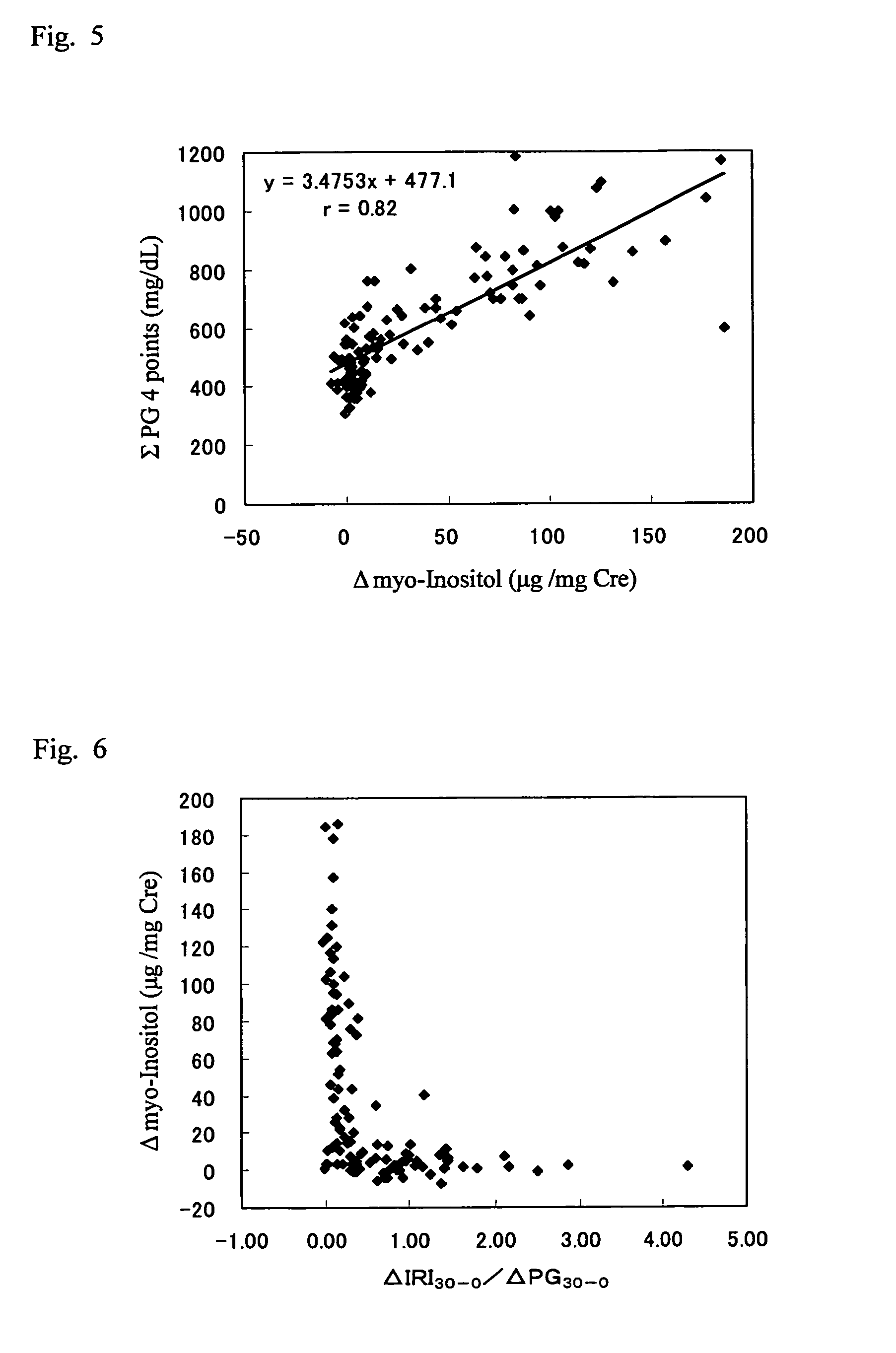
It is intended to provide a noninvasive method of conveniently detecting mild impaired glucose tolerance and / or insulin hyposecretion at the early stage with the use of an enzyme. Namely, mild impaired glucose tolerance and / or hyposecretion at the early stage are detected by quantifying myoinositol secreted into the urine before loading glucose and after loading glucose for a definite period of time with the use of a reagent and comparing the increase (or the increase ratio) in the myoinositol content thus measured with a characteristic level which has been preliminarily determined in normal subjects.
Owner:ASAHI KASEI PHARMA
Chickpea Extracts As Therapeutic Agents And Foods In The Treatment And Prevention Of Obesity And Non-Insulin-Depenent Diabetes
InactiveUS20080138453A1Good resultRemarkable effectBiocideMetabolism disorderLDL - Low density lipoproteinHigh fat diet
This invention discloses the uses of chickpea (Cicer arietinum) extract in the preparation of food for the prevention and treatment of obesity and non-insulin-dependent diabetes (NIDD). The invention provides a therapeutic agent and food containing chickpea extract for the prevention and treatment of obesity and NIDD. In this invention, the effect of chickpea extract to the high fat diet fed mice tested by the experiment proves that the chickpea extract can significantly decrease the levels of triglyceride, cholesterol and low density lipoproteins caused by high fat diet taken in a long period of time. Additionally, it is found in the experiments that the chickpea extract can improve the hyposensitivity to insulin. The preparation of the extract is also disclosed herein.
Owner:JUMPSUN BIO MEDICINE SHANGHAI
Pyrimidyl indoline compound
It is intended to provide a pyrimidyl indoline compound which structurally differs from compounds used as active ingredients in conventional oral hypoglycemic agents and has excellent hypoglycemic effect. The present invention provides a compound represented by the general formula (I) or a pharmaceutically acceptable salt thereof:
Owner:DAIICHI SANKYO CO LTD
Method of detecting mild impaired glucose tolerance or insulin secretory defect
InactiveUS7452687B2Simplified determinationGood reproducibilityOrganic chemistryMicrobiological testing/measurementIGT - Impaired glucose toleranceDefinite period
It is intended to provide a noninvasive method of conveniently detecting mild impaired glucose tolerance and / or insulin hyposecretion at the early stage with the use of an enzyme. Namely, mild impaired glucose tolerance and / or hyposecretion at the early stage are detected by quantifying myoinositol secreted into the urine before loading glucose and after loading glucose for a definite period of time with the use of a reagent and comparing the increase (or the increase ratio) in the myoinositol content thus measured with a characteristic level which has been preliminarily determined in normal subjects.
Owner:ASAHI KASEI PHARMA
Composition for treating diabetes and metabolic diseases and a preparation method thereof
ActiveUS20140350304A1Inhibiting glycemiaPromote absorptionOrganic chemistryMetabolism disorderDiseaseBlood sugar
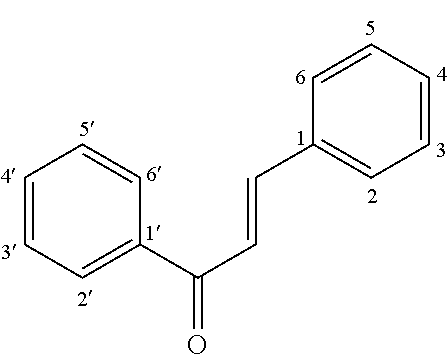
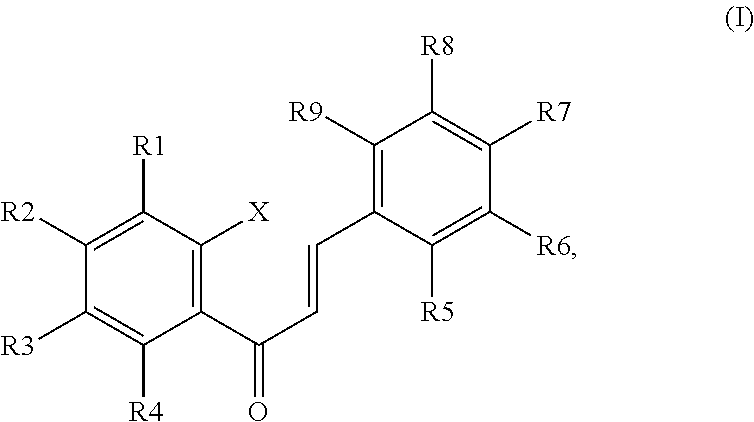
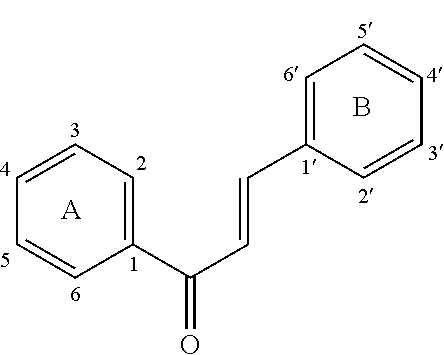
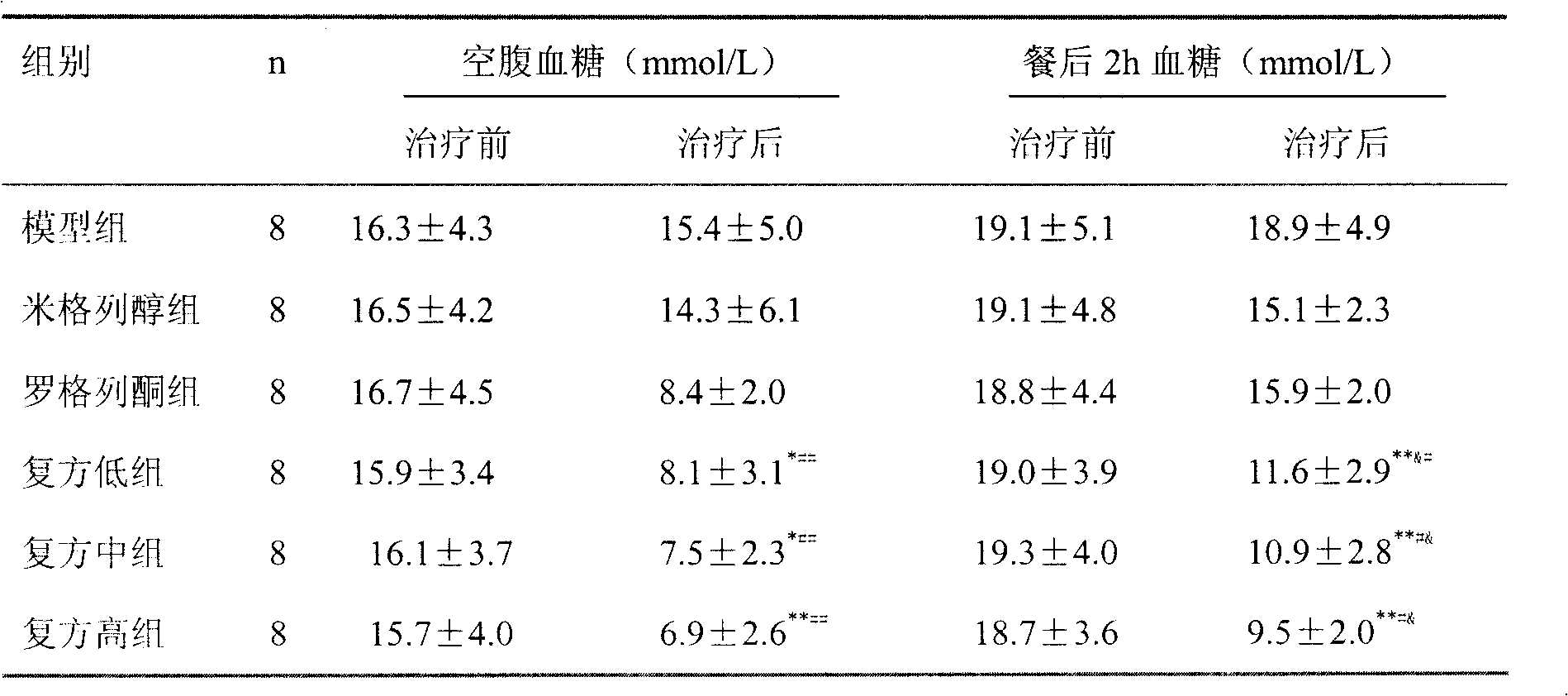
Disclosed is a chalcone composition for treating diabetes and metabolic syndromes. In particular, the chalcone compound bound with 2-halogen in ring A significantly decreases the blood glucose level in the in vitro anti-diabetic effect experiment. In the in vivo animal model, the leading chalcone compound can prevent the progression of diabetes and control the blood glucose level, and there is no significant difference in the gains in body weight. Throughout the seven-week administration, there are no hepatic or renal toxicity observed.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Medicine composition containing insulin intensifier and miglitol
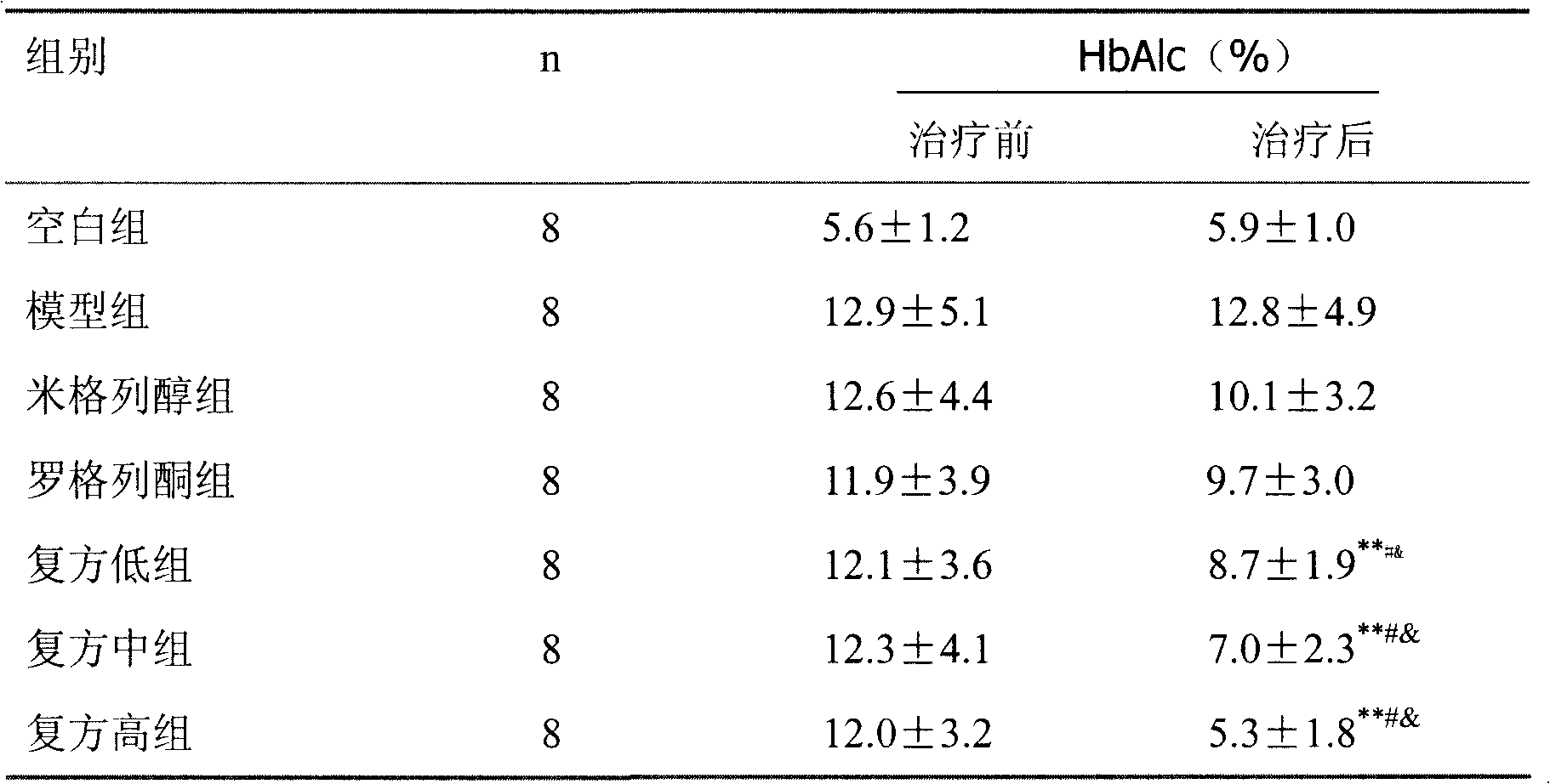
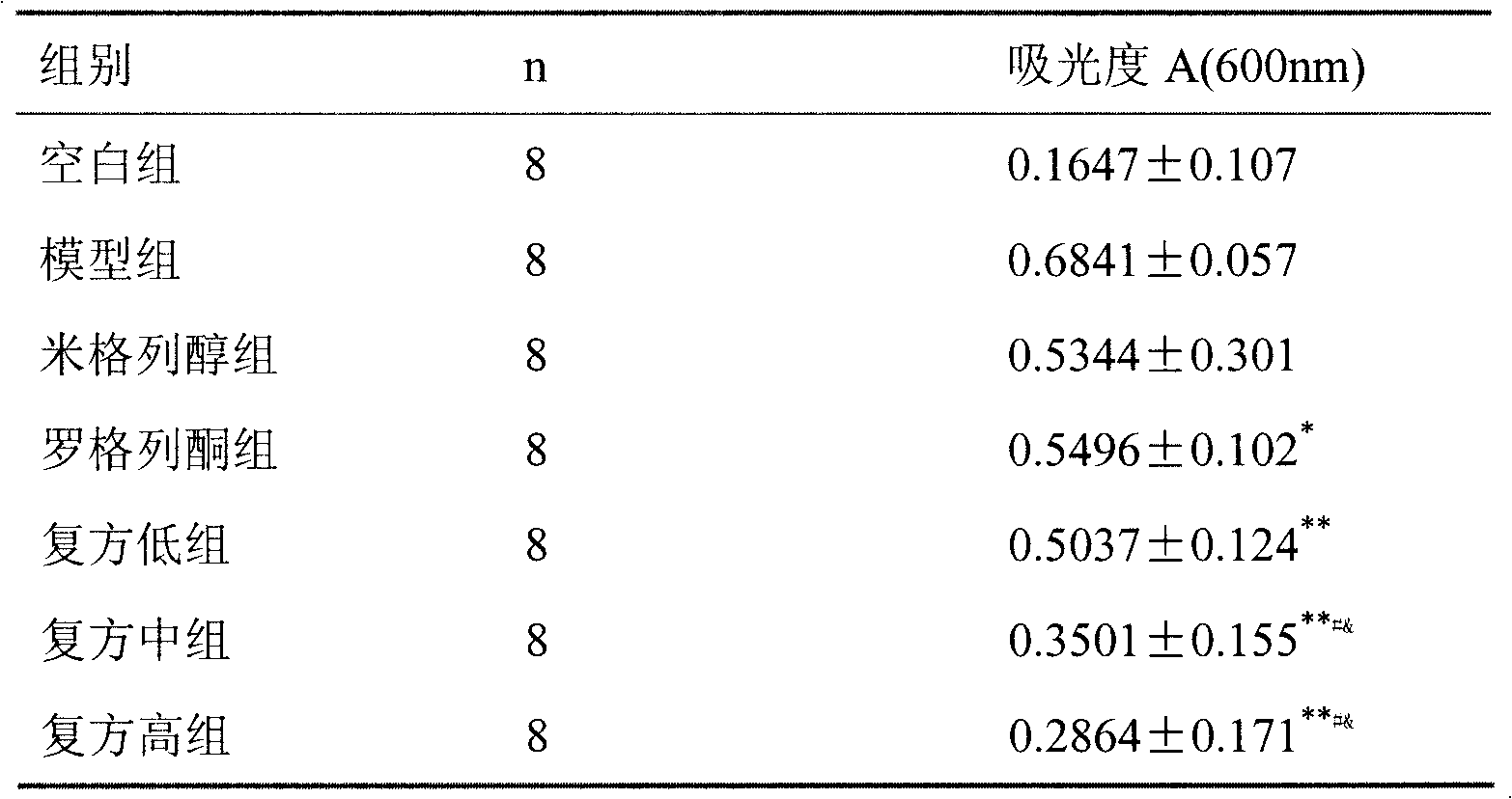
ActiveCN101121004BReduce complicationsImprove risk factorsMetabolism disorderPharmaceutical non-active ingredientsMiglitolAcute hyperglycaemia
The present invention relates to an oral blood-sugar-reducing compound medicinal preparation, which consists of an insulin sensitizer, a miglitol and auxiliary materials. Compared with the prior art, the present invention is characterized in that under a circumstance of a same curative effect, a separate dosage of the insulin sensitizer or the miglitol is reduced; at the same time compared with other hypoglycemic drugs, a side effect of the present invention is reduced; the insulin sensitizer and the miglitol have a synergistic effect: the insulin sensitizer and the miglitol respectively takecurative actions towards a patient with hyperglycemia synchronously according to the different pharmacological effects and directly provide the patient or a doctor with a scientific combined medication to improve the curative effect and provide a clinic or the patient with convenience.
Owner:LUNAN PHARMA GROUP CORPORATION
Antidiabetic oxazolidinediones and thiazolidinediones
InactiveUS20070173434A1Efficacious in treatmentLowering glucose, lipids, and insulinBiocideSenses disorderThiazolidinesCholemia
Phenoxyphenyl and phenoxybenzyl oxazolidine-2,4-diones and thiazolidine-2,4-diones of formula (I) are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
Cylcoalkenyl derivatives useful as agonists of the GPR120 and/or GPR40 receptors
ActiveUS11230526B1Impaired glucose toleranceElevate fasting glucoseOrganic chemistryMetabolism disorderDyslipidemiaPharmaceutical drug
The present invention is directed to cycloalkenyl derivatives, pharmaceutical compositions containing them and their use in the treatment of disorders and conditions modulated by the GPR120 and / or GPR40 receptors. More particularly, the compounds of the present invention are agonists of GPR120 and / or GPR40, useful in the treatment of, for example, obesity, Type II Diabetes Mellitus, dyslipidemia, etc.
Owner:JANSSEN PHARMA NV
Composition for amelioration/prevention of adverse side effect in steroid therapy
InactiveCN103120655AImprove side effectsImprovement or suppression of side effectsOrganic active ingredientsAntipyreticDiseaseSide effect
Owner:EA PHARMA CO LTD
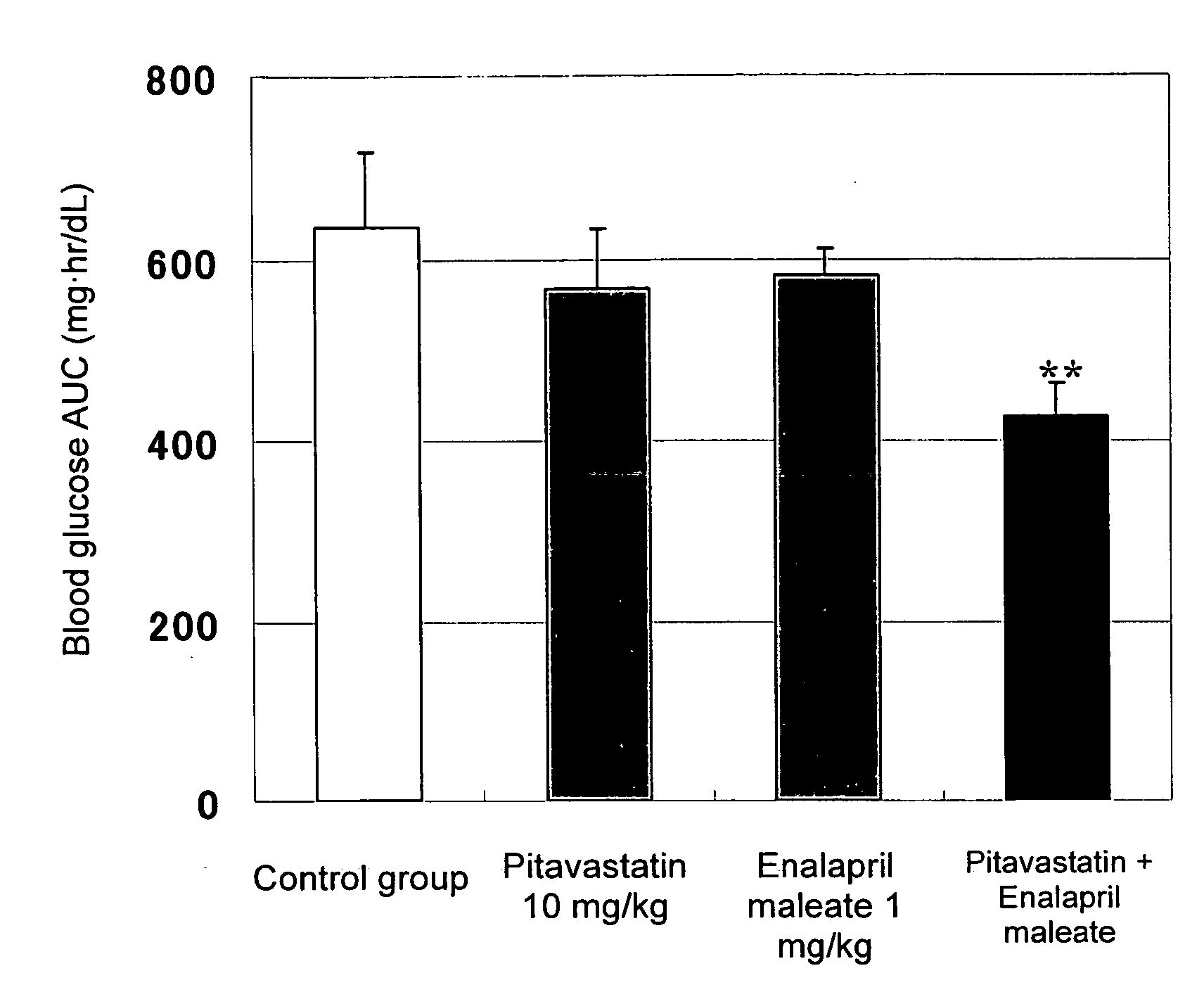
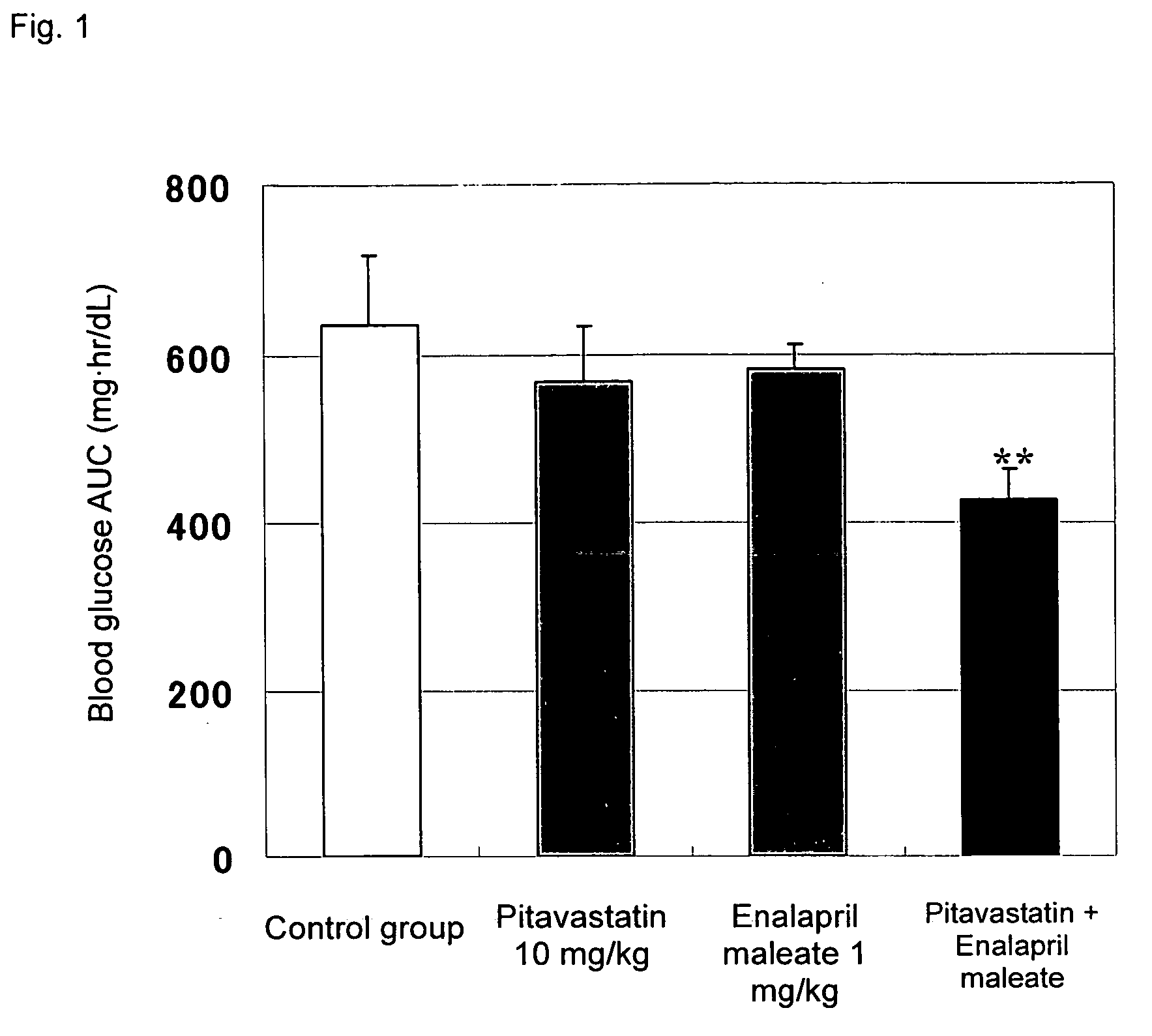
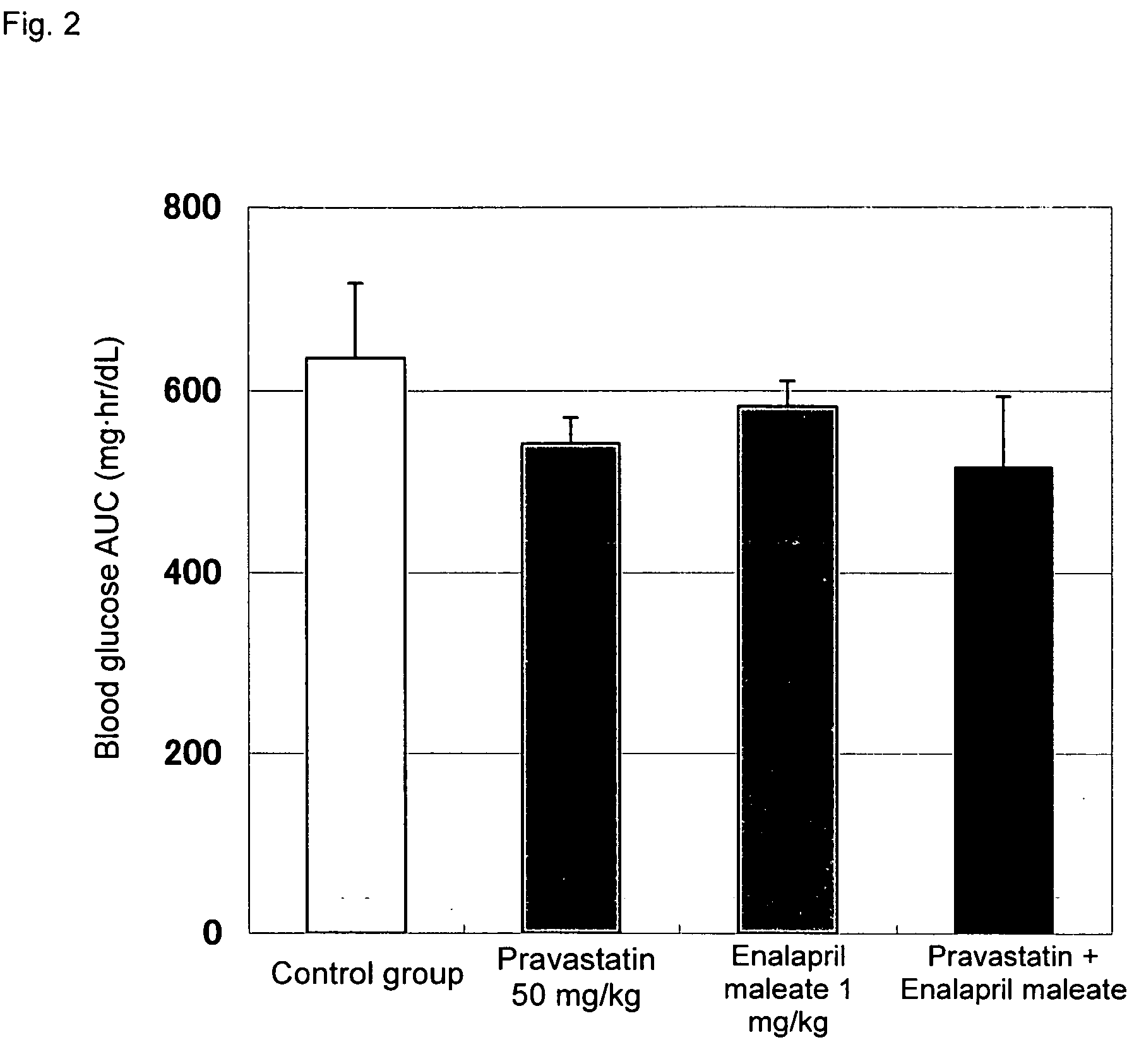
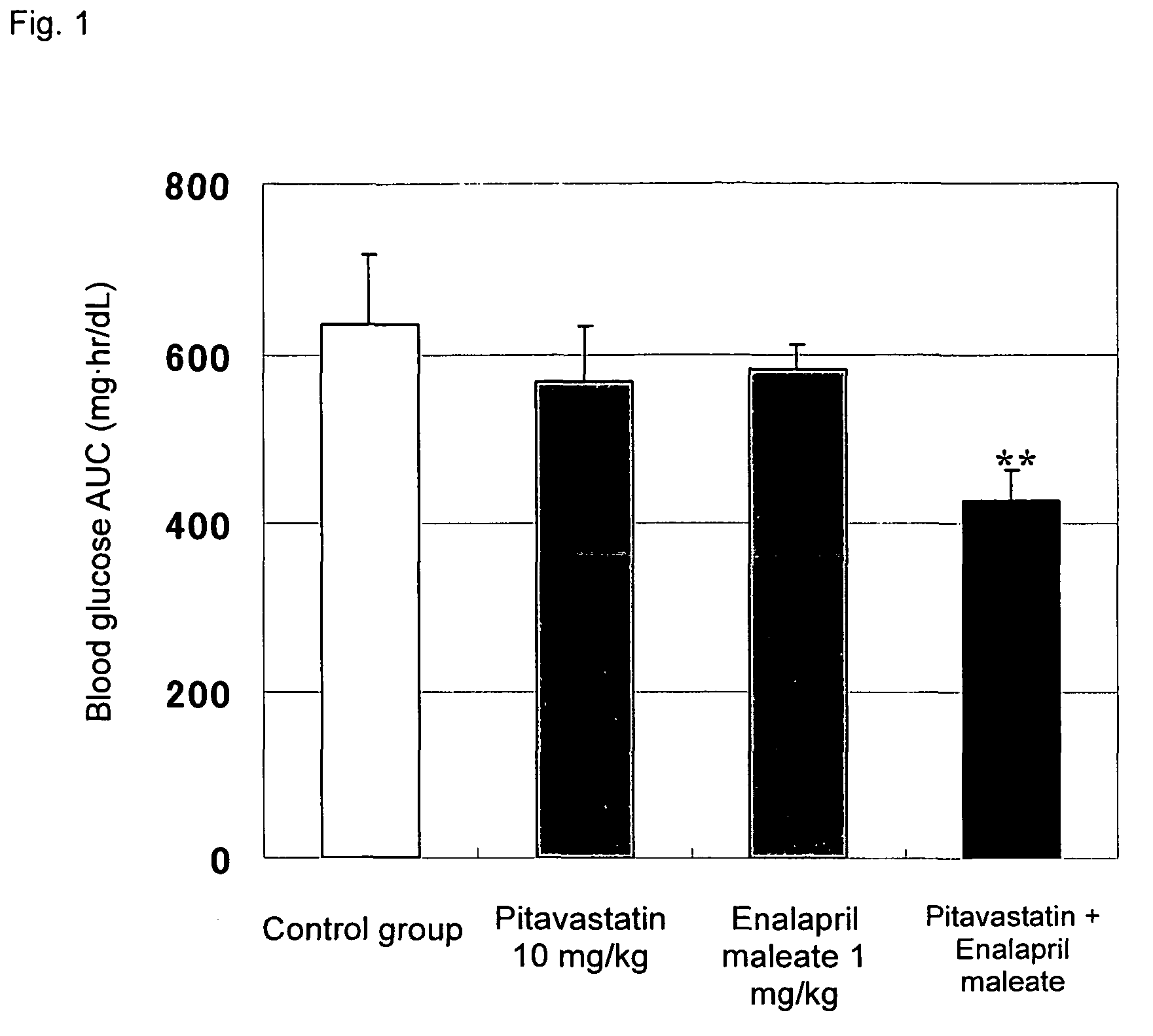
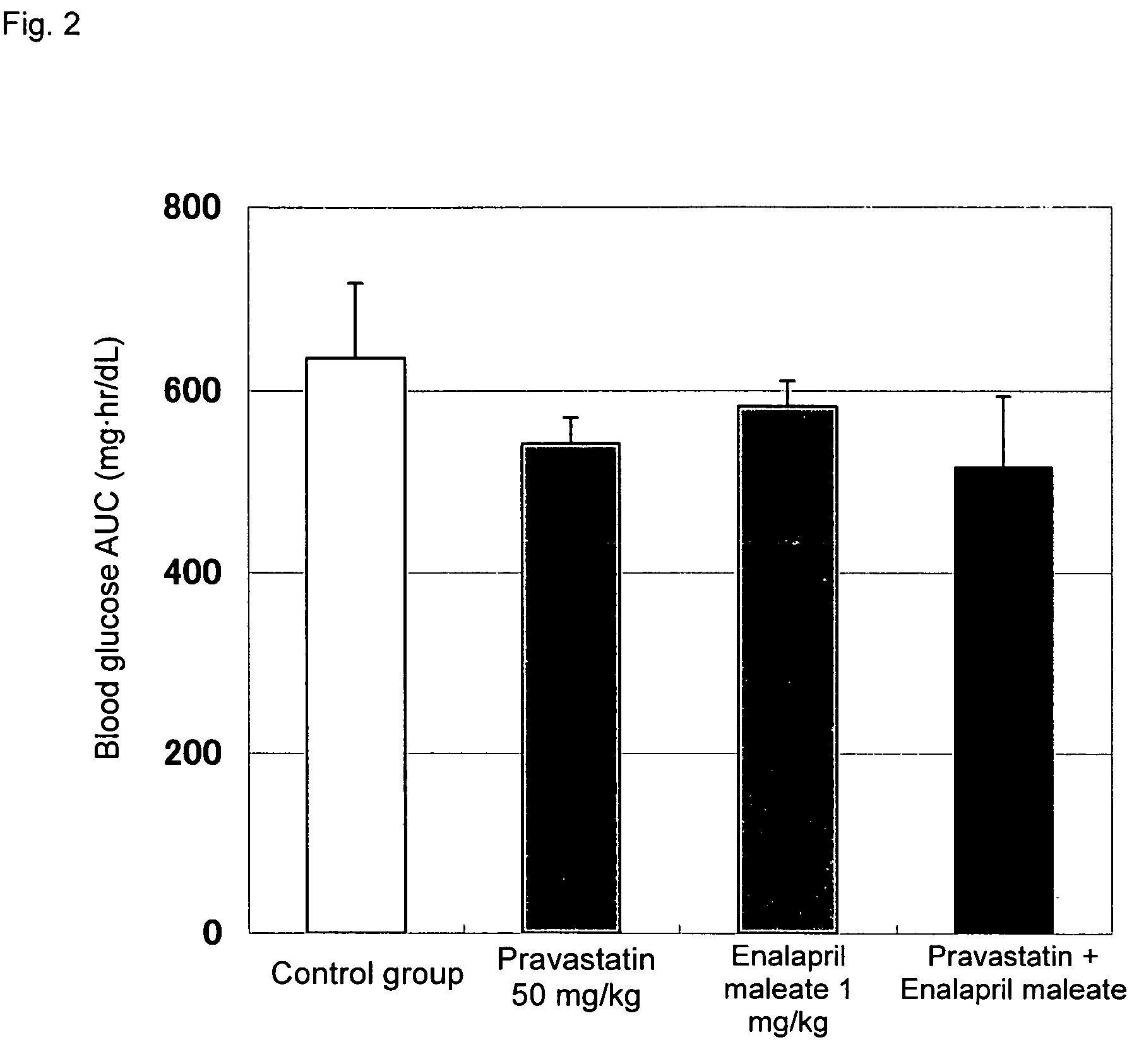
Method for the treatment of diabetes
InactiveUS20070179194A1Eliminate side effectsGood effectBiocideMetabolism disorderDiabetes mellitusPitavastatin
The present invention provides a method for treatment of diabetes, comprising administering a pitavastatin, and in combination therewith, enalapril or a salt thereof.
Owner:KOWA CO LTD +1
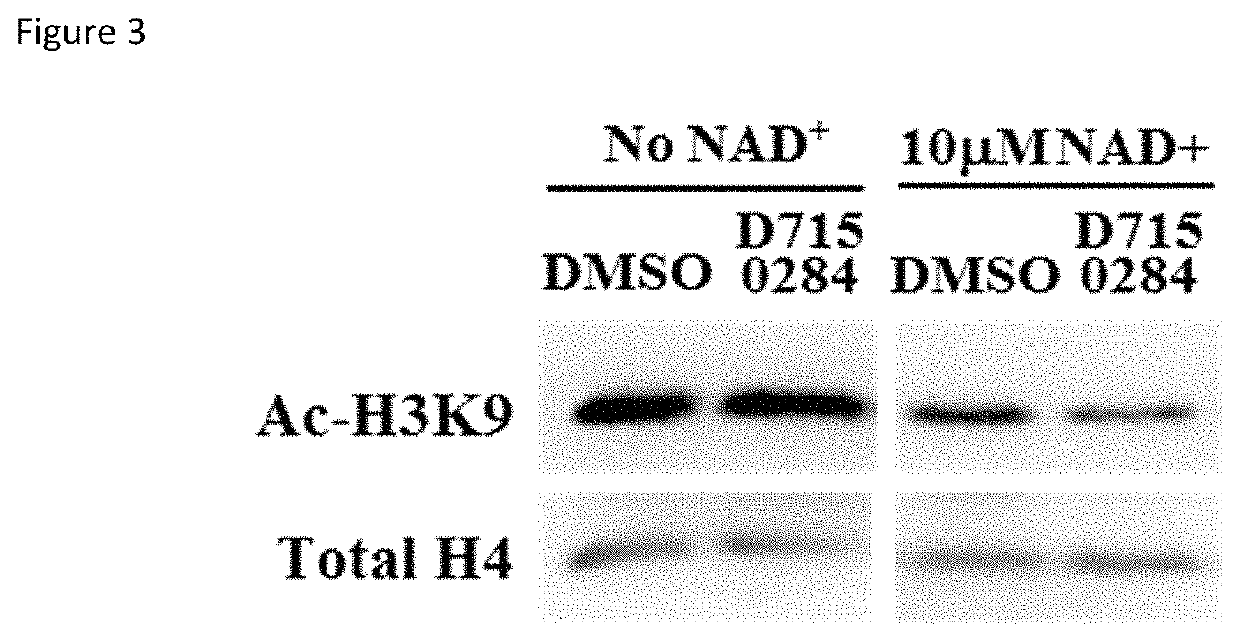
Further molecules, compositions and methods for modulation of SIRT6
ActiveUS11278525B1Loss of protectionEasily damagedOrganic active ingredientsMetabolism disorderPharmacophoreDrug efficiency
A SIRT6 activating molecule as shown in Tables 1-3 and 9, or a compound according to a pharmacophore as described herein.
Owner:SIRTLAB CORP
Therapeutic polyamine compositions and their synthesis
InactiveUS20140057877A1Chromium concentration were decreasedIncrease excretionBiocideCopper organic compoundsAntidoteRisk stroke
This invention relates to a process of synthesis and composition of open chain (ring), closed ring, linear branched and or substituted polyamines, polyamine derived tyrosine phosphatase inhibitors and PPAR partial agonists / partial antagonists via a series of substitution reactions and optimizing the bioavailability and biological activities of the compounds. Polyamines prevent the toxicity of neurotoxins and diabetogenic toxins including paraquat, methyphenyl pyridine radical, rotenone, diazoxide, streptozotocin and alloxan. These polyamines can be utilized to treat neurological, cardiovascular, endocrine acquired and inherited mitochondrial DNA damage diseases and other disorders in mammalian subjects, and more specifically to the therapy of Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease, Binswanger's disease, Olivopontine Cerebellar Degeneration, Lewy Body disease, Diabetes, Stroke, Atherosclerosis, Myocardial Ischemia, Cardiomyopathy, Nephropathy, Ischemia, Glaucoma, Presbycussis, Cancer, Osteoporosis, Rheumatoid Arthritis, Inflammatory Bowel Disease, Multiple Sclerosis and as Antidotes to Toxin Exposure.
Owner:MURPHY MICHAEL A +1
Indoles having anti-diabetic activity
ActiveUS20050277685A1Useful in treatment and controlEfficacious in treatmentBiocideSenses disorderBlood lipidsCholemia
Indoles having aryloxyalkanoic acid substituents or arylalkanoic acid substituents are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME LLC
Method for the treatment of diabetes
InactiveUS8685952B2Impaired glucose toleranceTo promote metabolismBiocideMetabolism disorderDiabetes mellitusPitavastatin
Owner:KOWA CO LTD +1
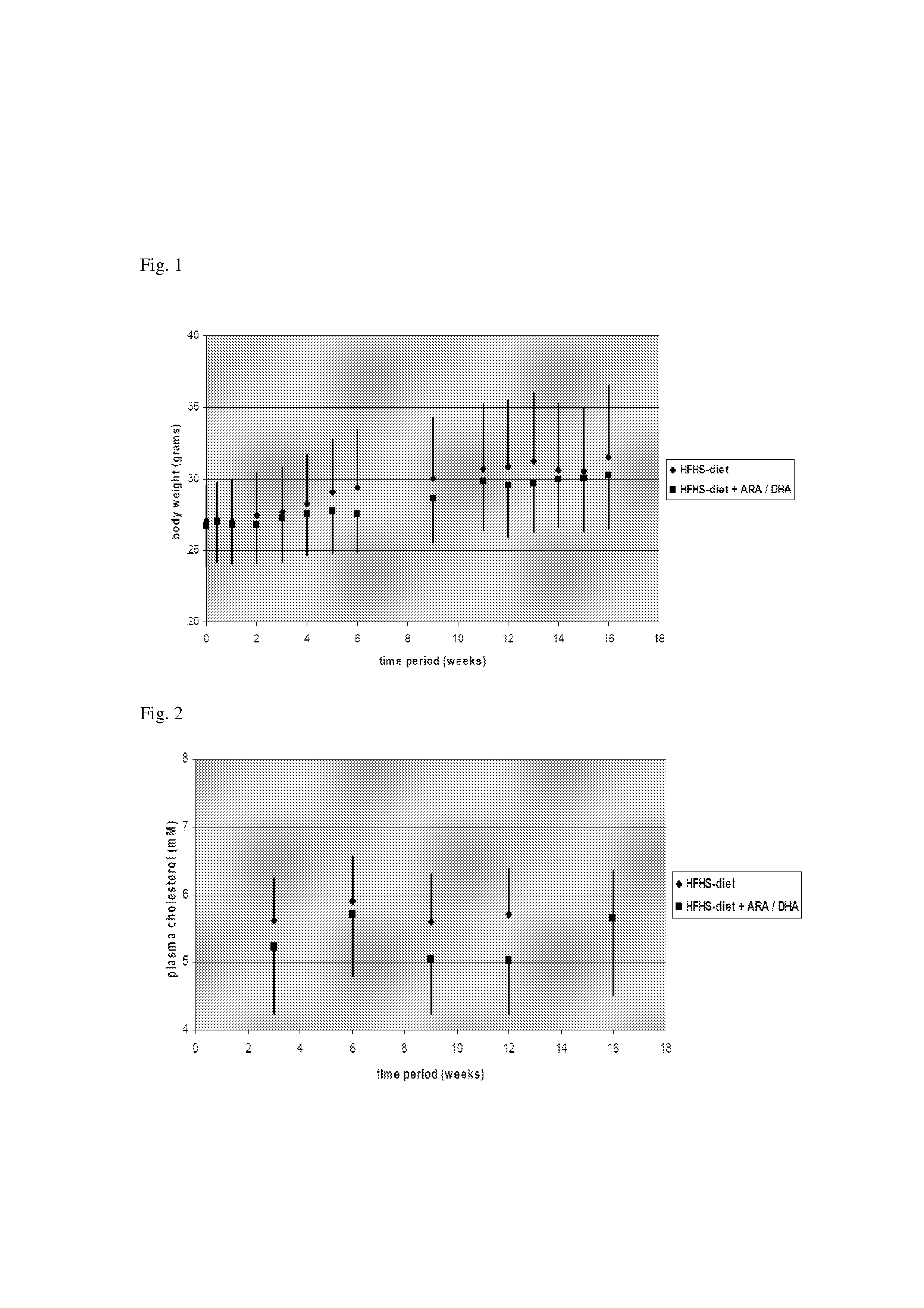
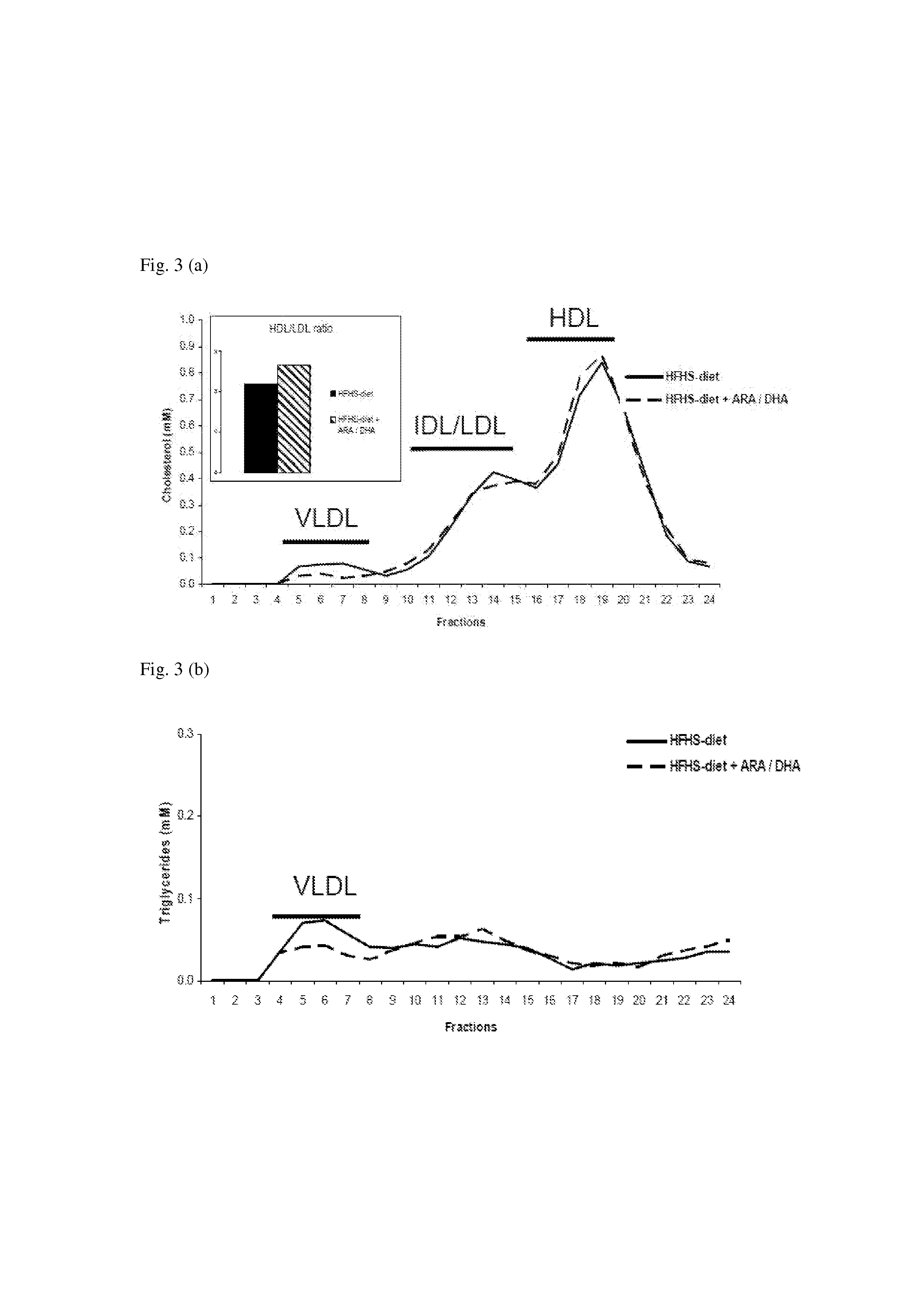
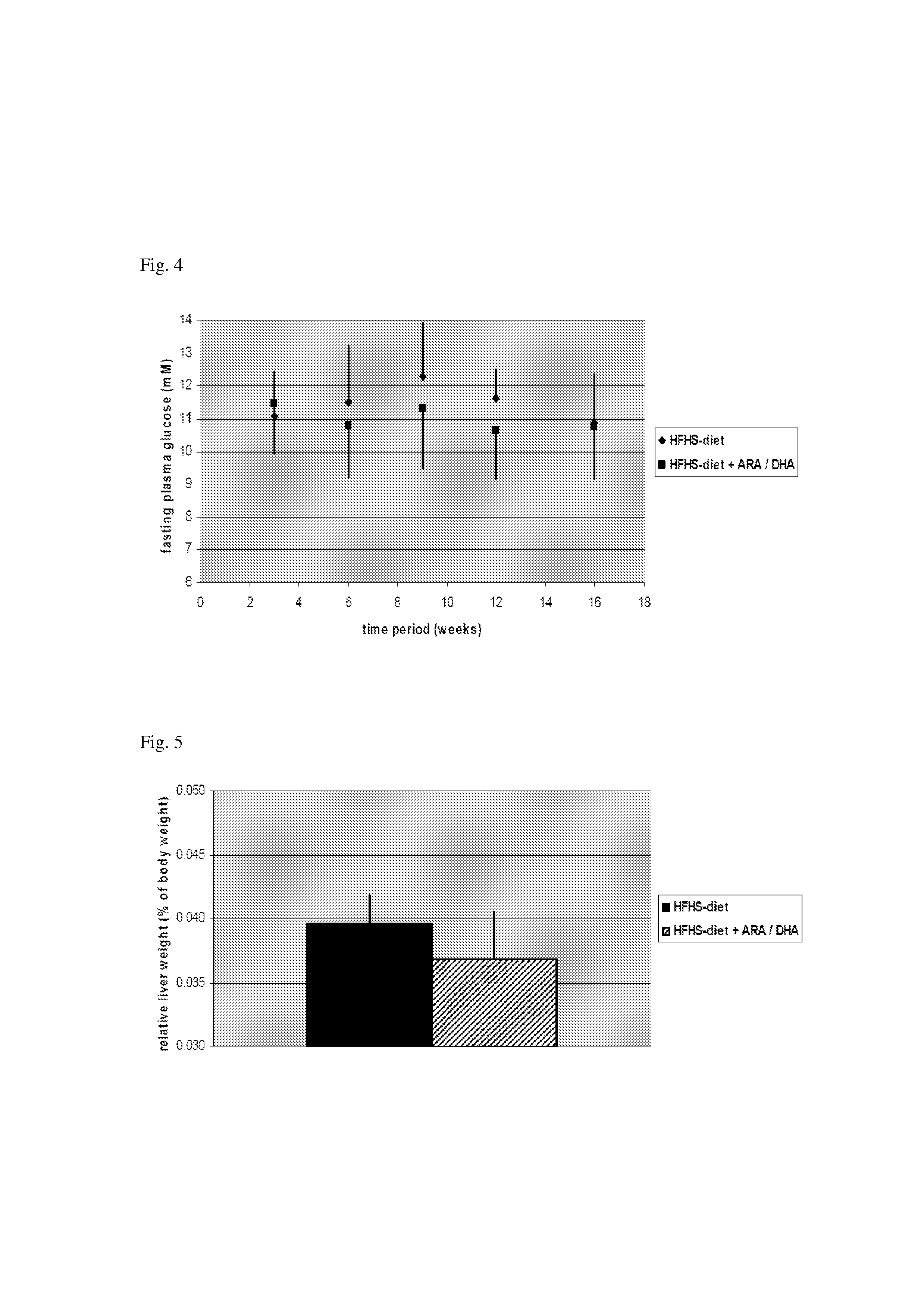
Nutritional Compensation For Western-Type Diet
InactiveUS20130178530A1Increased HDL/LDL cholesterol ratioReduced VLDL-cholesterol levelBiocideMetabolism disorderDocosahexaenoic acidHigh fat high sugar
Disclosed is the use of a composition comprising docosahexaenoic acid (DHA) and / or arachidonic acid (ARA) in human subjects that are on the typical ‘Western-type” high-fat high-sugar diet. It was found, in a humanized animal model, that said composition is capable of compensating for one or more of the adverse health effects of said diet. Particularly, an effect was found in body weight reduction, without a lowering of food intake. The composition can be administered as an oral (pharmaceutical) dosage unit, as a nutritional supplement, or as a component in a food or drink.
Owner:MEAD JOHNSON NUTRITION
Composition for amelioration/prevention of adverse side effect in steroid therapy
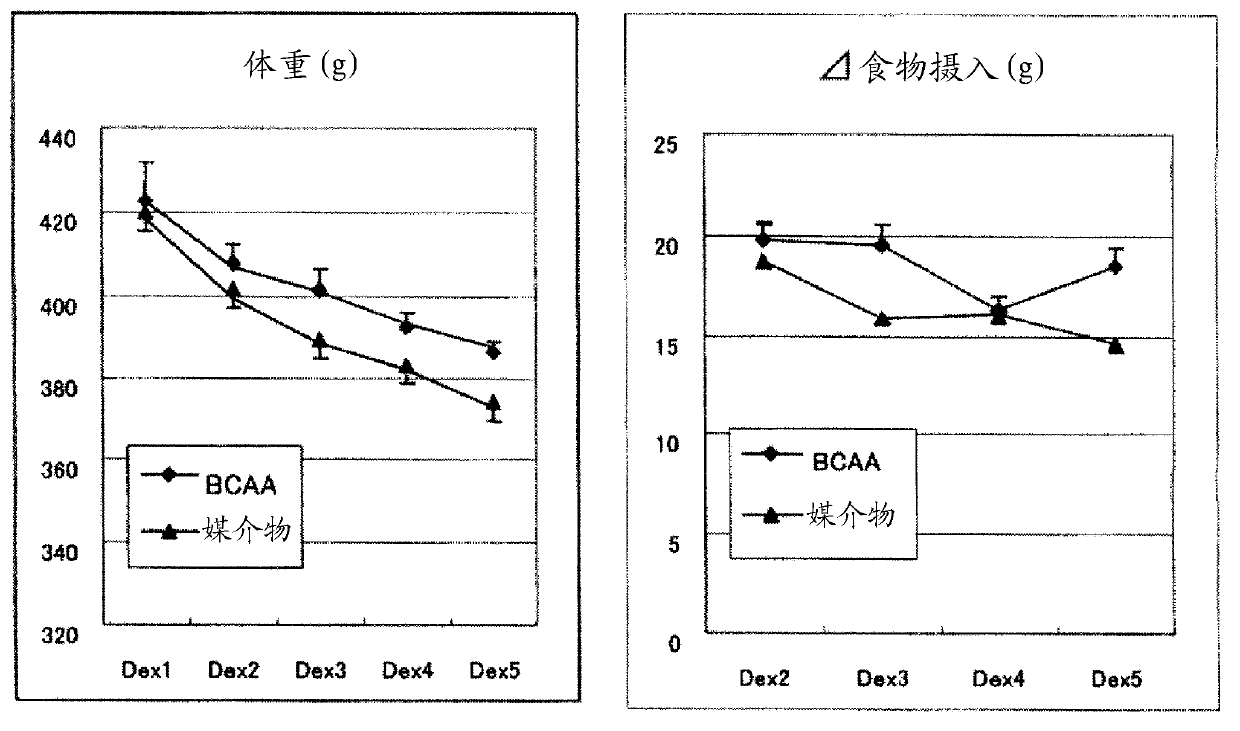
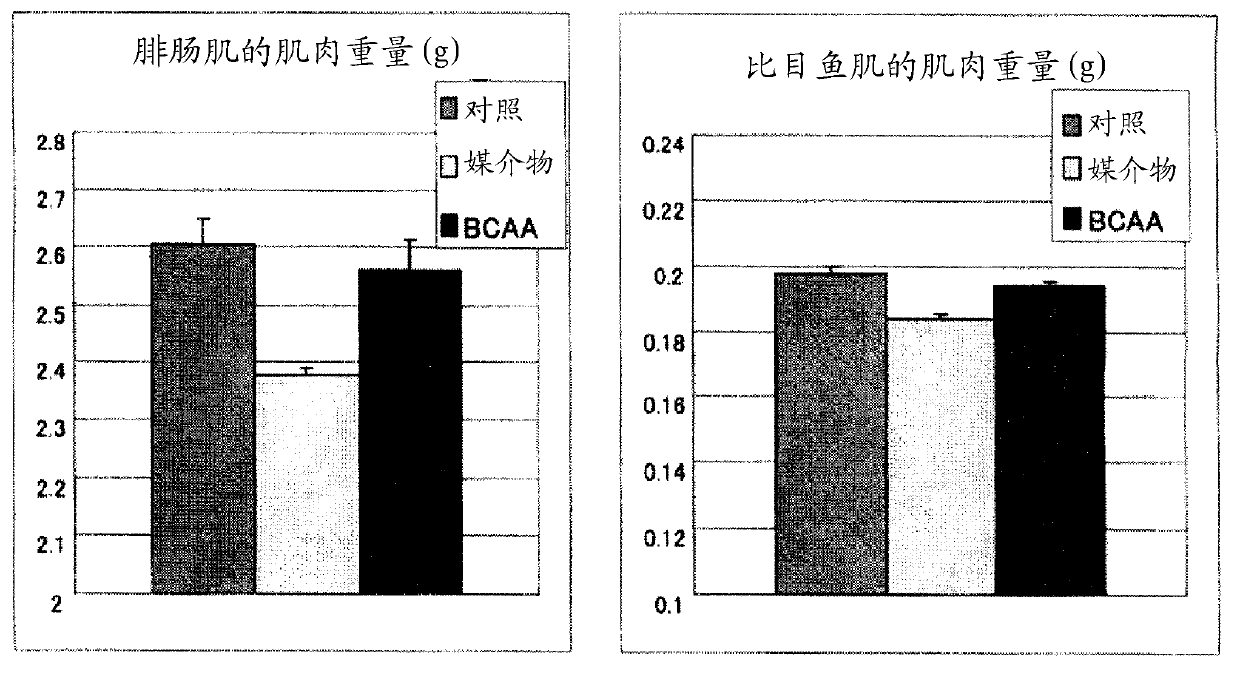
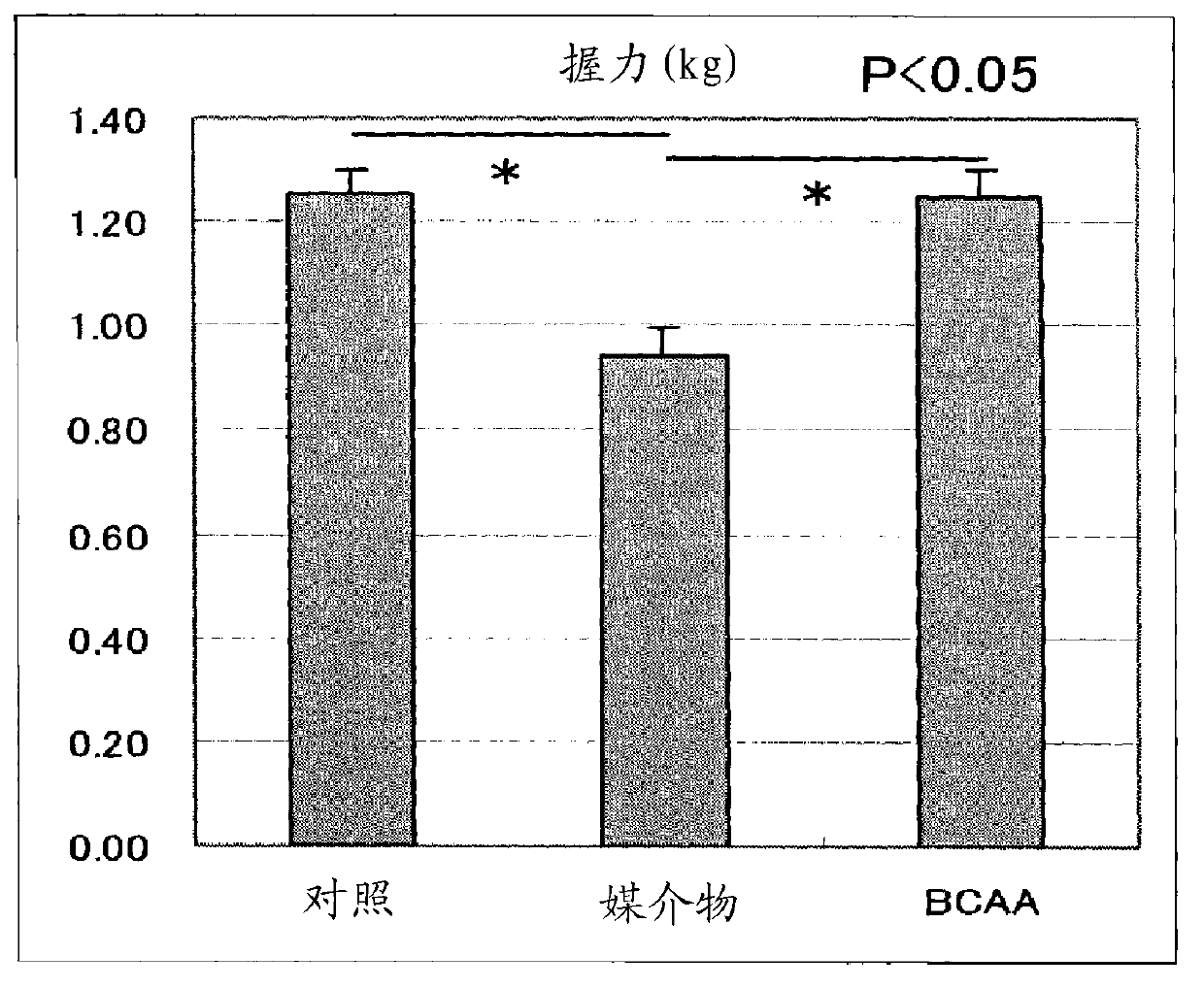
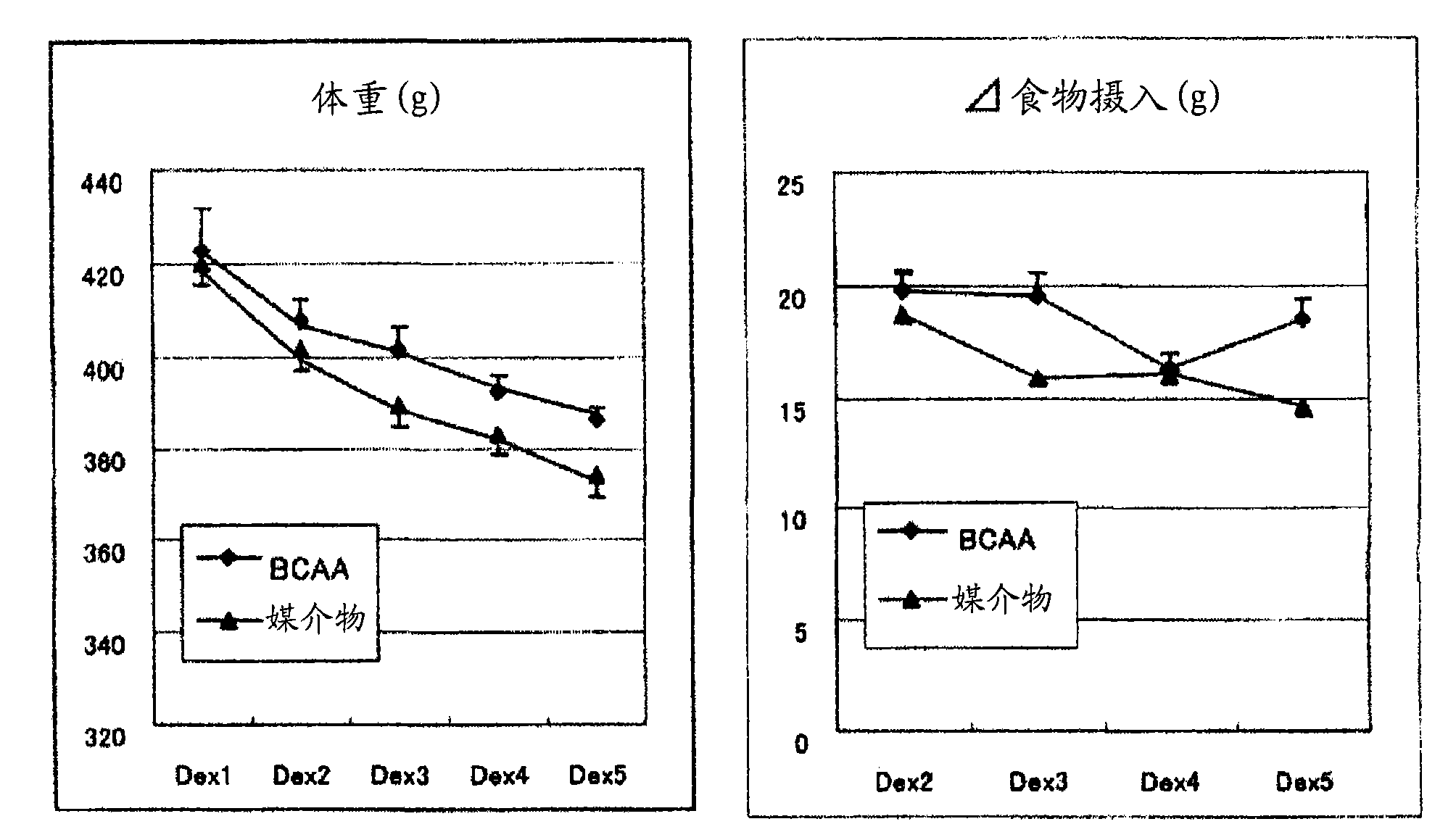
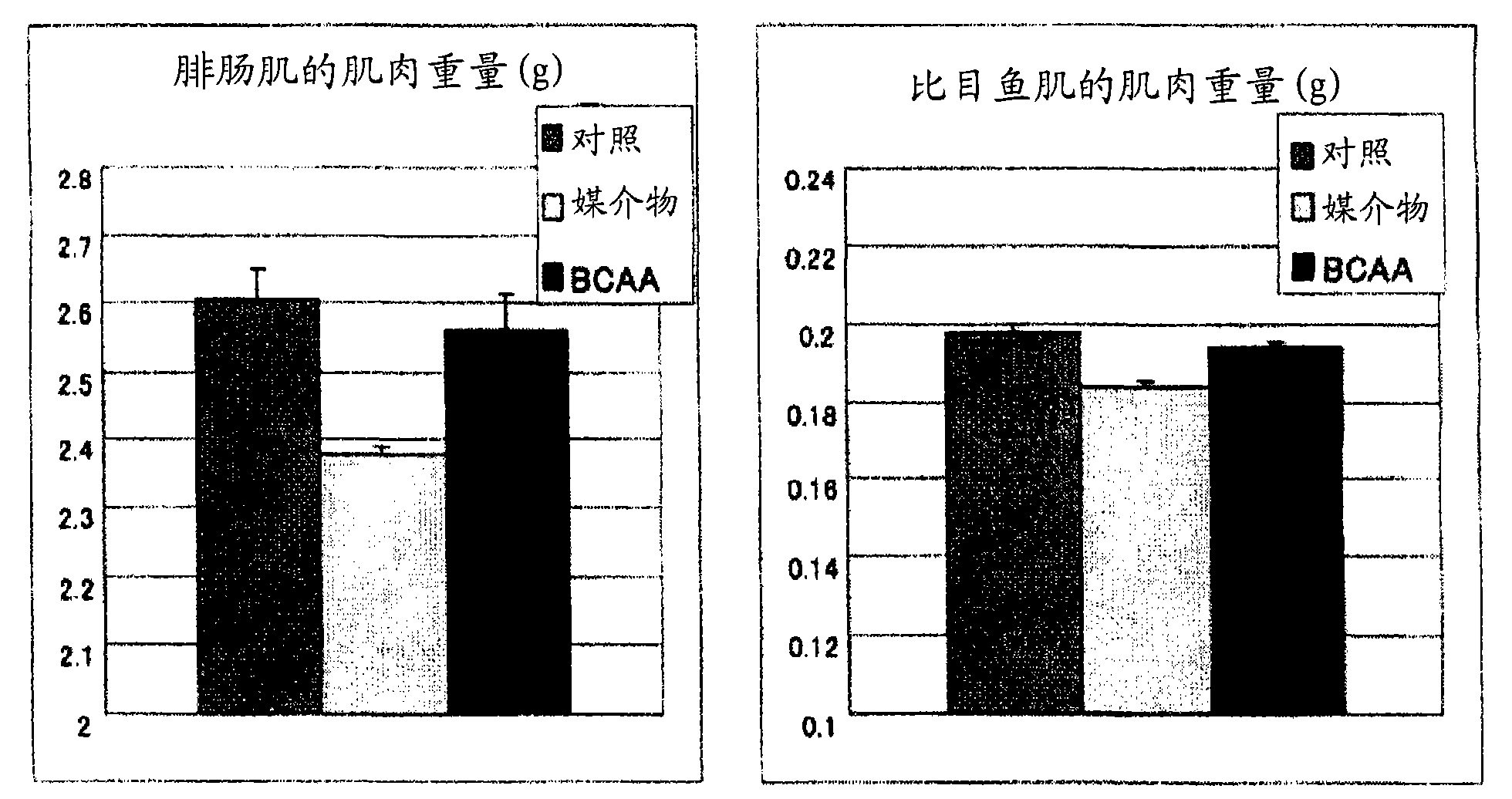
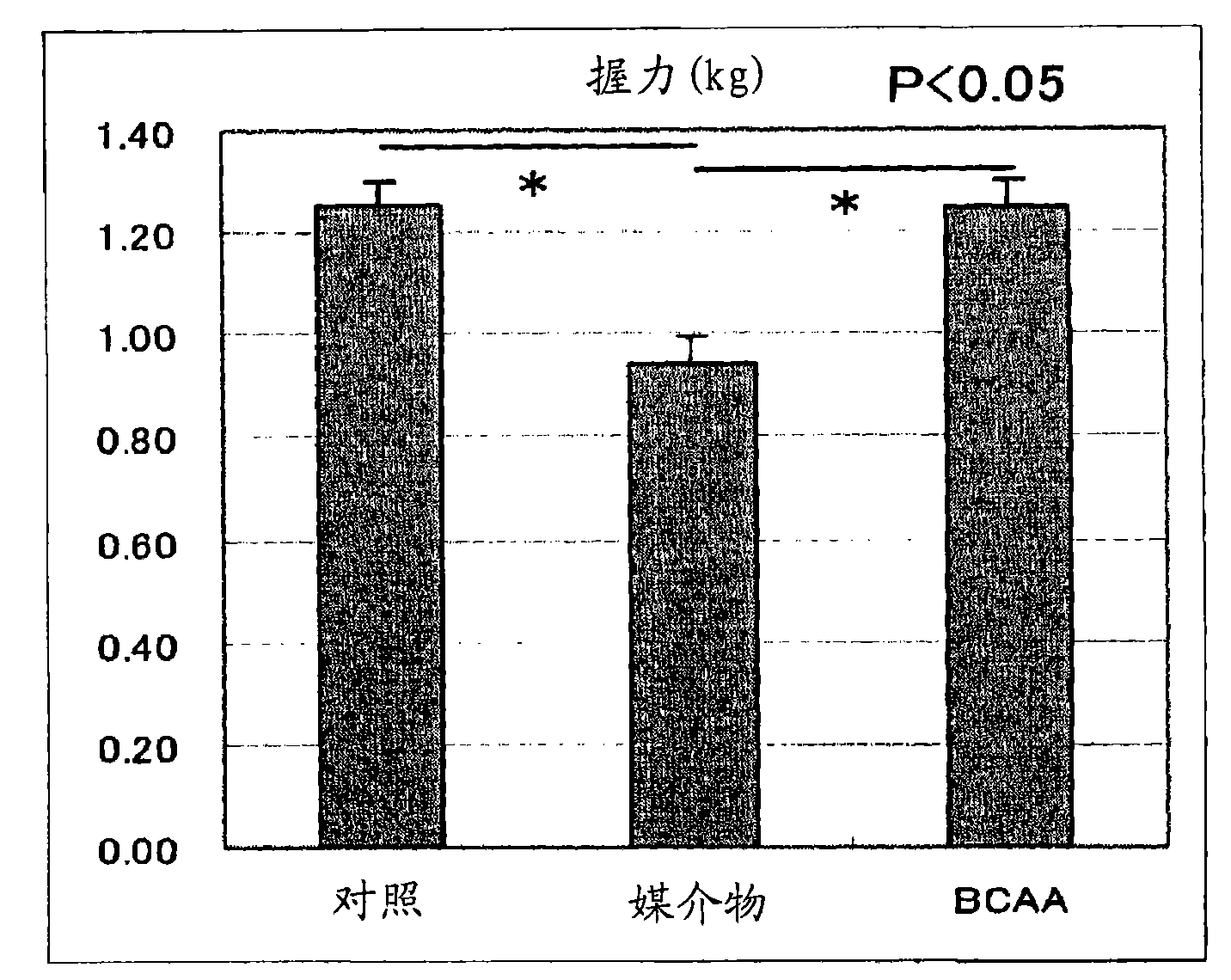
InactiveCN101600423AImprove side effectsImprovement or suppression of side effectsOrganic active ingredientsMuscular disorderDiseaseSide effect
Provided is a composition containing isoleucine, leucine and valine as active ingredients for improving or suppressing side effects associated with a steroid treatment and a composition for suppressing muscular atrophy-related gene. The composition improves or suppresses side effects in a steroid treatment such as muscular atrophy, muscular pain, arthritic pain, impaired glucose tolerance, decreased bone metabolism, impaired immunity, loss of appetite, body weight loss, fatigability and the like, and further suppresses muscular atrophy associated with various diseases. In addition, the composition suppresses muscular atrophy associated with promoted expression of muscular atrophy-related gene associated with glucocorticoid excess or renal failure pathology and the like. Therefore, the composition is effective form improving the QOL of patients.
Owner:AJINOMOTO CO INC
Novel compounds capable of antagonizing islet amyloid polypeptide (IAPP) induced beta-cell damage and impaired glucose tolerance
ActiveUS20180179276A1Impaired glucose toleranceIncrease secretionCompounds screening/testingMetabolism disorderIGT - Impaired glucose toleranceCell damage
Described are molecules specifically binding to human islet amyloid polypeptide (hIAPP) also known as amylin, particularly human-derived antibodies as well as fragments, derivatives and variants thereof for antagonizing islet amyloid polypeptide (IAPP) induced β-cell damage and impaired glucose tolerance which are symptoms typically associated with diabetes mellitus type 2 (T2D).
Owner:UNIV ZURICH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives](https://images-eureka.patsnap.com/patent_img/90a40ec1-a353-4bb5-951d-923da8e44698/US08080580-20111220-D00001.png)
![Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives](https://images-eureka.patsnap.com/patent_img/90a40ec1-a353-4bb5-951d-923da8e44698/US08080580-20111220-D00002.png)
![Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives](https://images-eureka.patsnap.com/patent_img/90a40ec1-a353-4bb5-951d-923da8e44698/US08080580-20111220-D00003.png)