Vesiculins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0211]In this example vesiculin was isolated using the following materials, including βTC6-F7 cells. Optiprep™ was obtained from Total Lab Systems, the New Zealand distributors for Axis-Shield products. Acetonitrile used was either HiperSolv grade from BDH or UltimAR grade from Mallinckrodt. Trifluoroacetic acid (Protein Sequencer Grade TFA) was obtained from Applied Biosystems. The 5 μm Jupiter C18 column, 250×2.0 mm was purchased from Phenomenex. Mass spectrometry standards consisted of insulin (RP-HPLC purified from Actrapid® Recombinant Human Insulin [Novo Nordisk Pharmaceuticals]), and synthetic somatostatin (H-2260) purchased from Bachem. The MS matrix used was α-cyano-4-hydroxycinnamic acid (α-CHC) and was obtained from Hewlett Packard. Guanadine HCl, iodoacetic acid, modified RPMI (R1383) and Dulbecco's modified Eagles medium (DMEM; D5523) was purchased from Sigma while fetal bovine serum was sourced from Invitrogen. Dithiothreitol was purchased from Boehringer and sequencin...
example 2
[0216]This Example relates to the identification and characterization of a vesiculin.
[0217]Identification of Granule Proteins—Peptides eluted from RP-HPLC were subjected to N-terminal sequencing on an Applied Biosystems Procise™ 492 protein sequencer using chemicals supplied by the manufacturer (Applied Biosystems).
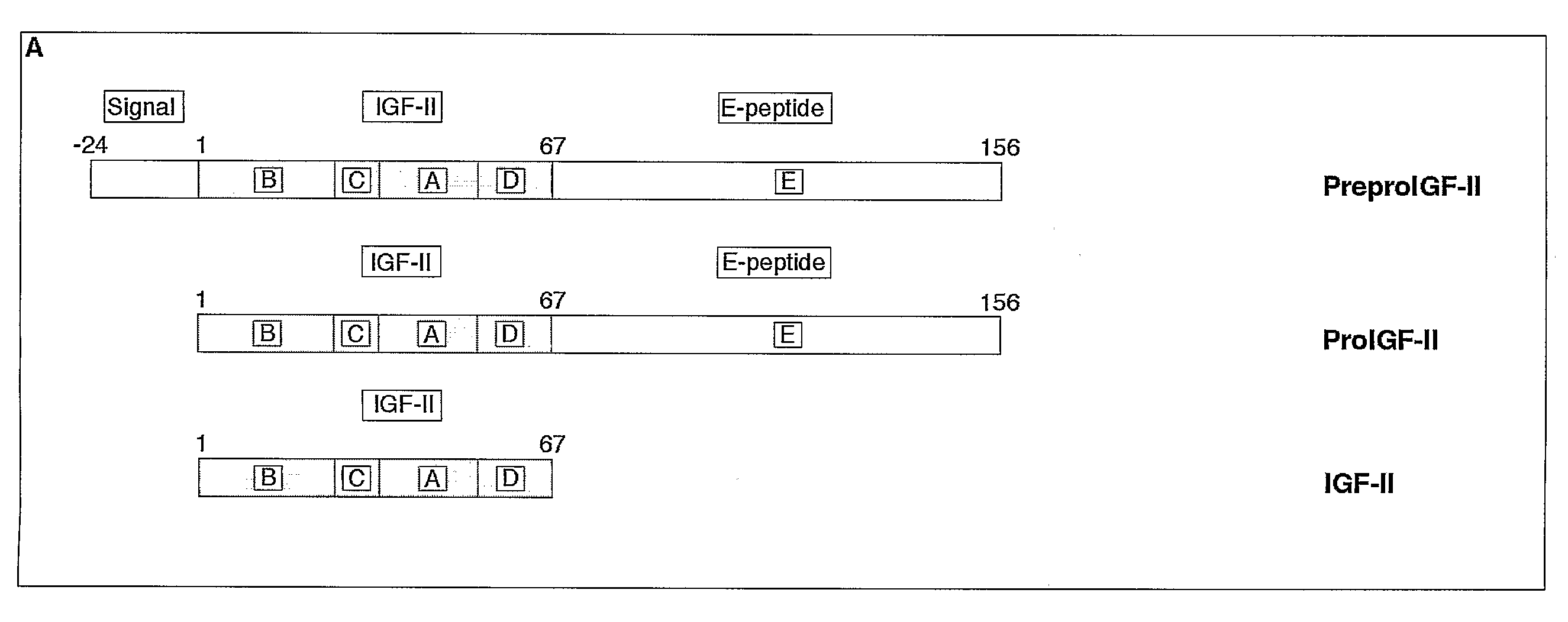
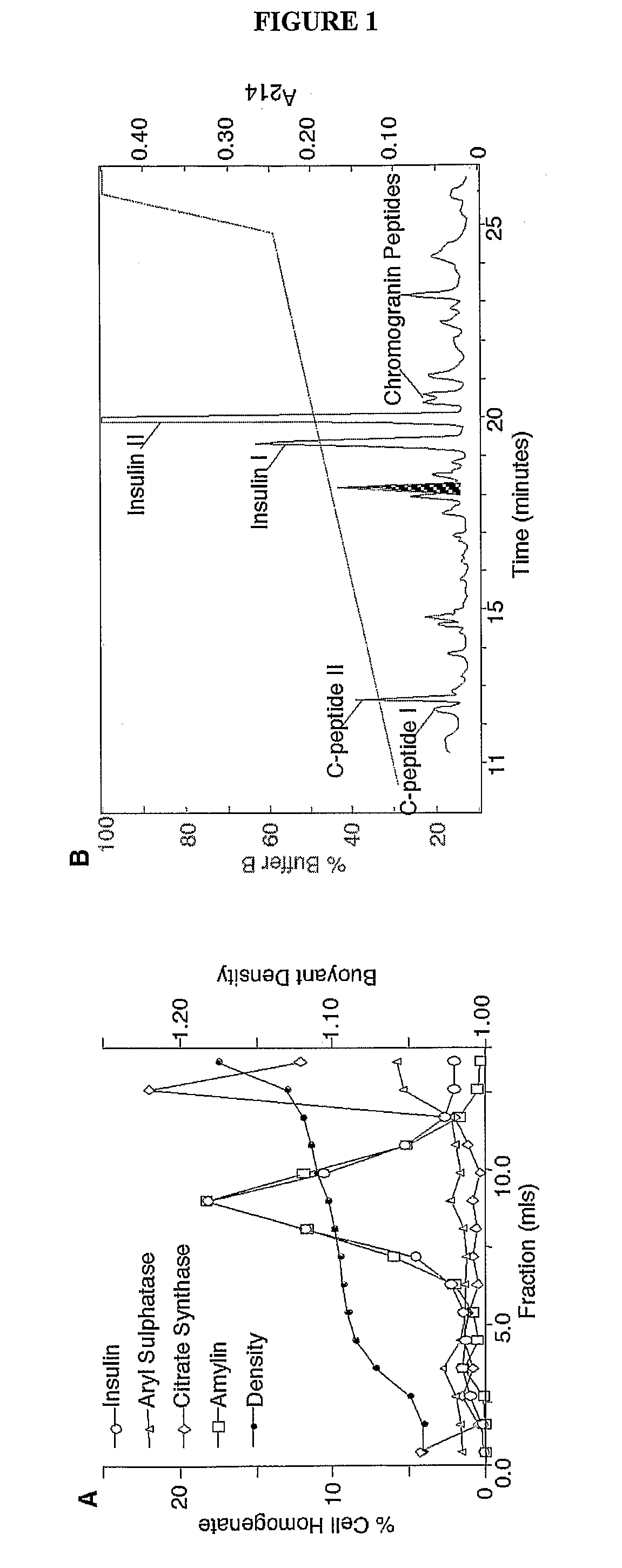
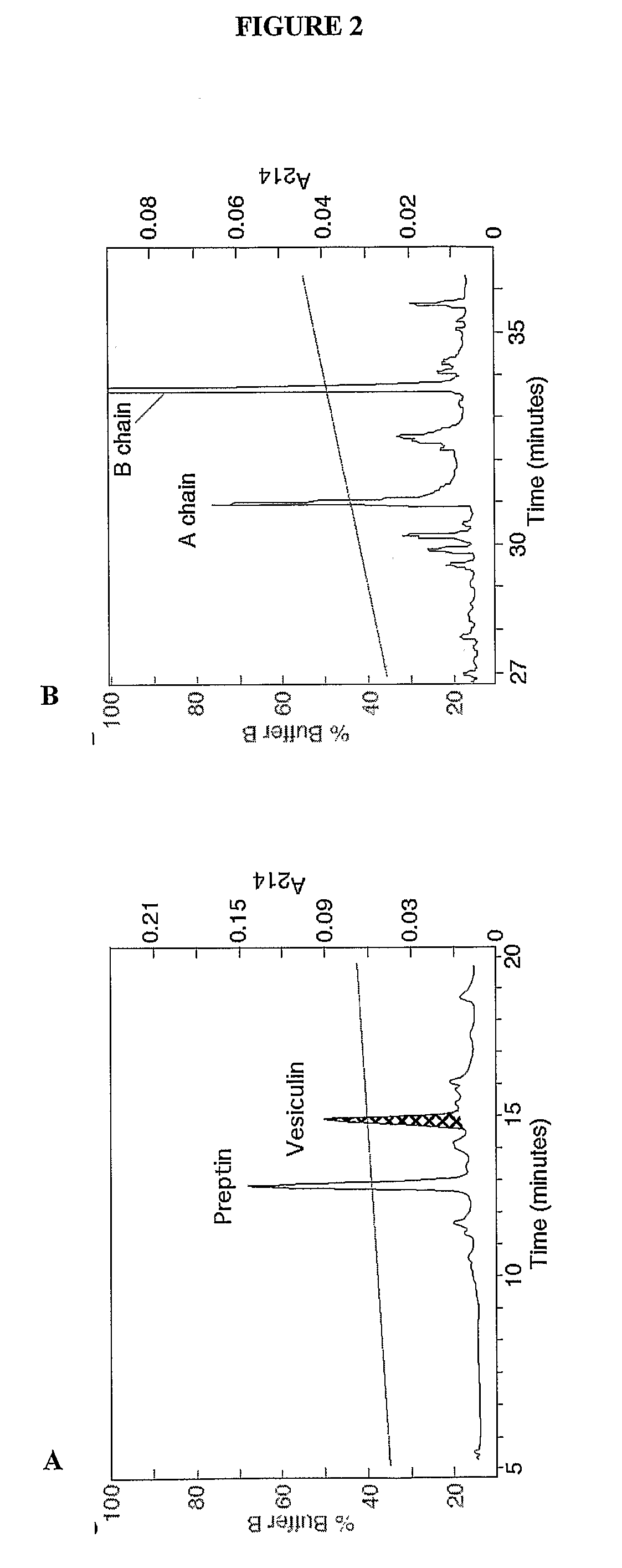
[0218]Molecular Weight Determination—Molecular weights of purified proteins were determined by MALDI-TOF mass spectrometry (Hewlett Packard G2025A). Samples and external standards (10 μM insulin and somatostatin) were mixed 1:1 with α-CHC (33 mM in 1:2 mixture of acetonitrile:1.33% TFA) and co-crystallised after vacuum drying (Hewlett Packard G2024A Sample prep accessory). Mass / charge ratios (m / z) of unknown proteins were generated by accumulating the data from 50 laser shots (Table 1). In this manner, the presence of C-peptide I and II fragments, amylin, ubiquitin, preptin, an IGF-II-like peptide, insulins I and II, neuropeptide Y, chromogranin A, and calreticulin (FIG. ...
example 3
[0225]This Example describes experiments demonstrating vesiculin bioactivity, here the incorporation of glucose into muscle glycogen. The soleus muscle was dissected from anaesthetized fasted male Wistar rats (200 g±20 g). Each muscle was then teased longitudinally into 3 equal strips with a final radius of approximately 1.5 mm. An average of six muscle strips were transferred into flasks, containing 10 ml of carbogen-saturated nDMEM and peptide (vesiculin, insulin-II, or commercial human IGF-II (GroPep)) at concentrations ranging from 0.71 nM to 237 nM, before the addition of 10 μl of (0.5 μCi) D-[14C(U)]-Glucose. Flasks were then incubated in a shaking water bath for 120 minutes at 30° C. under a stream of carbogen.
[0226]After incubation, the muscle strips were removed and blotted dry before being frozen instantly in liquid nitrogen and freeze-dried for 24 hours. The dried weight of muscle strip was recorded before the muscle strips were solubilised in 250 μl of 60% KOH (70° C. wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com