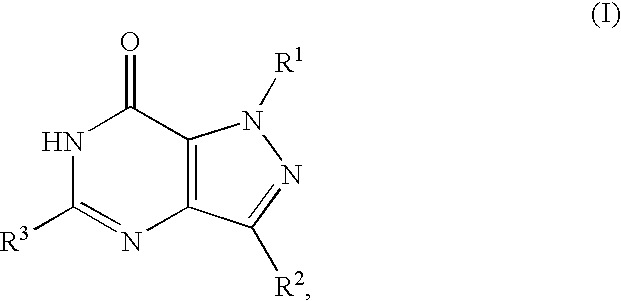
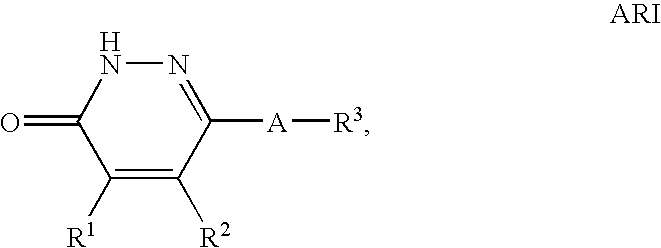Treatment of insulin resistance syndrome and type 2 diabetes with PDE9 inhibitors
a technology of insulin resistance syndrome and pde9 inhibitor, which is applied in the direction of drug composition, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of impaired insulin signaling for glucose uptake in insulin resistant individuals, huge population at risk of complications, and extremely common obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 160
3-Cyclopentyl-5-(2-trifluoromethoxy-benxyl)-1,6-dihydro-pyrazolo[4,3-d]pyrimidin-7-one
5-Cyclopentyl-4-[2-(2-trifluoromethoxy-phenyl)-acetylamino]-1H-pyrazole-3-carboxylic acid amide (120 mg, 0.303 mmol) and potassium tert-butoxide (102 mg, 0.909 mmol) were suspended in isopropylalcohol (5 ml) and the reaction was heated to reflux, under nitrogen, for 18 hours. The reaction mixture was concentrated under reduced pressure and the residue was partitioned between ethyl acetate (20 ml) and water (20 ml). The aqueous phase was removed, acidified to pH 2 with 2N HCl, and extracted with ethyl acetate (2×15 ml). The combined organic extracts were washed with saturated sodium carbonate solution (3×10 ml), dried over MgSO4, concentrated under reduced pressure and the residue was purified by flash column chromatography on silica gel eluting with dichloromethane: methanol (95:5, by volume) to give 3-cyclopentyl-5-(2-trifluoromethoxy-benxyl)-1,6-dihydro-pyrazolo[4,3-d]pyrimidin-7-one (21 mg) as...
example 161
3-Isobutyl-5-(2-trifluoromethoxy-benxyl)-116-dihydro-pyrazolo[4,3-d]pyrimidin-7-one
5-Isobutyl-4-[2-(2-trifluoromethoxy-phenyl)-acetylamino]-1H-pyrazole-3-carboxylic acid amide (140 mg, 0.365 mmol) and potassium tert-butoxide (123 mg, 1.09 mmol) were suspended in isopropylalcohol (6 ml) and the reaction was heated to reflux, under nitrogen, for 18 hours. The reaction mixture was concentrated under reduced pressure and the residue was partitioned between ethyl acetate (20 ml) and water (20 ml). The aqueous phase was removed, acidified to pH 2 with 2N HCl, and extracted with ethyl acetate (2×15 ml). The combined organic extracts were washed with saturated sodium carbonate solution (3×10 ml), dried over MgSO4, concentrated under reduced pressure and the residue was purified by flash column chromatography on silica gel eluting with dichloromethane:methanol (95:5, by volume) to give 3-isobutyl-5-(2-trifluoromethoxy-benxyl)-1,6-dihydro-pyrazolo[4,3-d]pyrimidin-7-one (27 mg) as an off-whi...
example 162
3-Pyridin-3-yl-5-(2-trifluoromethoxy-benxyl)-1,6-dihydro-pyrazolo[4,3-d]pyrimidin-7-one
5-Pyridin-3-yl-4-[2-(2-trifluoromethoxy-phenyl)-acetylamino]-1H-pyrazole-3-carboxylic acid amide (345 mg, 0.85 mmol) and potassium tert-butoxide (286 mg, 2.55 mmol) were suspended in isopropylalcohol (5 ml) and the reaction was heated to 55° C. under nitrogen for 18 hours. The reaction mixture was concentrated under reduced pressure and the residue was partitioned between ethyl acetate (20 ml) and water (20 ml). The aqueous phase was removed, acidified to pH 2 with 2N HCl, and extracted with ethyl acetate (2×15 ml) and dichloromethane (2×15 ml). The combined organic extracts were dried over MgSO4, concentrated under reduced pressure and the residue was purified by flash column chromatography on silica gel eluting with a solvent gradient of dichloromethane:methanol (99:1 changing to 95:5, by volume). The product was triturated with methanol (3 ml), dichloromethane (3 ml) and diethylether (3 ml) t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight loss | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| insulin resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com