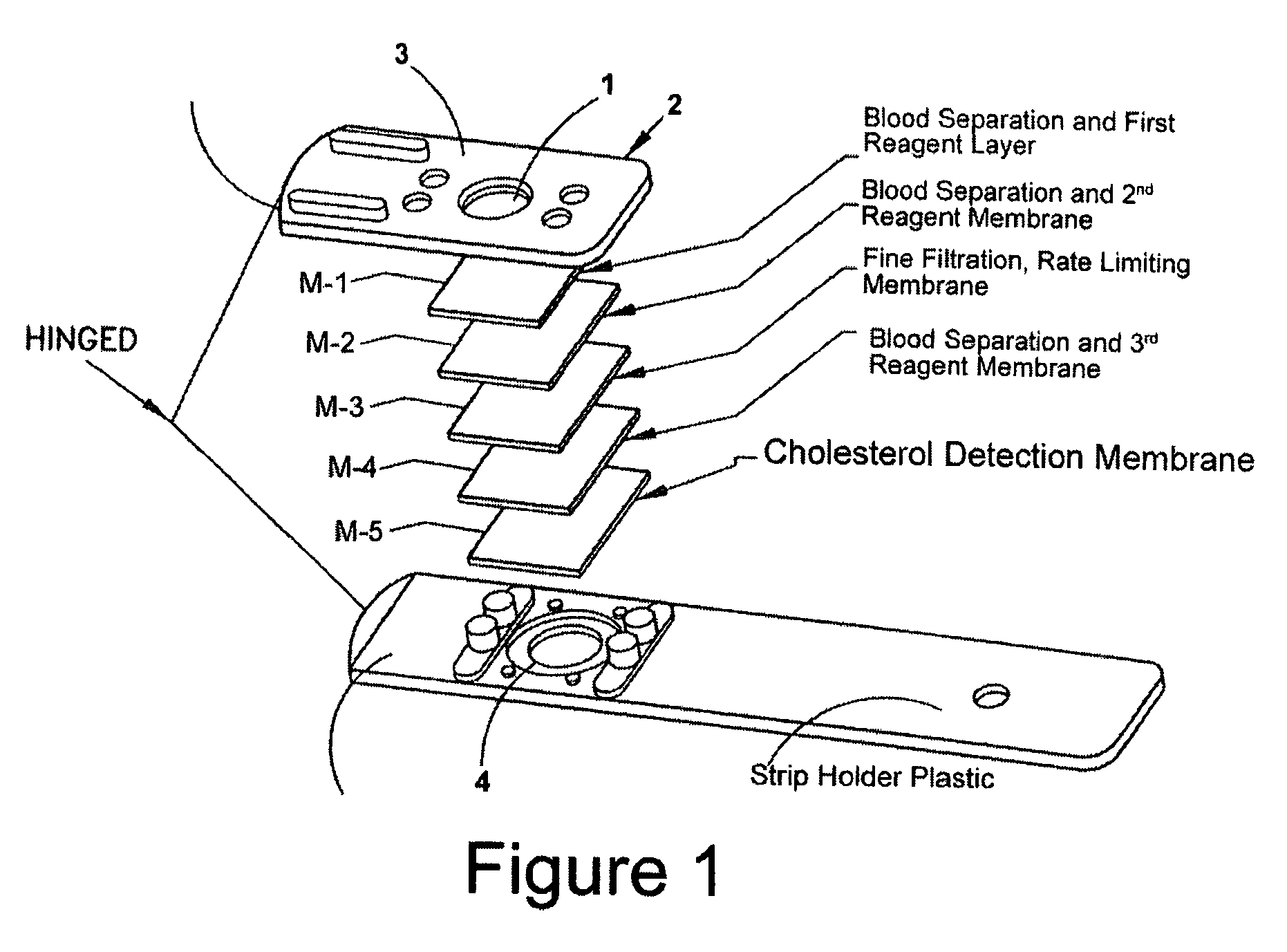
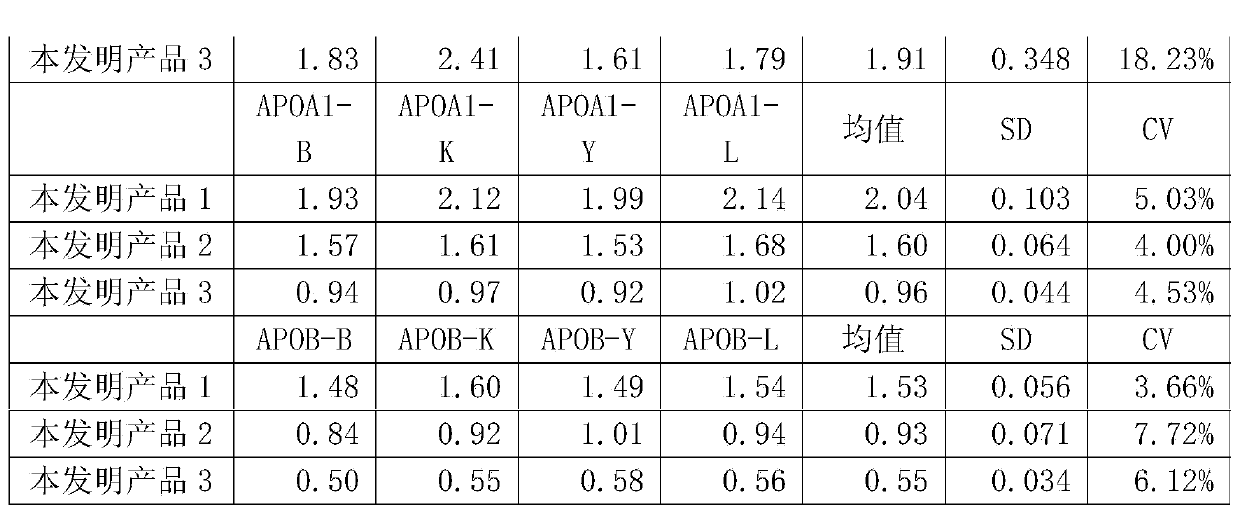
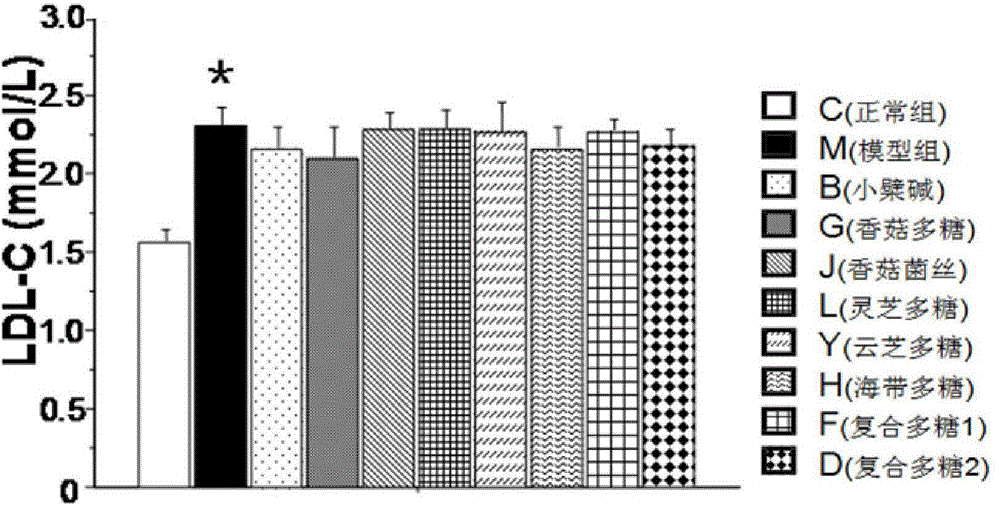
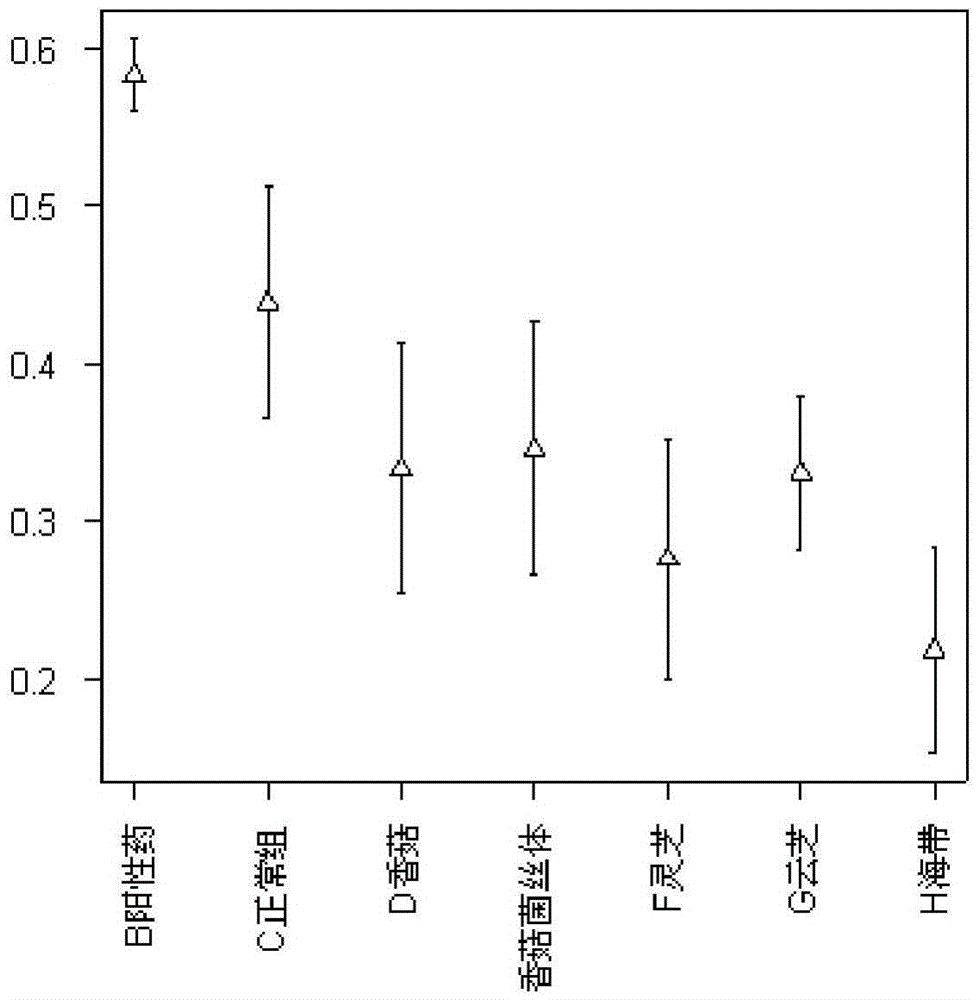
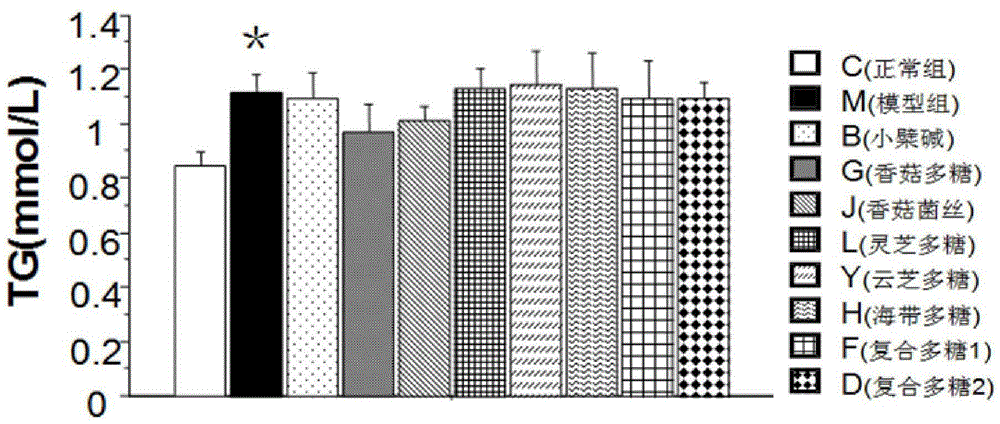
Patents
Literature
428 results about "LDL - Low density lipoprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low-density lipoprotein (LDL) refers to a class and range of lipoprotein particles, varying in their size (18-25 nm in diameter) and contents, which carry cholesterol in the blood and around the body, for use by cells.
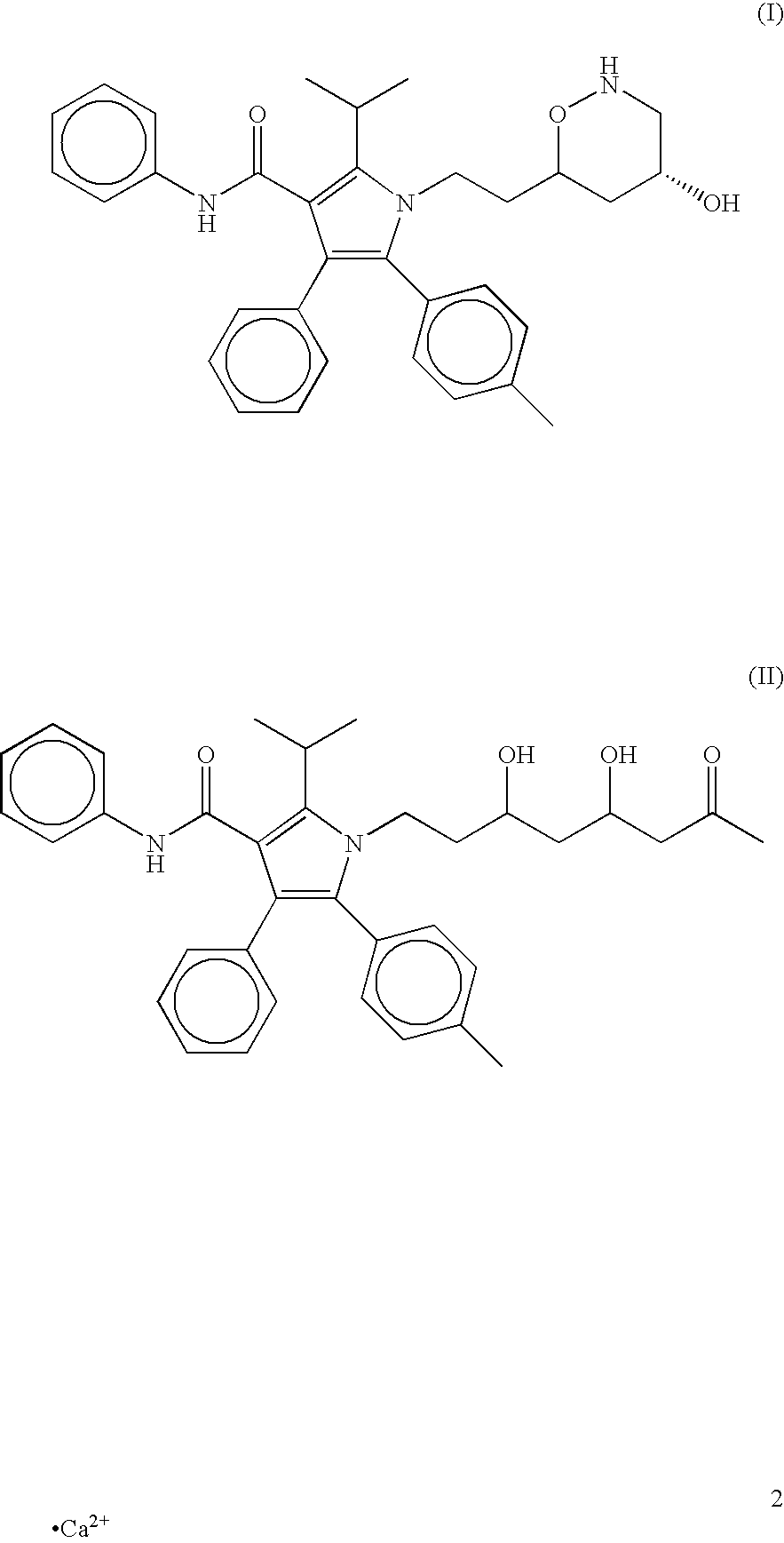
Atorvastatin formulation
InactiveUS20090311322A1Reduce impactReduces food effectBiocidePowder deliveryBioavailabilityAtorvastatin
Provided are atorvastatin compositions which reduce the effect of food on the bioavailability of atorvastatin and methods for making such compositions. Also provided are methods of reducing low density lipoprotein by administering the compositions of the invention.
Owner:TEVA PHARMA IND LTD
Method for decreasing low density lipoprotein
The present invention relates to a method for decreasing elevated serum / plasma LDL-cholesterol levels or LDL-cholesterol levels and CRP levels in a mammal in need thereof. The methods comprises administering an effective amount of a tetracycline formulation. In one embodiment, the tetracycline formulation is a non-antibacterial tetracycline. In another embodiment, the tetracycline formulation is an antibacterial tetracycline at a sub-antibacterial amount.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +2
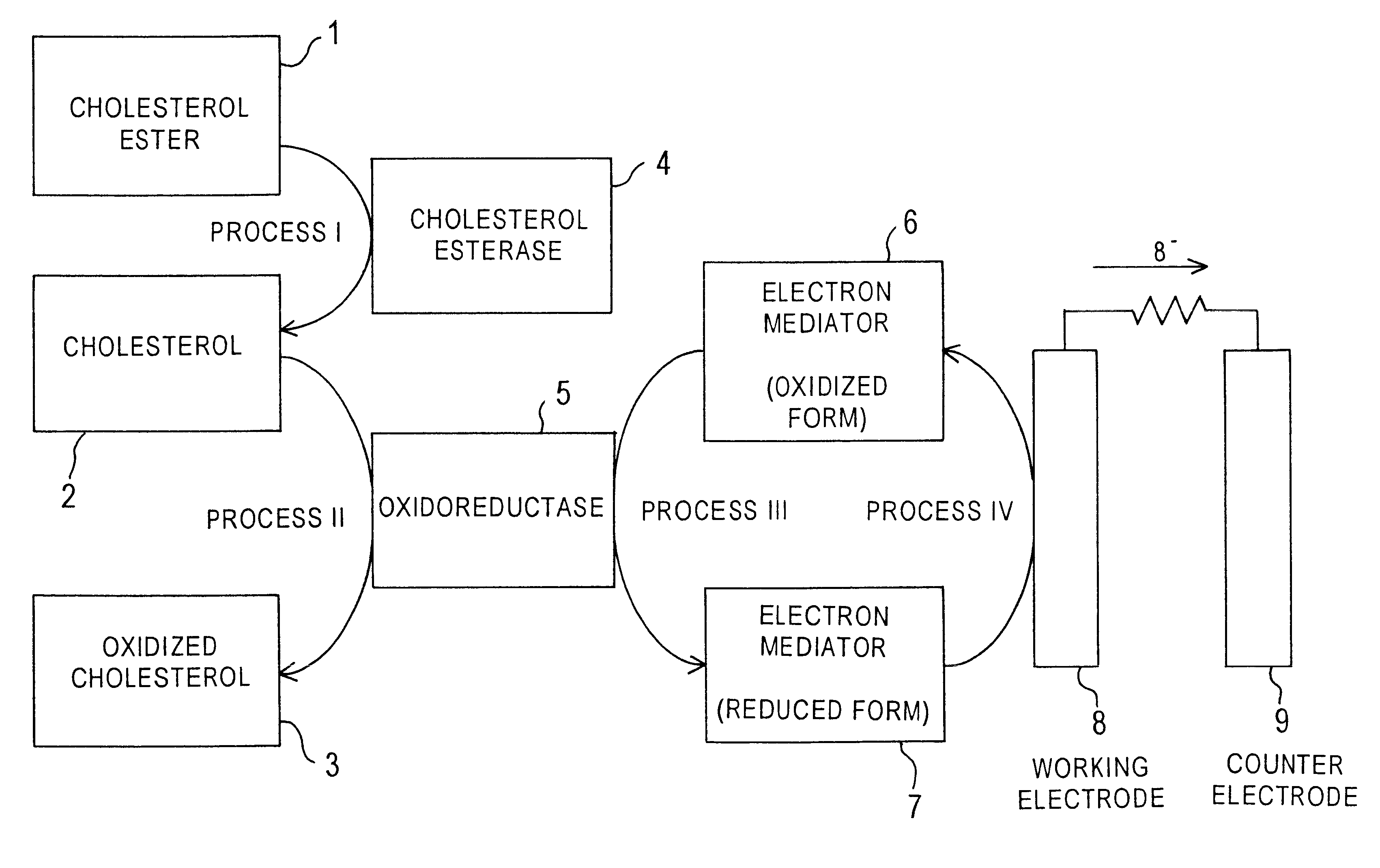
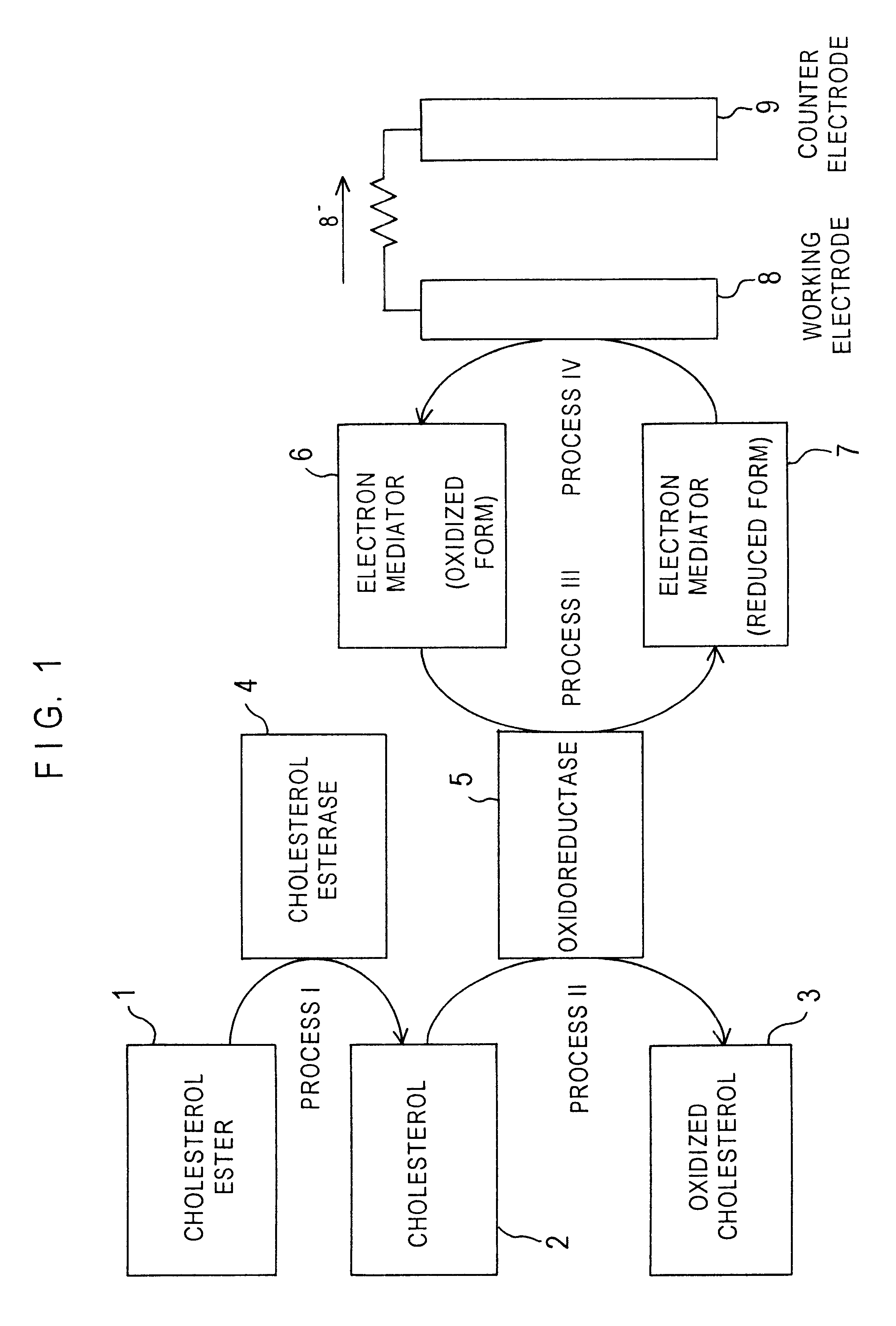
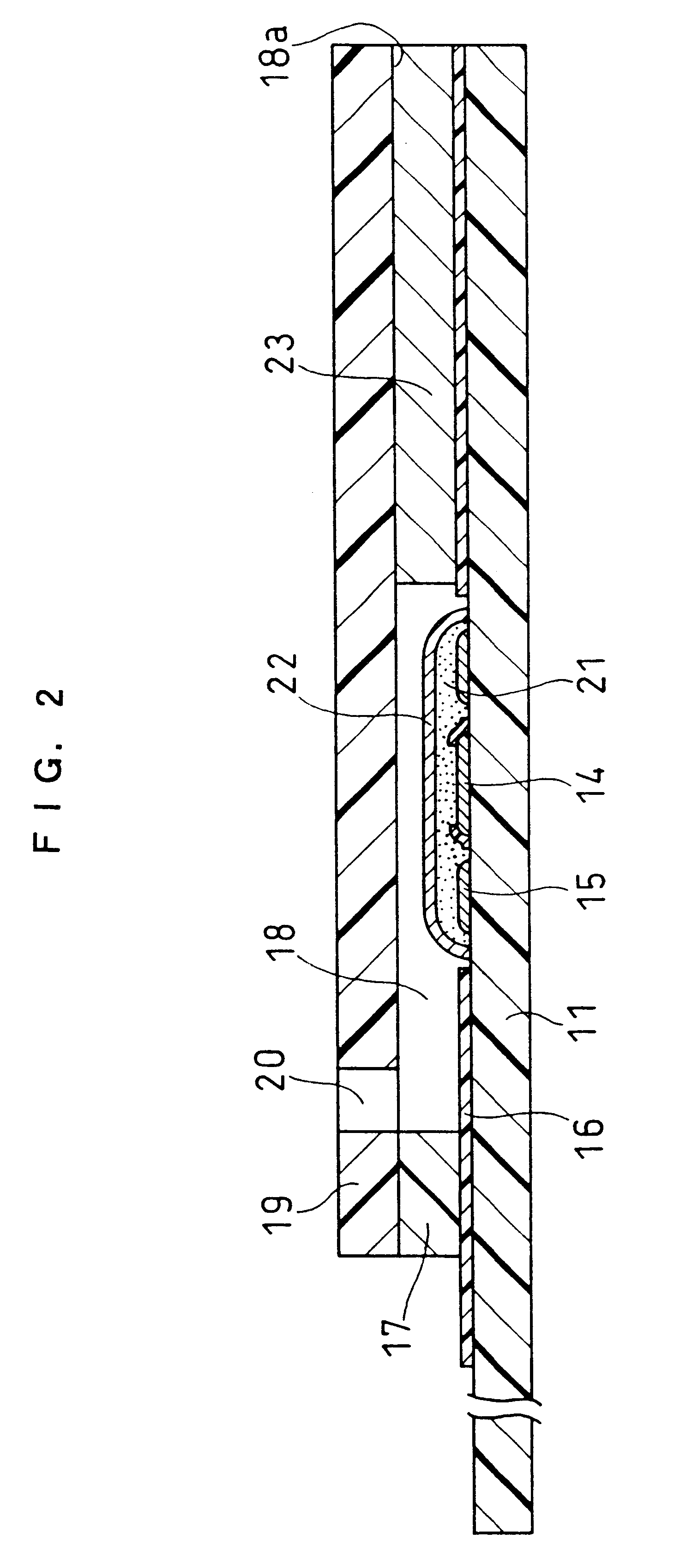
Cholesterol sensor and method of determining cholesterol
InactiveUS6342364B1Good reproducibilityImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsOxidoreductaseWater soluble
The present invention provides a sensor that electrochemically determines cholesterol in low density lipoprotein by only one feed of a sample. The sensor has: an electrode system that is mounted on an electrically insulating base plate and includes at least a working electrode and a counter electrode; an enzyme layer formed on the base plate with the electrode system; and a reagent layer that is arranged before the enzyme layer in a sample solution supply path to the electrode system. The enzyme layer includes at least an oxidoreductase and an electron mediator. The reagent layer includes a reagent that depresses reactivity of cholesterol in lipoproteins other than the low density lipoprotein with the oxidoreductase, for example, a reagent that attaches to lipoproteins other than the low density lipoprotein to form a water-soluble complex.
Owner:PHC HLDG CORP
Statin and Omega-3 Fatty Acids For Lipid Therapy
InactiveUS20090239927A1Lower triglyceride levelsBiocideMetabolism disorderLipid formationTG - Triglyceride
A method of lipid therapy, comprising providing a subject group having a baseline triglyceride level of 200 to 499 mg / dl and being at or near its low-density lipoprotein cholesterol (LDL-C) level goal, and reducing the triglyceride level and the non-high-density lipoprotein cholesterol (non-HDL-C) level of the subject group as compared to treatment with a 3-hydroxy-3-methyl glutaryl coenzyme A (HMG CoA) inhibitor alone, by administering to the subject group an effective amount of an HMG CoA inhibitor and a composition comprising omega-3 fatty acids.
Owner:BOBOTAS GEORGE +3
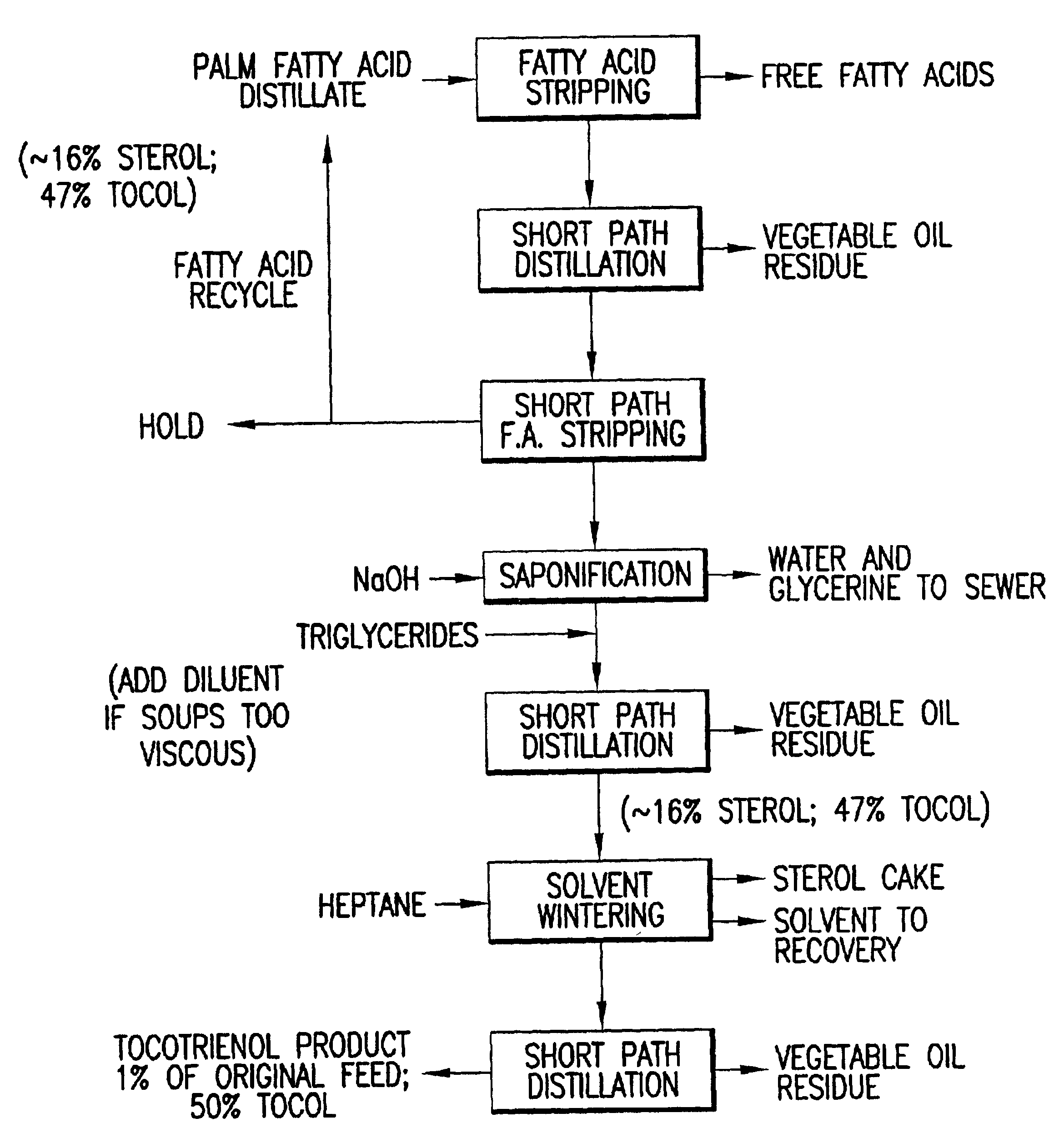
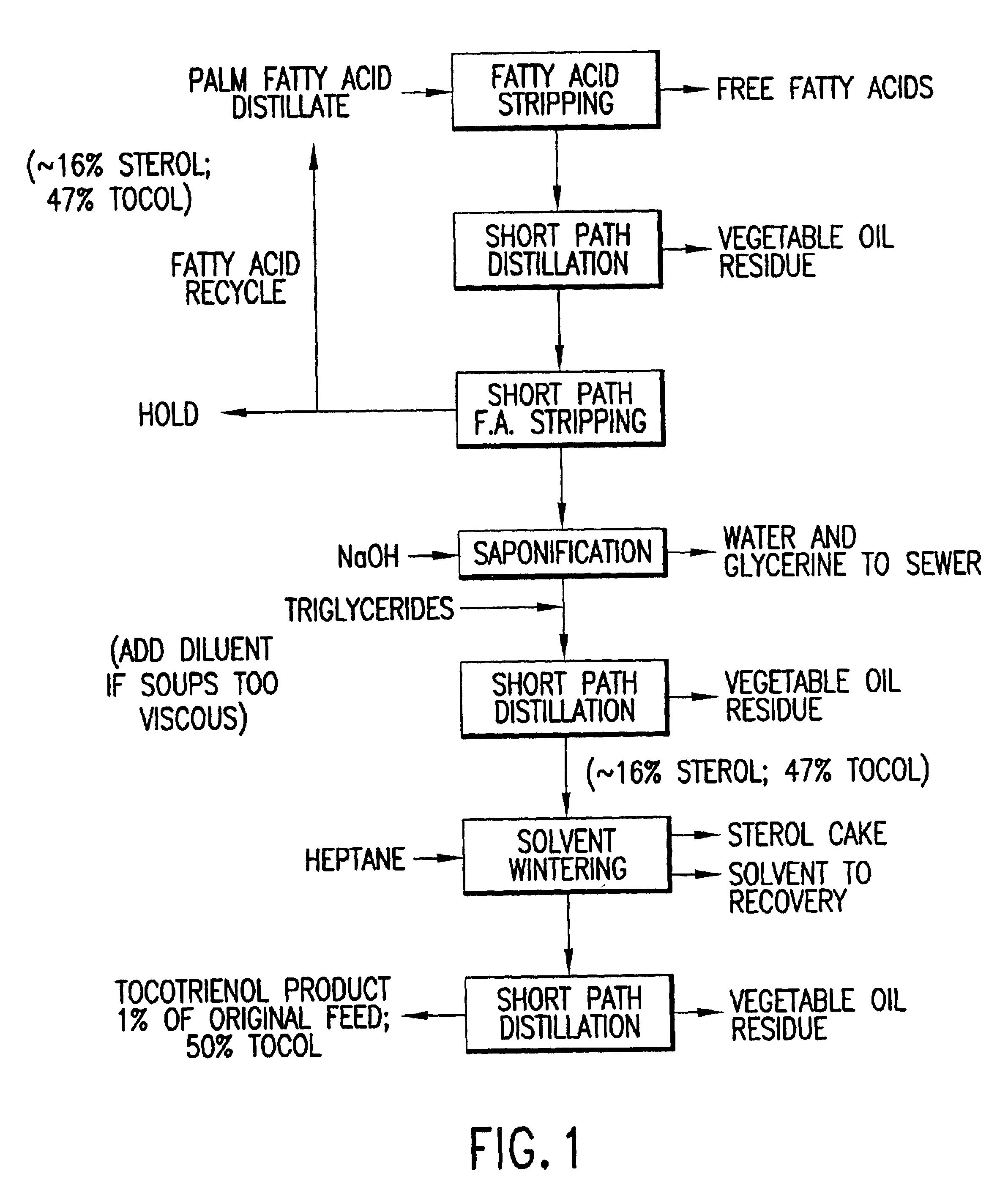
Process for the production of tocotrienols
This invention relates to processes for the production of tocotrienol compounds from biological sources such as palm oil, cereals, grains, and grain oils. The tocotrienol products are recovered in high yields. These tocotrienols are useful as pharmaceuticals, in foodstuffs and as dietary supplements. These compositions are hypocholesterolemic, antioxidizing, antithrombotic, antiatherogenic, antiinflammatory and immunoregulatory in nature. Tocotrienols are known to lower the levels of low density lipoproteins in the bloodstream.
Owner:ARCHER DANIELS MIDLAND CO
Cattle freezing seminal fluid dilution and method for producing the same
InactiveCN101220345AImprove qualityImprove survival rateDead animal preservationTissue cultureFiltrationGlycerol
The invention discloses a frozen bovine semen diluent, and the consumption amounts of all the raw materials in 100ml of the diluent are: 0.8 to 1.2g of fructose, 1.4 to 1.6g of sodium citrate, 2.3 to 2.6g of TRIS, 7.5 to 9.5g of low-density lipoprotein, 5 to 8ml of glycerol, 0.085 to 0.12 million IU of penicillin and the rest is distilled water. The preparation method is that: the fructose, the sodium citrate and the TRIS are dissolved in the distilled water to be prepared into a base liquid; penicillin G sodium and the low-density lipoprotein are added in the base liquid to prepare a I liquid; the glycerol is added in the I liquid to prepare a II liquid; then the pH value is adjusted to 6 to 7.5, then a filtration and a sterilization are carried out, and the liquid is cooled until reaching the room temperature and then arranged in a refrigerator of 2 - 5 DEG C for standby. The frozen bovine semen diluent of the invention has good and reliable effect, which can provide high-quality and excellent straw frozen semen for bovine artificial insemination and have very broad market application prospect.
Owner:NORTHWEST A & F UNIV
Synergistic effects of amlodipine and atorvastatin metabolite as a basis for combination therapy
The combination of amlodipine with atorvastatin metabolite shows a synergistic antioxidant effect on lipid peroxidation in human low-density lipoproteins and membrane vesicles enriched with polyunsaturated fatty acids. Inhibition of oxy-radical damage by this drug combination was observed at therapeutic levels in a manner that could not be reproduced by the combination of amlodipine with other statins or the natural antioxidant, vitamin E. The basis for this potent activity is attributed to the chemical structures of these compounds and their molecular interactions with phospholipid molecules, as determined by x-ray diffraction analyses. This combination therapy can be used to treat cardiovascular disorders, especially coronary artery disease, by increasing the resistance of low-density lipoproteins and vascular cell membranes against oxidative modification.
Owner:MASON R PRESTON
Gene transfer method with the use of serum-free medium
InactiveUS6287864B1Effective maintenanceImprove efficiencyBiocideMicroorganismsVirus-RetrovirusGene transfer
A method for transferring a gene into target cells by a retrovirus with the use of serum-free medium. This method comprises infecting target cells with a retrovirus in serum-free medium optionally containing low-density lipoprotein and / or cytokines in the presence of a functional substance such as fibronectin in an amount effective in elevating the gene transfer efficiency of the retrovirus into the target cells by co-localizing the retrovirus and the target cells.
Owner:TAKARA HOLDINGS
Casein-derived antioxidant peptide and preparation method thereof
ActiveCN105254714AInhibit oxidation functionPeptide/protein ingredientsPeptide preparation methodsAntioxidant capacityProteinase activity
The invention relates to the technical field of bioactive peptides, in particular to a casein-derived antioxidant peptide mixture. Casein is hydrolyzed with protease, various pre-products of the casein antioxidant peptide are obtained, then separation, desalination and concentration are performed through ion exchange column chromatography, and the casein antioxidant peptide rich in basic amino acid is obtained. The casein antioxidant peptide has higher cell antioxidant capacity and low-density lipoprotein oxidation inhibition capacity and can still keep good antioxidant capacity after being digested and absorbed by gastrointestinal tracts, so that the casein antioxidant peptide rich in basic amino acid can be applied to fields of food and healthcare products and has very high social and economic benefit.
Owner:CHINA AGRI UNIV
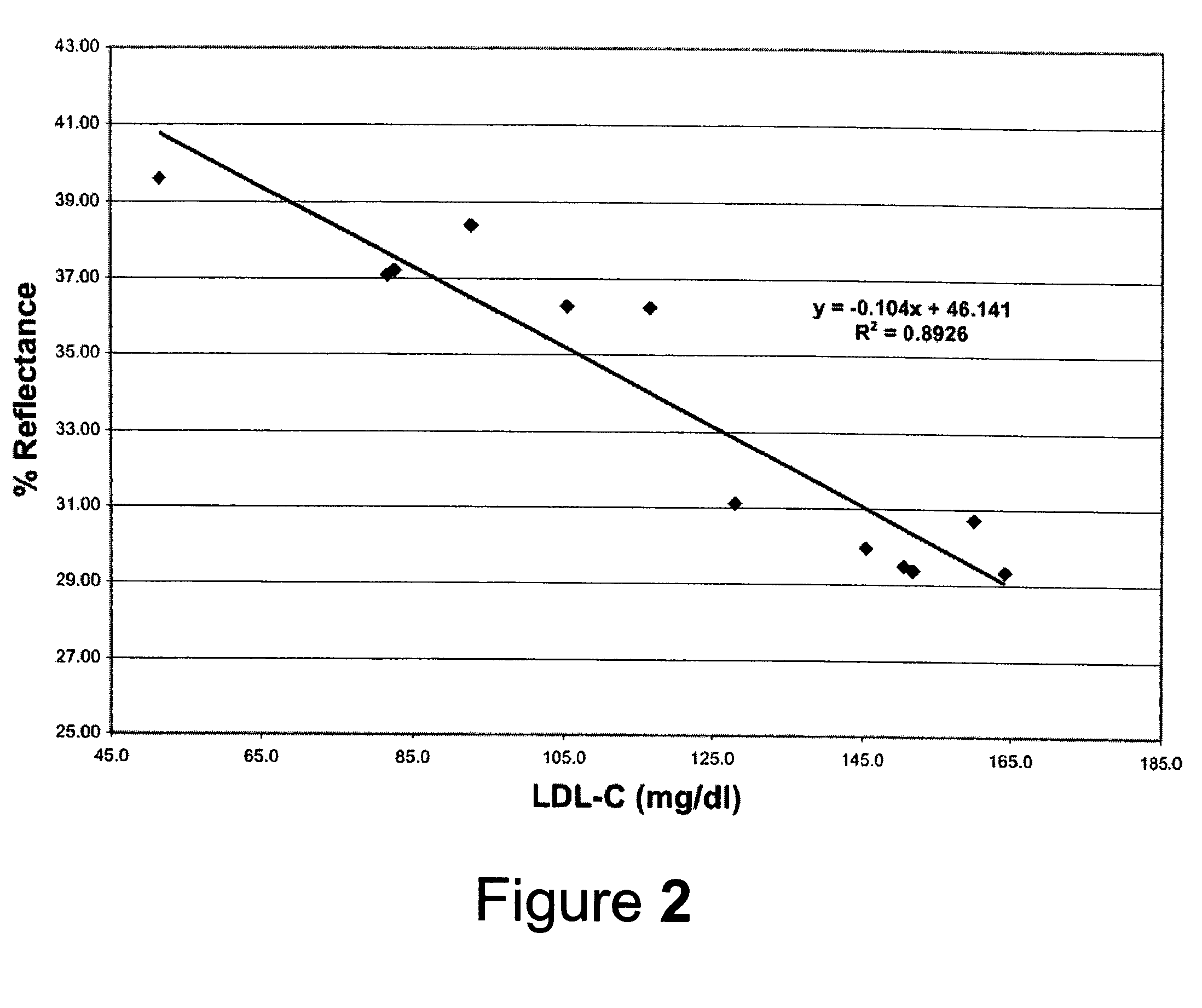
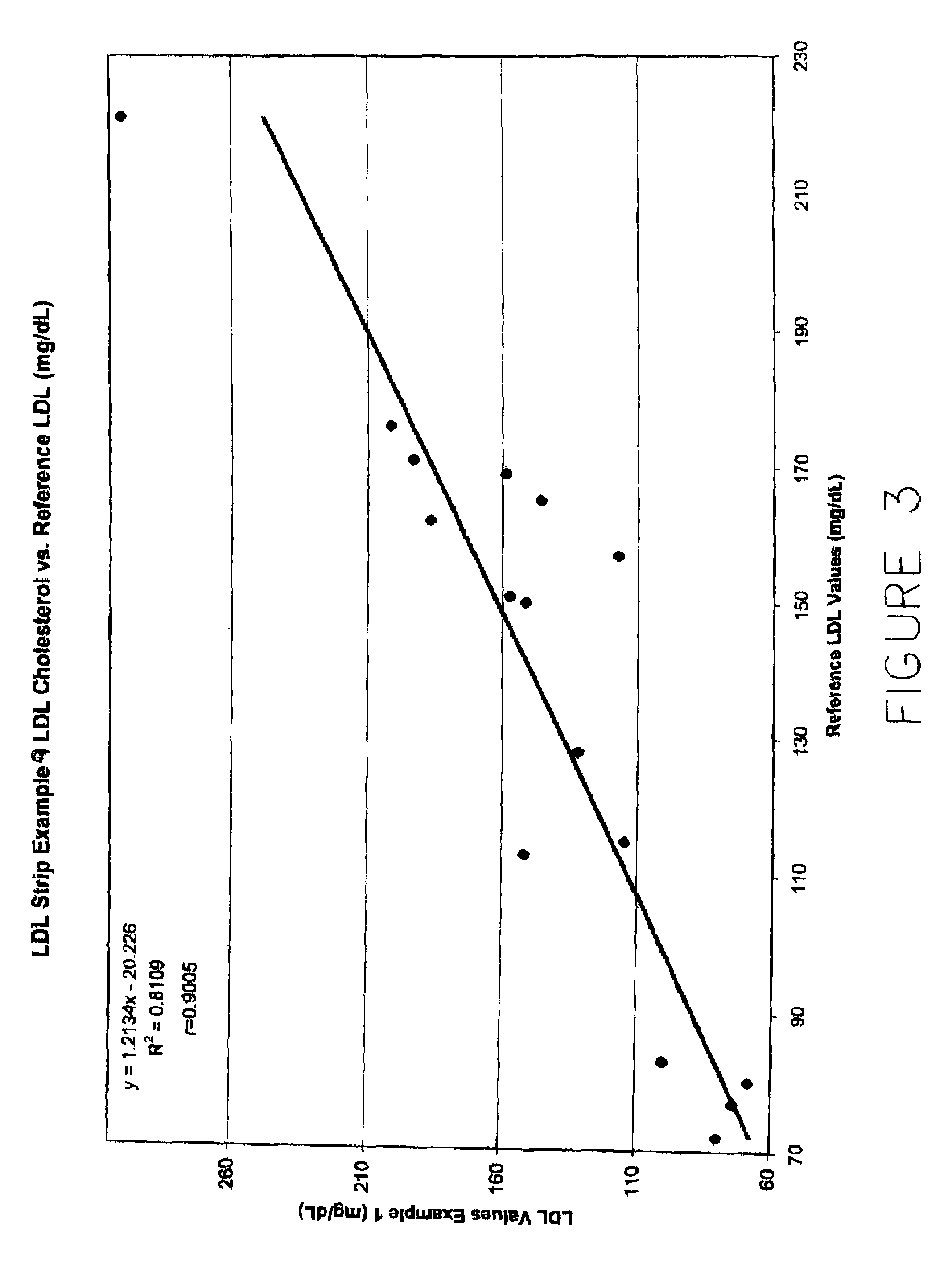
Direct measurement of chlolesterol from low density lipoprotein with test strip
ActiveUS7435577B2Improve solubilityReduce solubilityBioreactor/fermenter combinationsBiological substance pretreatmentsVery low-density lipoproteinRoom temperature
Cholesterol from Low Density Lipoproteins (LDL-C) is measured directly with a test strip at room temperature using a reagent that takes advantage of the varying surface charge density on LDLs and non-LDLs to selectively make LDL-C available for testing.
Owner:POLYMER TECH SYST
Application of lactobacillus plantarum in reducing blood fat and assisting fat-reducing
InactiveCN103598594AStrong bile salt toleranceLower levelMilk preparationMulti-step food processesSerum igeMicroorganism
The invention relates to an application of lactobacillus plantarum in preparation of food, health food, a health product or a medicine with a function of reducing blood fat or assisting fat-reducing. The collection number of the lactobacillus plantarum is CGMCC No.8198. the lactobacillus plantarum was collected in China General Microbiological Culture Collection Center (CGMCC) on Sep. 17, 2013. The address is 1 Beichen West Road, Chaoyang District, Beijing. The strain achieves the effect of reducing blood fat by reducing the level of serum cholesterol, triglyceride and low-density lipoprotein cholesterin. L.plantarum CGMCC No.8198 and its fermented products also play a role in controlling and reducing weight, and have an effect of assisting fat-reducing. The invention provides a biological therapy for effectively reducing serum cholesterol and assisting fat-reducing. The biological therapy has long-term eating safety and has a considerable application prospect.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Prepn process of beta-hydroxyl-beta-methyl butyric calcium (HMB-Ca)
InactiveCN1417190ASimple preparation processHigh purityCarboxylic acid salt preparationIsobutanolHaloform reaction
The present invention belongs to an organic compound preparing technology. Beta-hydroxyl-beta-methyl butyric calcium (HMB-Ca) is prepared using 4-methyl-4-hydroxy-2-propione as raw material and wateras solvent and through haloform reaction in water solution of NaOBr, acidification, isobutanol extraction, reaction of HMB acid in isobutanol extract with Ca(OH)2 to produce salt HMB-Ca. The said process has high product purity and less environmental pollution. The product HMB-Ca may be used in promoting the growth of muscles, strengthening immunity, reducing cholesterin and low-density lipoprotein level, preventing coronary heart disease and cardiac vascular disease, strengthening body's nitrogen-fixing capacity and maintaining body's protein level.
Owner:MANTE SHANGHAI BIAOLOGICAL SCI TECH
Reagent combination and method for direct test strip measurement of cholesterol from low density lipoproteins at ambient temperatures
ActiveUS20050170447A1Promote disseminationBioreactor/fermenter combinationsBiological substance pretreatmentsReagent stripDirect test
Cholesterol from Low Density Lipoproteins (LDL-C) is measured directly with a test strip at room temperature using a reagent that takes advantage of the varying surface charge density on LDLs and non-LDLs to selectively make LDL-C available for testing.
Owner:POLYMER TECH SYST
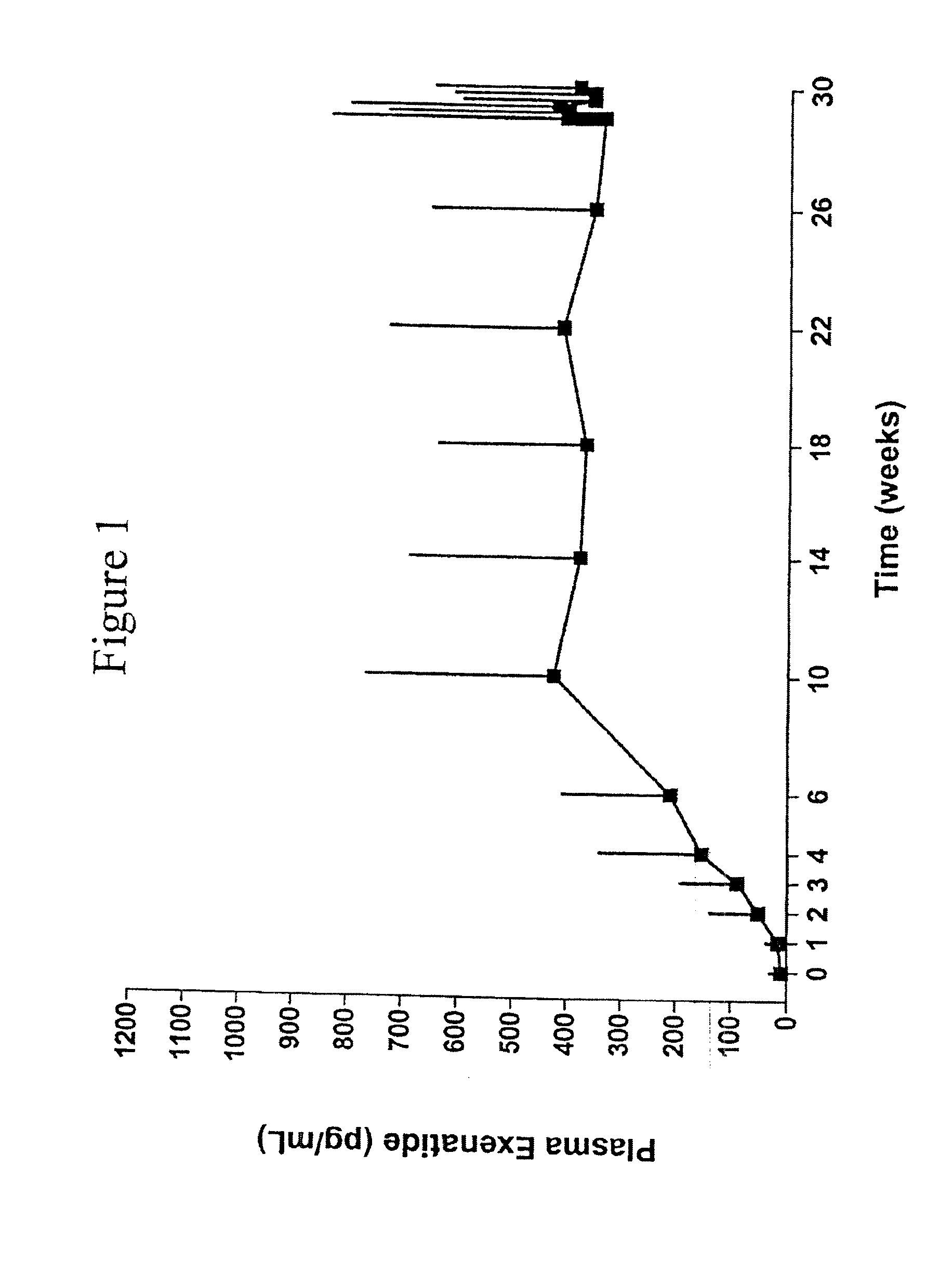
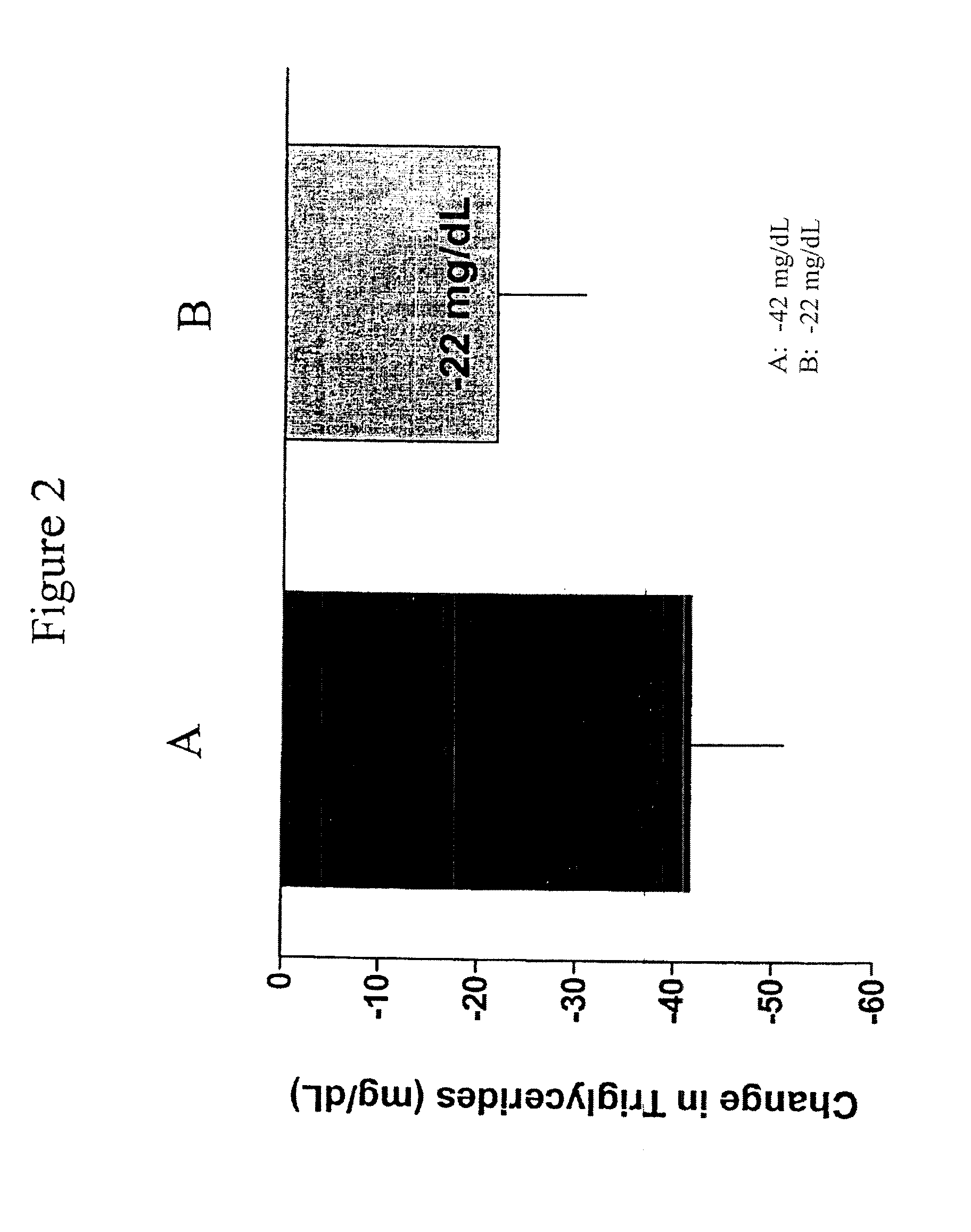
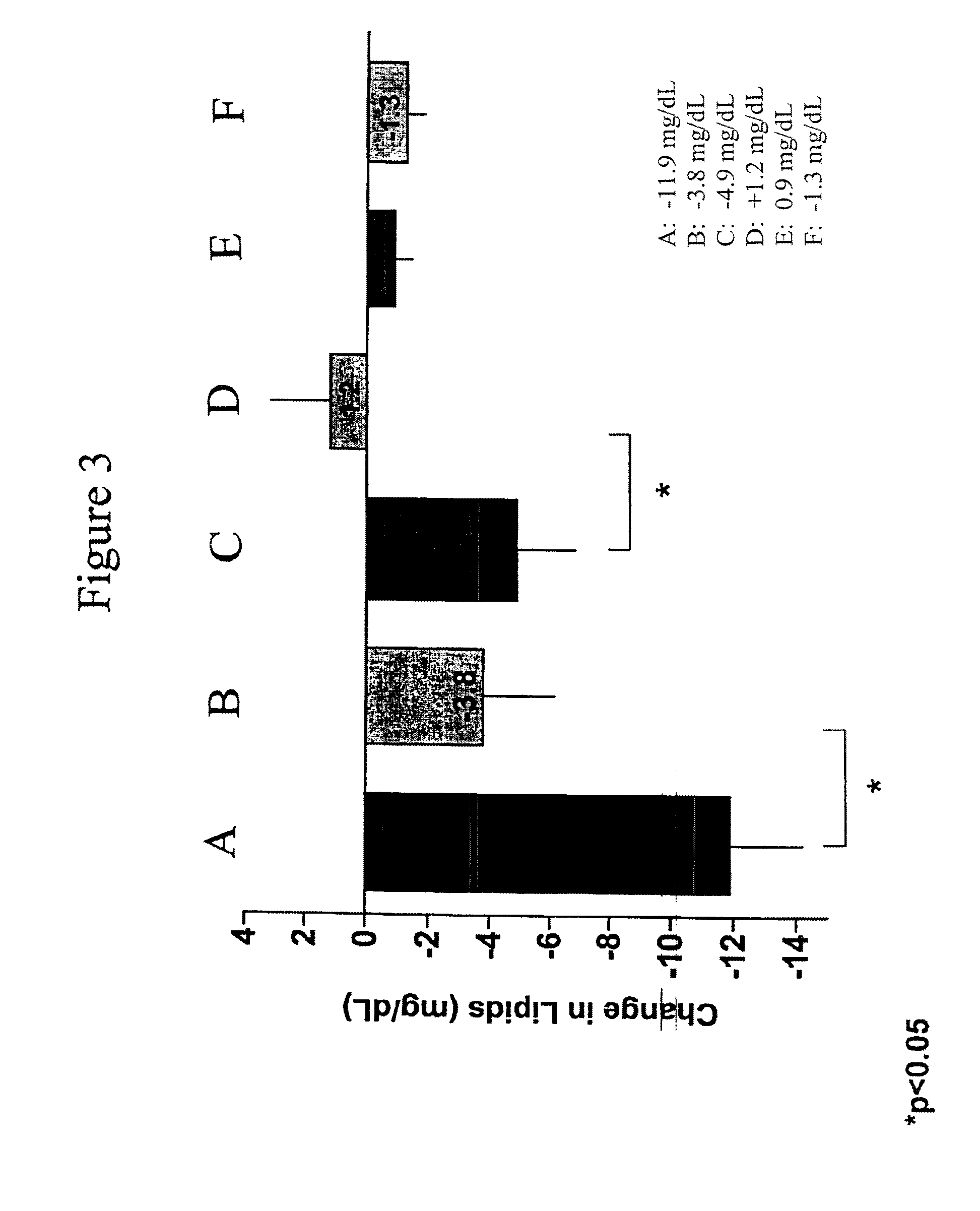
Exendins to lower cholesterol and triglycerides
InactiveUS20110263496A1Lower cholesterol levelsStable statePeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Compositions and methods for reducing or controlling blood cholesterol, lipoproteins, triglycerides, and sugar and preventing or treating cardiovascular diseases
InactiveUS20040253327A1Reduce riskLower blood sugar levelsBiocideUnknown materialsGuar gumHigh-density lipoprotein
This invention provides compositions and methods related to the administration of psyllium husk, Î<2>-sitosterol, guggul tree extract, guar gum and chromium as a combination to reduce or control blood cholesterol, triglycerides, low density lipoproteins, blood sugar or increasing or controlling high density lipoproteins in a mammal, to reduce arterial plaque build-up, atherosclerosis, in a mammal which may be associated with cardiovascular, cerebrovascular, peripheral vascular, or intestinal vascular disorders.
Owner:PBN PHARMA
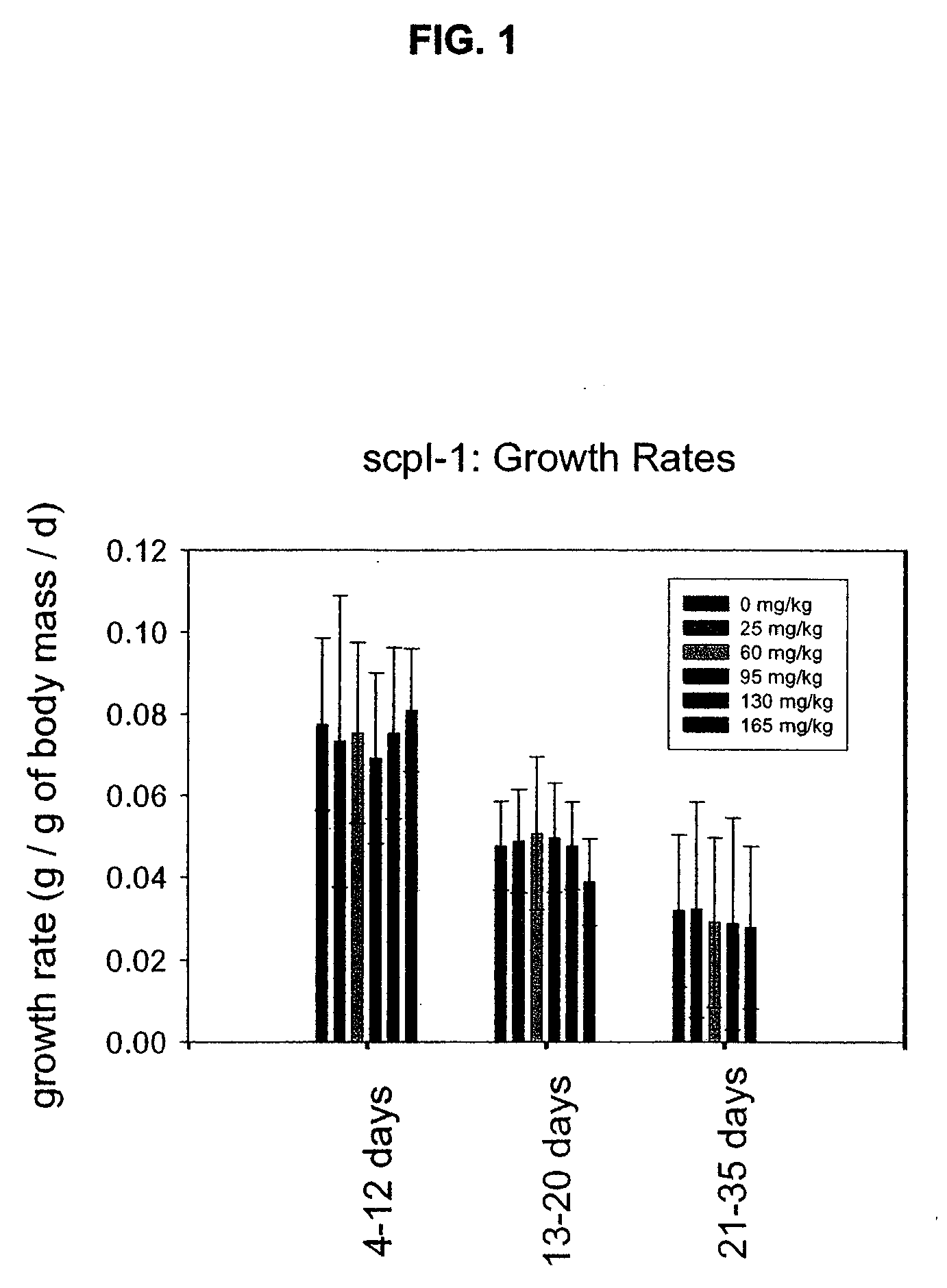
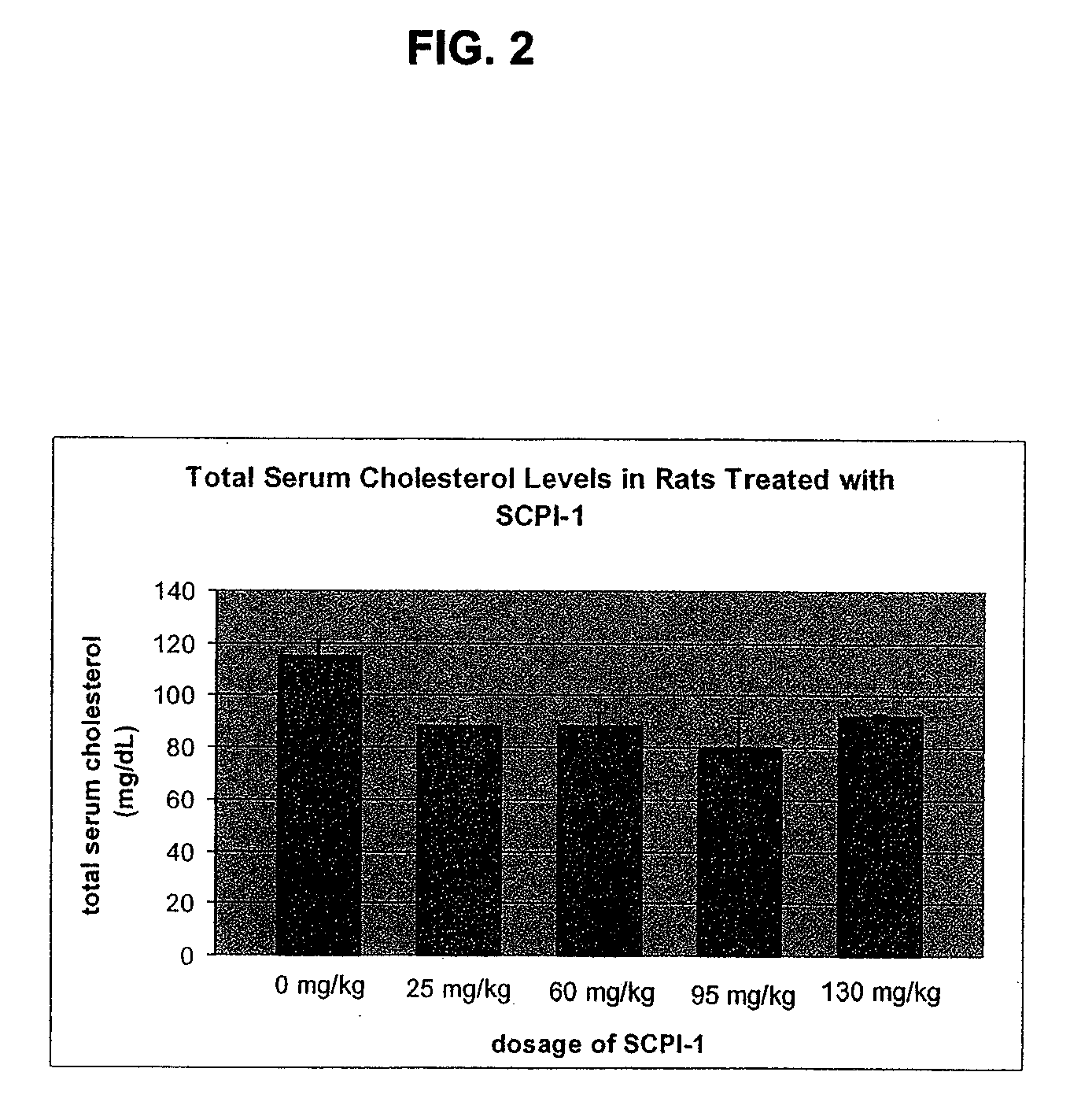
Sterol Carrier Protein-2 Inhibitors for Lowering Cholesterol and Triglyceride Levels in Mammals
InactiveUS20080194658A1Lowering serum total cholesterol concentrationLowering serum low density lipoprotein cholesterol concentrationBiocideOrganic active ingredientsHydrobromideSerum ige
Methods of treating high serum levels of total cholesterol, low density lipoprotein and triglycerides in a mammal by administering therapeutically effective doses of N-(4-{[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]amino}phenyl)acetamide or a salt thereof such as a hydrochloride or hydrobromide salt.
Owner:ZAMA JAPAN
Human blood fat (serum/plasma) quality management reference kit and preparation method
ActiveCN103472240ANo medium effect interferenceSolve medium effect interferenceBiological testingAntigenLow density lipoprotein cholesterol
The invention provides a human blood fat (serum / plasma) quality management reference kit. The kit takes human serum (plasma) as a matrix; a human blood fat component (human apolipoprotein A1 antigen, apolipoprotein B antigen, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and the like), a stable system and an anticorrosion system are added to the matrix; the stable system is any combination of a surfactant of Triton series, Tween series, EDTA (ethylene diamine tetraacetic acid) series and the like, a polymer of polyvinyl alcohol series, polyvinylpyrrolidone series, polyethylene glycol series and the like, and a phosphate buffer solution; and the anticorrosion system is any combination of sodium azide, gentamicin, amphomycin and ProClin series. The invention further provides a preparation method of the kit. The kit is free from medium effect interference, is used for quality control and evaluation between the detection systems, and more really reflects actual quality conditions of human serum (plasma) samples, which are detected by detection systems.
Owner:上海北加生化试剂有限公司
Application of lentinan in preparing medicine, healthcare product and food in treating or preventing disease caused by enteric flora disturbance
InactiveCN104546889AChange compositionReduce contentOrganic active ingredientsMetabolism disorderDiseaseDiabetes model
The invention provides application of lentinan in preparing medicines, healthcare products and food in treating or preventing diseases caused by enteric flora disturbance. The applicant of the invention adopts high-fat feed to induce a mouse obesity type-II diabetes model to observe the influence of polysaccharide in traditional Chinese medicines on lipid metabolism of mice through enteric florae, and by adopting the lentinan, enteric flora disturbance caused by diet can be adjusted, constitution of enteric florae can be remarkably improved, and the unbalance enteric florae in bodies can be recovered, so that the blood fat and the blood sugar indexes of obesity caused by enteric flora disturbance caused by diet can be adjusted, the content of triglyceride in blood plasma can be remarkably reduced, and the content of cholesterol, low density lipoprotein and free fatty acid can be reduced.
Owner:INFINITUS (CHINA) CO LTD
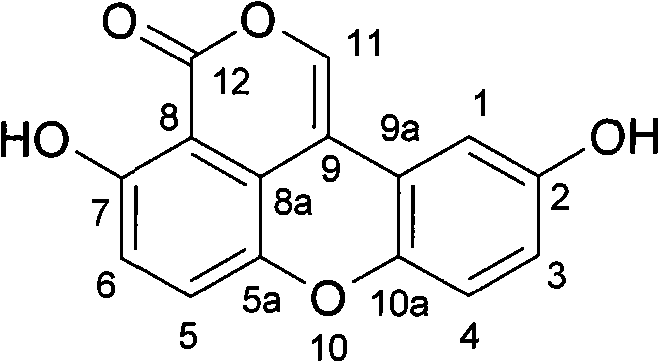
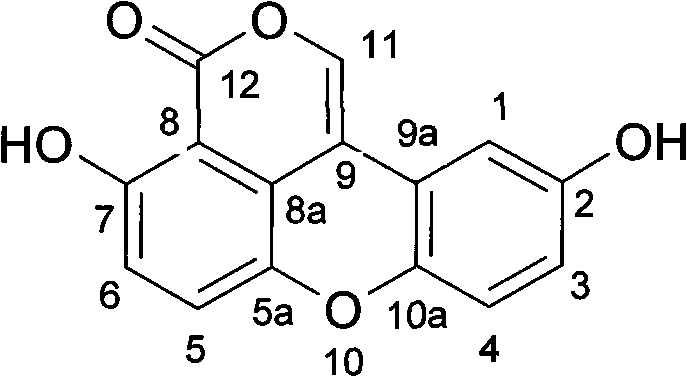
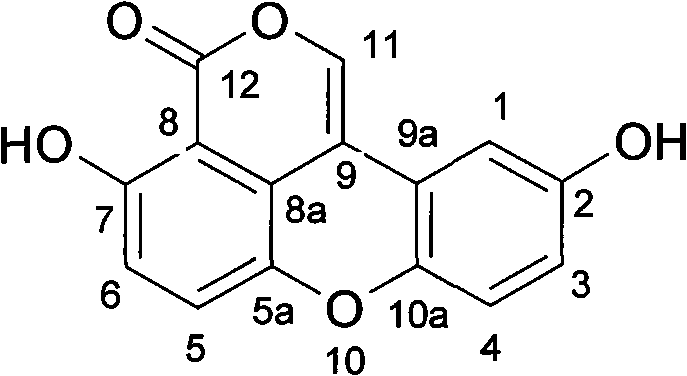
Antiatherosclerotic sparstolenin B compound and preparation method thereof
The invention discloses a new xanthene compound-sparstolenin B of which the chemical structural formula is as follows. The sparstolenin B is obtained by extracting and separating the dried tuber of Sparganium stoleniferum Buch-Ham. The sparstolenin B has the functions of inhibiting migration of arterial smooth muscle cells, inhibiting macrophage-mediated low-density lipoprotein oxidation and inhibiting platelet aggregation activity, and can be used as a medicament for curing and preventing atherosclerosis diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Lactobacillus plantarum ZJUF T17 and application thereof
ActiveCN108570436AImprove survival rateHas immune regulation functionMilk preparationBacteriaLow density lipoprotein cholesterolHigh survival rate
The invention discloses lactobacillus plantarum ZJUF T17 and application thereof and belongs to the technical field of biology. The lactobacillus plantarum ZJUF T17 is separated from sour dough and has a preservation number of CCTCC (China Center For Type Culture Collection) NO: M 2017342. The lactobacillus plantarum ZJUF T17 provided by the invention has a very high survival rate and a relativelystrong stomach and intestine adhesive force and can be used for remarkably inhibiting reproduction of gastrointestinal tract pathogenic bacteria; the lactobacillus plantarum can be used for remarkably reducing the content of cholesterol in blood serum and optimizing the ratio of high density lipoprotein cholesterol and low density lipoprotein cholesterol; the lactobacillus plantarum can be used for remarkably reducing the content of TNF (Tumor Necrosis Factor)-alpha in the blood serum and has an immunological regulation function; the lactobacillus plantarum has the capability of alleviating the increasing of body weight; furthermore, the lactobacillus plantarum has the potential of preparing fermented milk.
Owner:ZHEJIANG UNIV +1
Method for preparing spherical carbon aerogel with specific absorbability for low density lipoprotein
InactiveCN102091595APrevent collapseAvoid volumeOther chemical processesOther blood circulation devicesSupercritical dryingSorbent
The invention relates to a method for preparing spherical carbon aerogel with specific absorbability for low density lipoprotein, which comprises: mixing a phenol and an aldehyde according to a ratio to form a precursor, preparing nano silica sol as a template agent, performing a sol-gel reaction in a dispersed phase containing surfactant, performing supercritical drying, carbonizing and etching steps to obtain pure carbon aerogel spheres; and coating the carbon aerogel spheres with a polymer material with high biological compatibility and rich free radicals on surface, coupling the carbon aerogel spheres with anion groups by using a coupling agent, and drying to obtain the spherical carbon aerogel with specific absorbability for low density lipoprotein. Compared with the prior art, the spherical carbon aerogel has large internal and external specific surface area and a large amount of negative charges on surface, and therefore can absorb a large amount of low density lipoprotein (LDL) with positive charges on surface; meanwhile, the spherical carbon aerogel does not have obvious absorption effect on beneficial ingredients in blood such as high-density lipoprotein, a carrier and a genin both have high biological compatibility, and an absorbent can be used as a hemoperfusion absorbant for treating hyperlipemia.
Owner:EAST CHINA UNIV OF SCI & TECH
Compositions and methods for effecting the levels of high density lipoprotein (HDL) cholesterol and apolipoprotein AI very low density lipoprotein (VLDL) cholesterol and low density lipoprotein (LDL) cholesterol
InactiveUS7008776B1Reduced activityEnhances enzymatic reactionHydrolasesBiological material analysisVery low-density lipoproteinNeutralizing antibody
The present invention relates to compositions for use in raising or lowering the level of LIPG polypeptide in a patient. Embodiments of the composition include compositions comprising: an anti-sense nucleic acid; a neutralizing antibody; an intracellular binding protein; an inhibitor which inhibits the enzymatic activity of LIPG polypeptide; an inhibitor which inhibits the expression of LIPG gene; a ribozyme; an LIPG polypeptide; an enhancer which increases the enzymatic activity of LIPG polypeptide; or an enhancer which increases the expression of LIPG gene. The invention relates also to methods for using the above compositions.In addition, the invention relates to a method for diagnosing a predisposition to lower cholesterol, a method for determining whether a test compound can inhibit the enzymatic reaction between LIPG polypeptide and HDL cholesterol, and methods for determining whether a test compound can enhance the enzymatic reaction between LIPG polypeptide and LDL or VLDL cholesterol.
Owner:AVENTIS PHARMA INC +1
Reagent set and method for detecting cholesterol in a high-density lipoprotein or low density lipoprotein
InactiveUS7208287B2Raise the pHMicrobiological testing/measurementBiological testingVery low-density lipoproteinHigh-density lipoprotein
A method for quantitating a specific component in lipoproteins contained in a biological sample, for example, HDL (high-density lipoprotein), LDL (low-density lipoprotein) or VLDL (very low-density lipoprotein) by using a commonly employed automatic analyzer without centrifuging or making the reaction liquor cloudy due to complexes or aggregates. Namely, a controlling means, whereby an enzyme reaction can be carried out exclusively for the target component, is introduced into a method for enzymatically assaying a component in a specific lipoprotein fraction in the serum, thereby specifically assaying the component.
Owner:SYSMEX CORP
In-vitro diagnostic reagent for homogeneous method of low-density lipoprotein cholesterol (LDL-C) of serum
InactiveCN102041296AOvercome the shortcomingsMeet the requirements of large-scale assay samplesMicrobiological testing/measurementChemiluminescene/bioluminescenceVery low-density lipoproteinLuminous intensity
The invention relates to an in-vitro diagnostic reagent for a homogeneous method of low-density lipoprotein cholesterol (LDL-C) of serum, wherein the in-vitro diagnostic reagent is capable of being widely applied to the technical field of medicine and biochemistry and is characterized in that the in-vitro diagnosis is carried out by means of a method comprising the following steps of: step one, selectively cracking chyle particles (CM), very low density lipoprotein cholesterol (VLDL-C) and high density lipoprotein cholesterol (HDL-C) within the serum by using a group of surfactant comprising trimethyl-beta-cyclodextrin, ethylene oxide octadecyl amine, poloxamer F88 and Brij-58, then generating hydrogen peroxide (H2O2) during the catalytic reaction of cholesterol esterase (COE) and cholesterol oxidase (COD), and then discomposing the H2O2 by means of a chemiluminescence clearing system of hydrogen peroxide, wherein the LDL-C particles within the serum are still kept perfectly at the moment; step two, reacting the LDL-C by catalyzing with the COE and the COD under the effect of TritonX-100 so as to generate H202, then promoting a chemiluminescence quantitative system to produce chemiluminescence by catalyzing the H2O2 with POD, and quantitating the LDL-C after measuring luminous intensity. The measuring reagent provided by the invention has the advantages that the sensitivity is high, the capacity of resisting disturbance is strong, the purpose for detecting the LDL-C of serum in batch is realized on a microporous plate chemiluminescence apparatus by measuring chemiluminescence intensity, and the reagent is suitable for the application in clinical laboratory.
Owner:WENZHOU MEDICAL UNIV
Assays to predict atherosclerosis and dysfunctional high-density lipoprotein
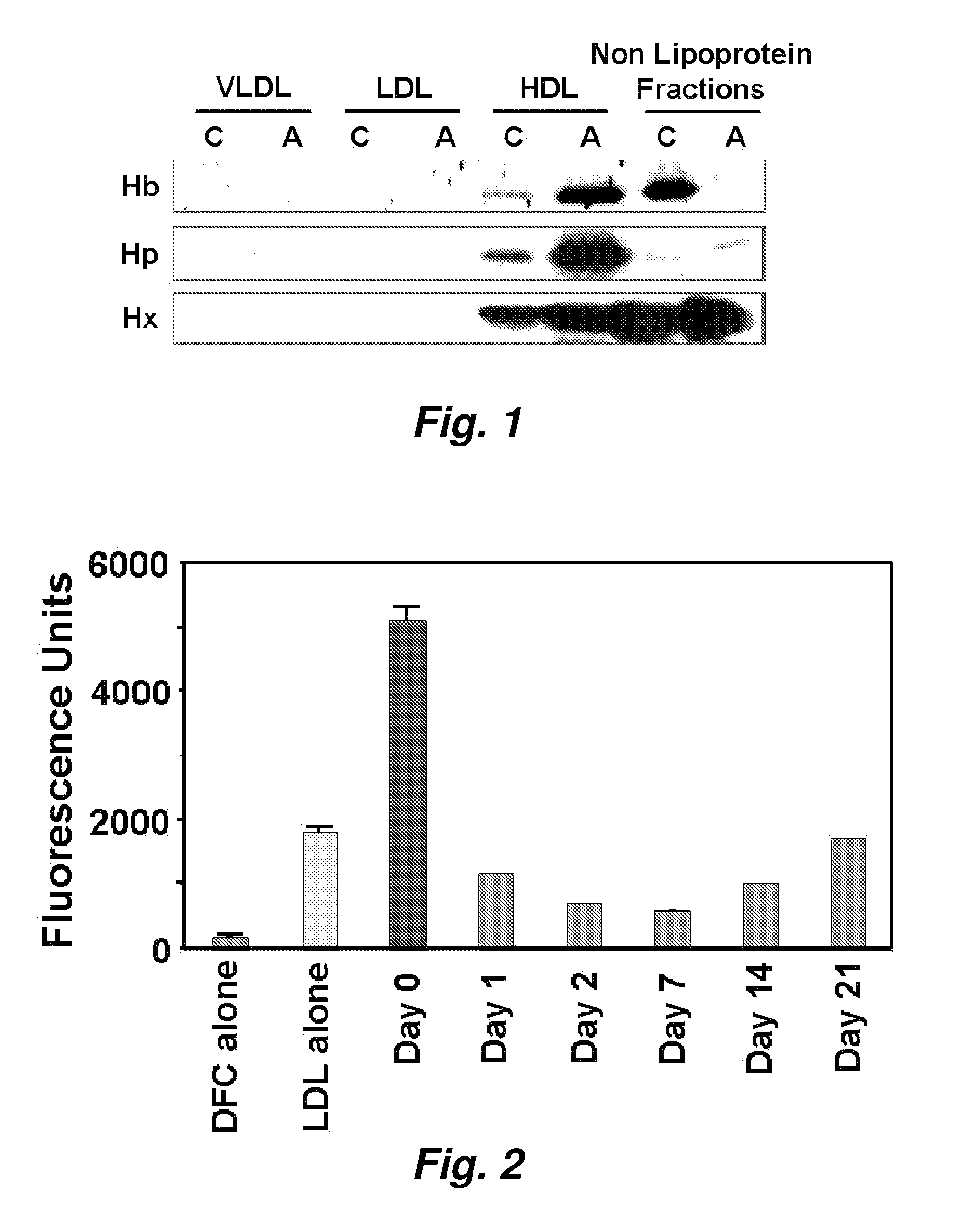
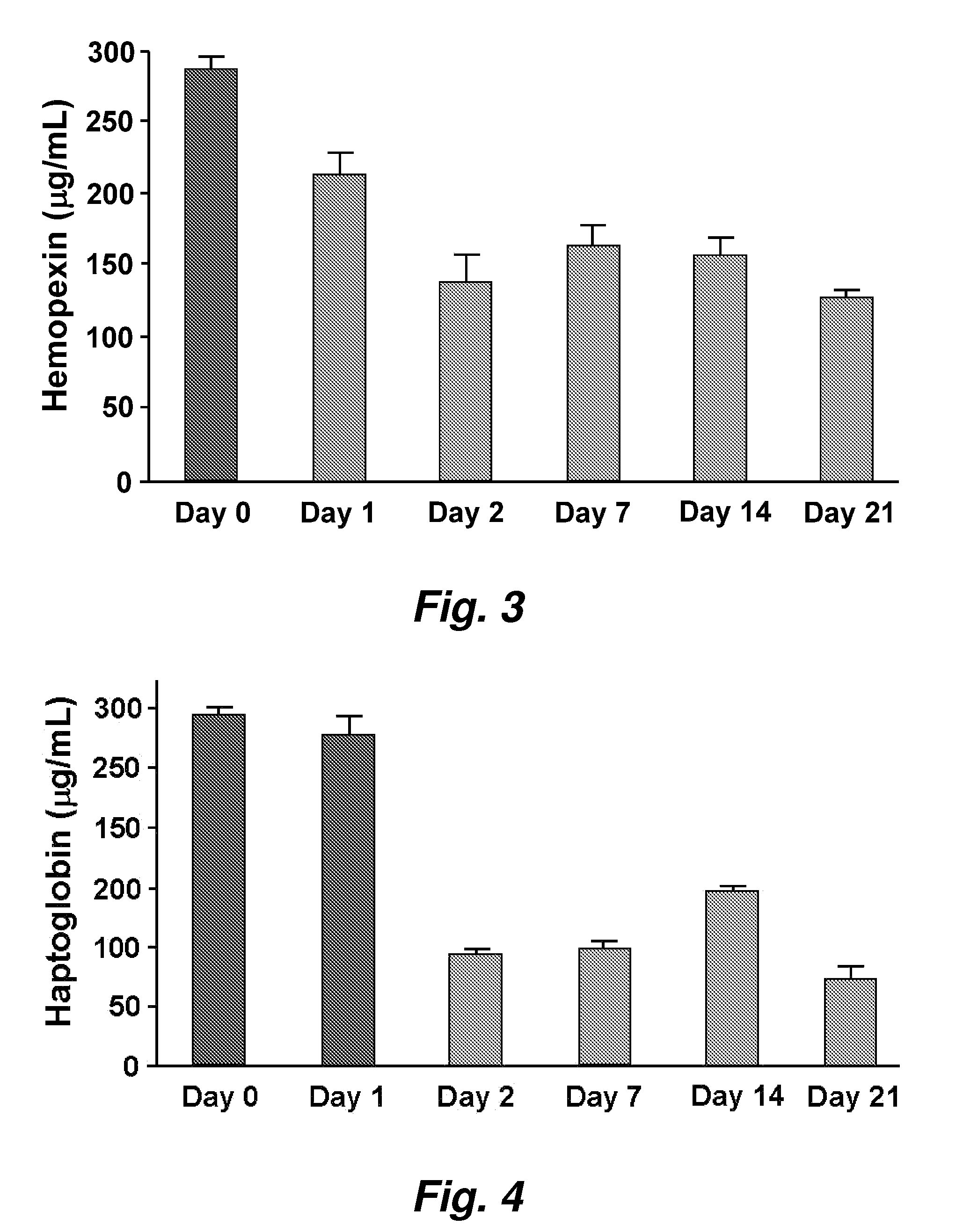
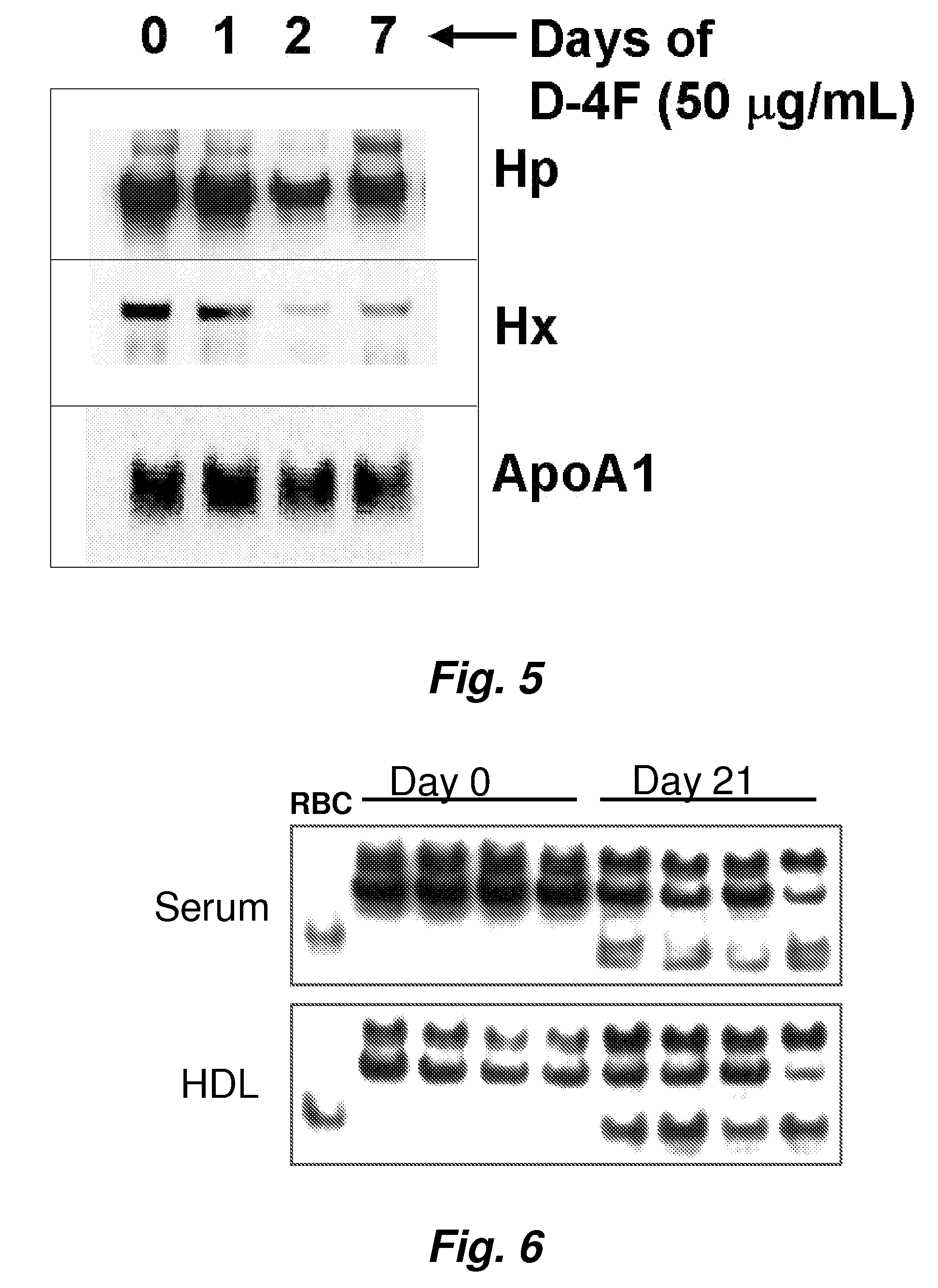
ActiveUS20070218501A1Bioreactor/fermenter combinationsBiological substance pretreatmentsNitric oxideAtheroma
This invention provides novel assays for the detection of dysfunctional HDL. The assays are good diagnostics and / or prognostics for atherosclerosis or other pathologies characterized by an inflammatory response. In certain embodiments the methods involve measurements of heme-related HDL-associated proteins (e.g., haptoglobin, hemopexin, etc.), and / or measurements of the relative distribution of HDL-associated proteins between HDL and the non-lipoprotein fractions of plasma / serum, and / or measurements of the ability of pro-inflammatory HDL to consume nitric oxide, and / or measurement of the ability of HDL to inhibit LDL aggregation.
Owner:RGT UNIV OF CALIFORNIA
Peanut water-soluble dietary fiber enzymatic extracting method
InactiveCN101664167APromote growth and reproductionReduce manufacturing costFood preparationFiberAlpha-amylase
The invention discloses a peanut water-soluble dietary fiber enzymatic extracting method which comprises the steps of: pretreating the prepared peanut powder by acetone, alcohol, protease and alpha-amylase, and hydrolyzing by using cellulose for three times; and concentrating hydrolysis liquid and then precipitating by absolute ethyl alcohol, centrifuging and drying to obtain the water-soluble dietary fiber. The product prepared by the invention contains 30-60% of non-starch polysaccharide, can reduce the content of low density lipoprotein (LDL) cholesterol in the blood, promotes the growth and the propagation of probiotics group in the intestinal tract, and can be taken as functional food for preventing and alleviating symptom as well as treating diseases such as cardiovascular diseases and the like in an auxiliary way. The method is low in production cost, high in fiber content and suitable for industrialized production.
Owner:SHANDONG PEANUT RES INST
Scutellarein derivative, its preparing process and application
The invention discloses a novel scutellarein derivative, the method for preparing the same and the application. It is proved through pharmacological test that the compound possesses obvious effect against lipid peroxidation, effect against cell damage induced by H2O2 and cell apoptosis induced by A beta, it can also inhibit Cu2+ from inducing oxidation and modification for low density lipoprotein. The compound can be used to prepare medicine that can prevent or treat neurodegenerative diseases such as Alzheimer's disease caused by free radical oxidation, and medicine that can treat inflammation, reduce blood fat and treat atherosclerosis.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
2,3,5,4'-tetrahydroxy diphenyl ethylene-2-1-beta-d-glucoside
InactiveCN1539844AGood water solubilityReduce dissolutionOrganic active ingredientsSugar derivativesBeta-DLipoprotein cholesterol
A 2,3,5,4'-tetrahydroxy distyrene-2-O-beta-D-glucoside used for hypoglycemic medicine is prepared from the decoction of fleece flower root through extracting, separating and purifying. It can suppress the increasement of serum cholesterol, decrease low-density serum lipoprotein cholesterol and improve activity of liver esterase.
Owner:广州中医药大学热带医学研究所
Preparation method of bitter gourd peptide arabinose composite tablet
InactiveCN103861086AGood for blood sugar controlBeneficial to blood lipid metabolismOrganic active ingredientsPeptide/protein ingredientsLow density lipoprotein cholesterolLiver and kidney
The invention relates to a preparation method of a bitter gourd peptide arabinose composite tablet. The bitter gourd peptide arabinose composite tablet consists of the following components in parts by weight: 8-15 parts of bitter gourd polypeptides, 15-25 parts of L-arabinose, 0.1-0.3 part of chromium-enriched yeast, 25-35 parts of inulin, 5-15 parts of phytosterol, 10-15 parts of D- mannitol, 10-20 parts of microcrystalline cellulose, 0.5-1 part of magnesium stearate, and 2-5 parts of hydroxypropyl methylcellulose. The preparation method has beneficial effects that the bitter gourd peptide arabinose composite tablet is prepared by adopting a plurality of components beneficial to blood sugar control and blood lipid metabolism; compared with a way of independently adopting the bitter gourd polypeptides, the inulin or the phytosterol, and the like, a way of combining the bitter gourd polypeptides, the inulin or the phytosterol, and the like is better in effect, beneficial to blood sugar control and blood lipid metabolism of a patient with diabetes mellitus II, and free of influences on functions of livers and kidneys. Besides, the preparation method can improve sensibility of insulin better and can remarkably lower total cholesterol and low-density lipoprotein cholesterol of the patient with diabetes mellitus II.
Owner:DONGYING BEIKANG BIOLOGICAL SCI & TECH
A kind of preparation method of low-density lipoprotein affinity adsorption hemodialysis membrane material
ActiveCN102258946ADoes not affect the performance of the bodyPerformance is not affectedSemi-permeable membranesOther chemical processesVery low-density lipoproteinEnvironmental resistance
The invention belongs to the fields of membrane surface engineering and bioseparation engineering and aims at providing a method for preparing a low-density lipoprotein affinity adsorption hemodialysis membrane material. The method provided by the invention comprises the following steps: prewashing the hemodialysis membrane material; placing the prewashed hemodialysis membrane material into a plasma processor cavity; introducing inert gas and activated gas until atmosphere ingredients are constant and then carrying out plasma processing; immersing into a heparin solution; adding a coupling agent to carry out an oscillation reaction; carrying out oscillation washing by utilizing a PBS (Phosphate Buffer Solution); and repeatedly washing with clean water and perform filter drying to obtain the low-density lipoprotein affinity adsorption hemodialysis membrane material. In the method provided by the invention, active reaction groups are introduced into by utilizing a constant-pressure and low-temperature plasma activation modification processing method; the reaction is only carried out on the surface of the material, the body performance of the material is not influenced, and simultaneously the method has the characteristics of high efficiency, low cost, low energy consumption, environmental friendliness and the like; the hydrophilia and blood compatibility of the surface of a macromolecule separation membrane material can be greatly improved; and the blockage effect caused by membrane pollution in the hemodialysis course can be effectively reduced.
Owner:江苏巨之澜科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com