Human blood fat (serum/plasma) quality management reference kit and preparation method
A quality management and kit technology, applied in the biological field, can solve problems such as quality comparison investigation and quality control that cannot be used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
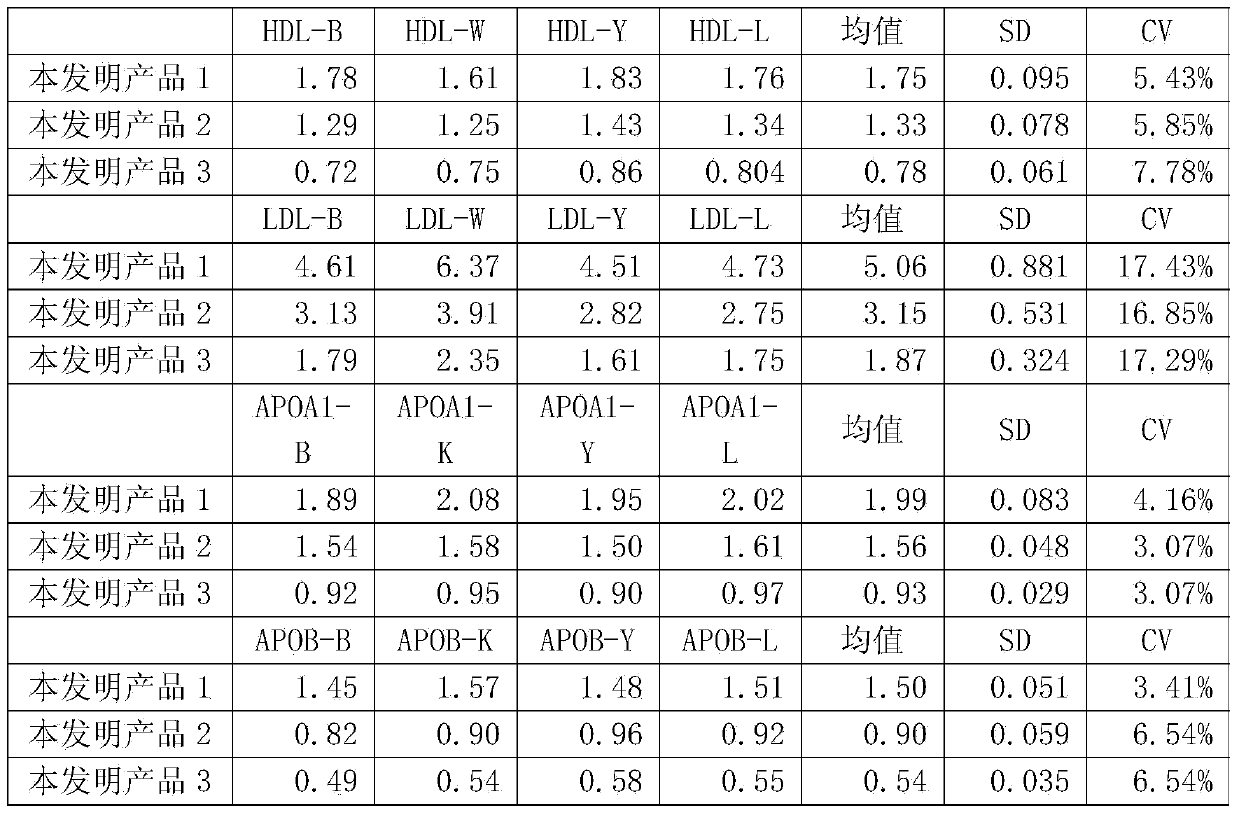
[0038] The present invention is a human blood lipid (serum / plasma) quality management reference kit, which contains a reference reagent, and the reference reagent is based on human serum (plasma), and is added with Contains human-derived blood lipid components, a stabilizing system and a preservative system. The human-derived blood lipid components are human-derived apolipoprotein A1 antigen (APOA1), apolipoprotein B antigen (APOB), high-density lipoprotein cholesterol (HDL ), low-density lipoprotein cholesterol (LDL), lipoprotein a (La), cholesterol (CHOL) and triglyceride (TG) any one or a combination of more than one, the stable system is Triton series surface Active agent, Tween series surfactant, EDTA series surfactant, polyvinyl alcohol series polymer, polyvinylpyrrolidone series polymer, polyethylene glycol series polymer and phosphate buffer,
[0039] Described stabilizer is made up of TritonX100, Tween20, EDTA, polyvinyl alcohol, polyvinylpyrrolidone, polyethylene gly...
Embodiment 2
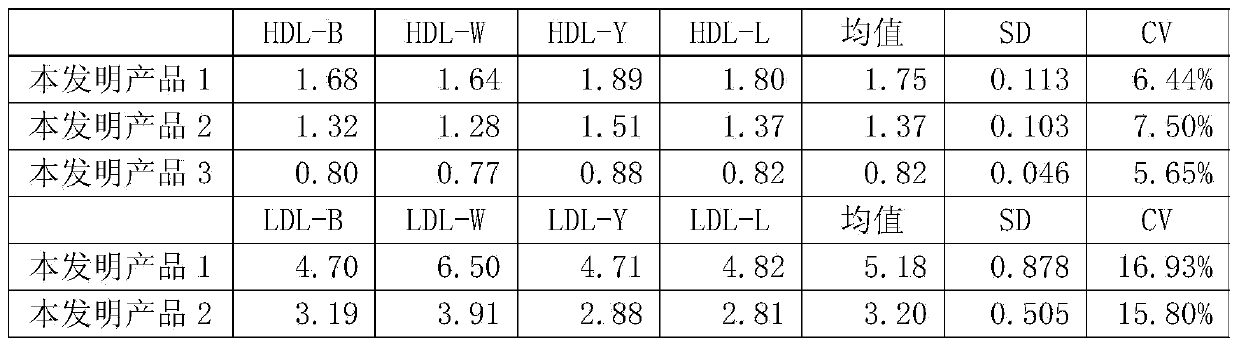
[0046] The present invention is a human blood lipid (serum / plasma) quality management reference kit, which contains a reference reagent, and the reference reagent is based on human serum (plasma), and is added with Contains human-derived blood lipid components, a stabilizing system and a preservative system. The human-derived blood lipid components are human-derived apolipoprotein A1 antigen (APOA1), apolipoprotein B antigen (APOB), high-density lipoprotein cholesterol (HDL ), low-density lipoprotein cholesterol (LDL), lipoprotein a (La), cholesterol (CHOL) and triglyceride (TG) any one or a combination of more than one, the stable system is Triton series surface Active agent, Tween series surfactants, EDTA series surfactants, polyvinyl alcohol series polymers, polyvinylpyrrolidone series polymers, polyethylene glycol series polymers and any one or more than one of phosphate buffer The combination.
[0047] Further, the stabilizer is composed of TritonX100, Tween40, and polyv...
Embodiment 3
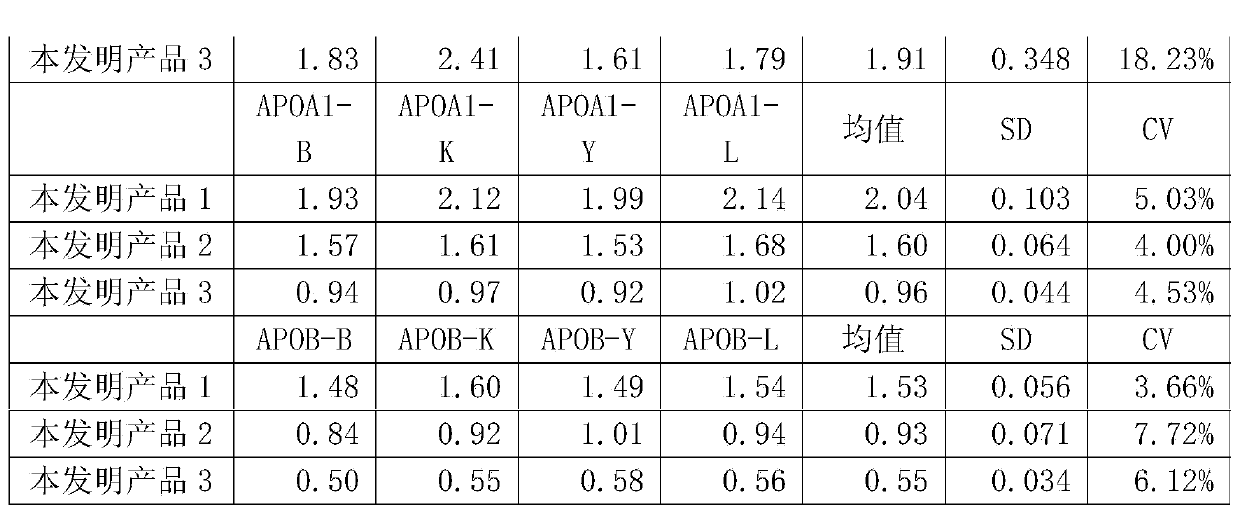
[0055] The present invention is a human blood lipid (serum / plasma) quality management reference kit, which contains a reference reagent, and the reference reagent is based on human serum (plasma), and is added with Contains human-derived blood lipid components, a stabilizing system and a preservative system. The human-derived blood lipid components are human-derived apolipoprotein A1 antigen (APOA1), apolipoprotein B antigen (APOB), high-density lipoprotein cholesterol (HDL ), low-density lipoprotein cholesterol (LDL), lipoprotein a (La), cholesterol (CHOL) and triglyceride (TG) any one or a combination of more than one, the stable system is Triton series surface Active agent, Tween series surfactants, EDTA series surfactants, polyvinyl alcohol series polymers, polyvinylpyrrolidone series polymers, polyethylene glycol series polymers and any one or more than one of phosphate buffer The combination.
[0056] Further, the stabilizer is composed of Tween80, EDTA-Na4, polyvinyl a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com