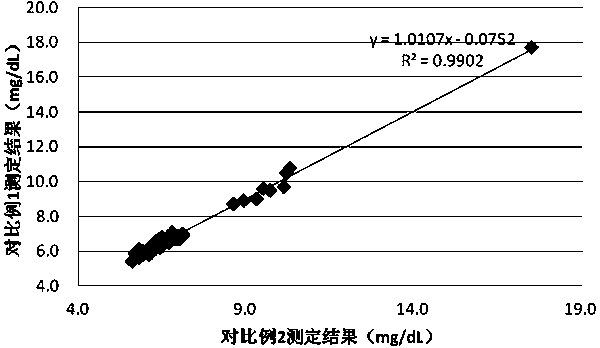
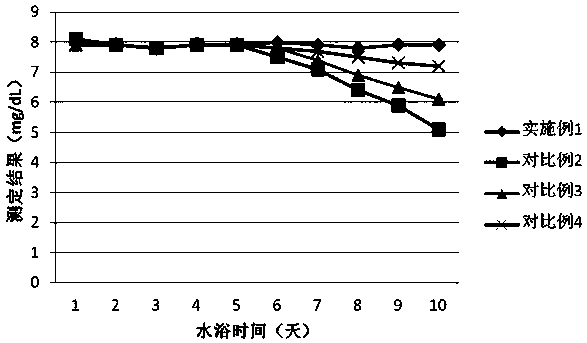
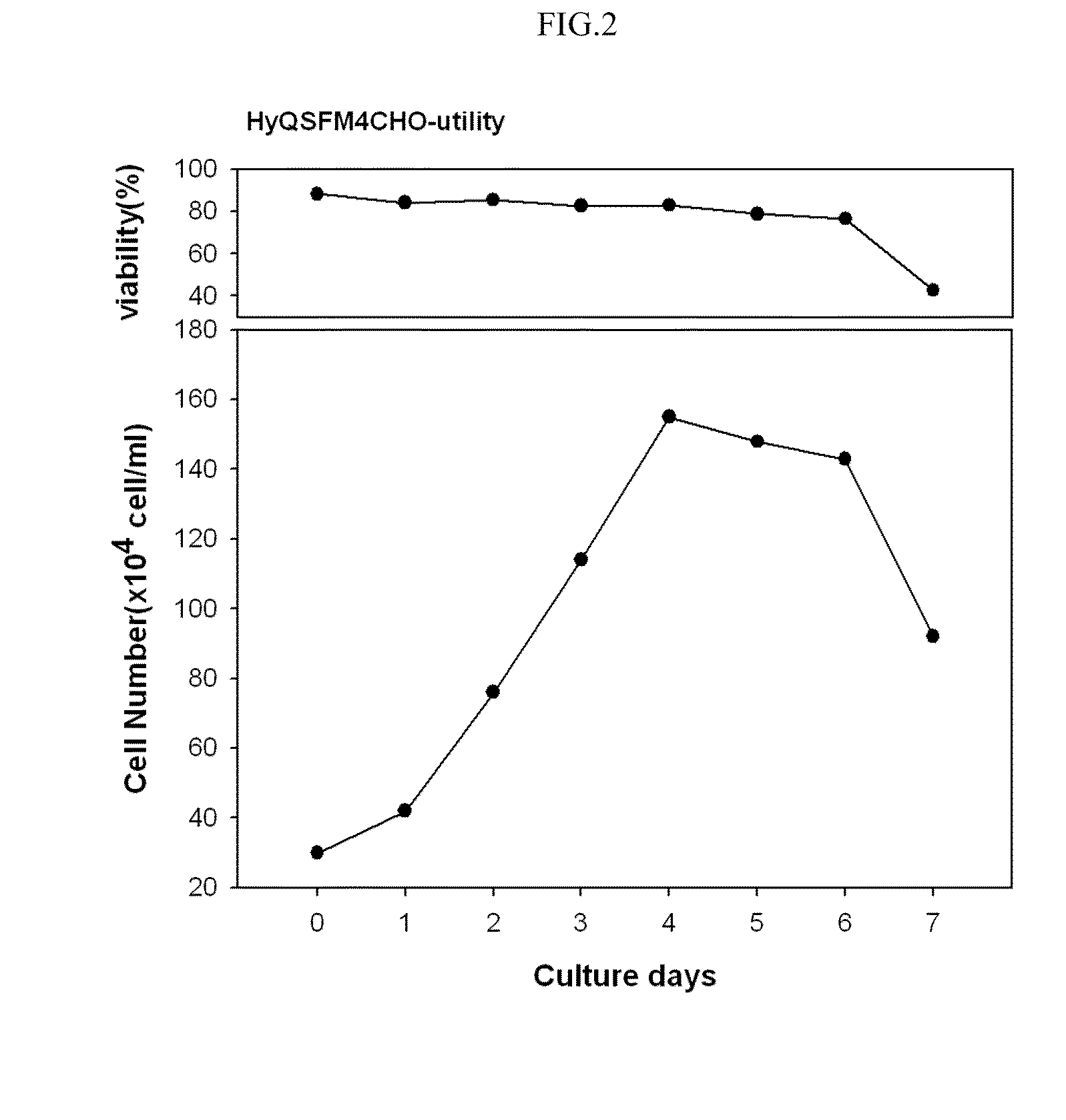
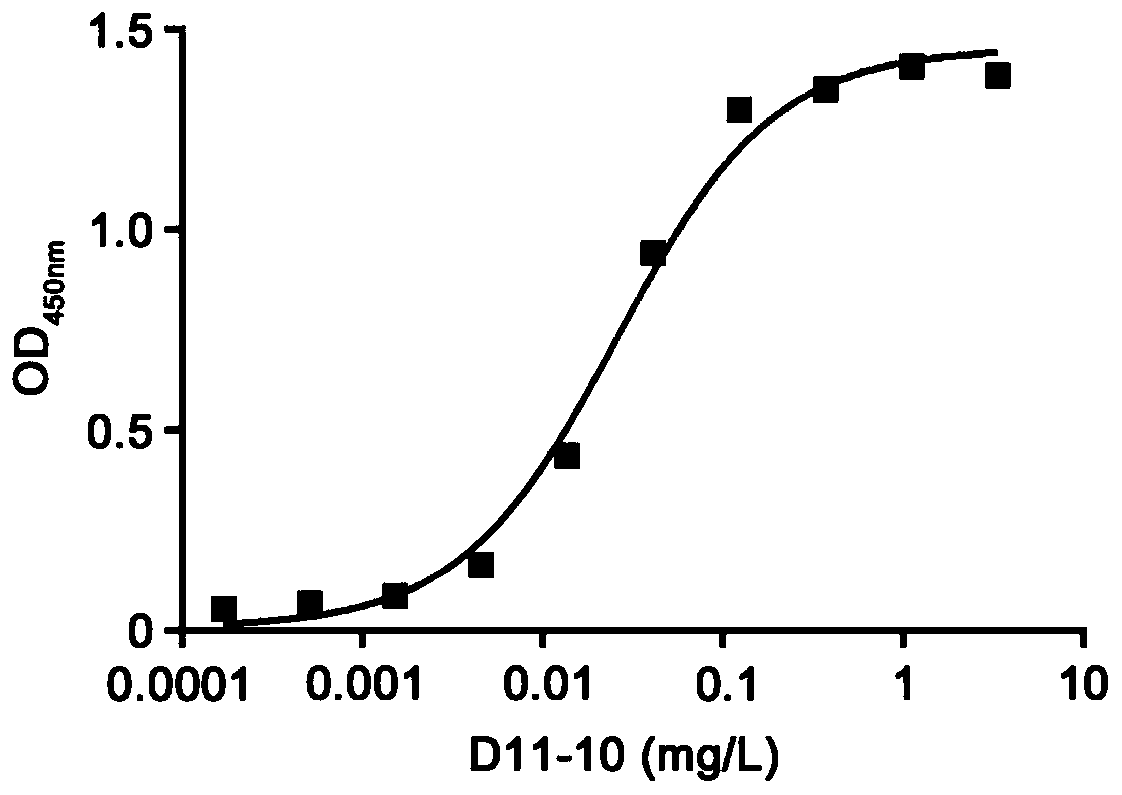
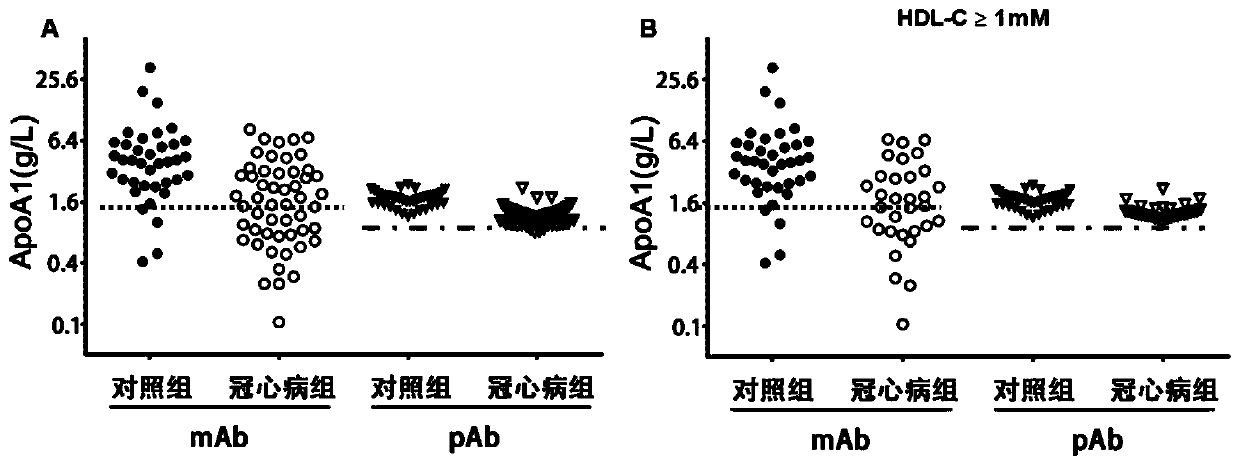
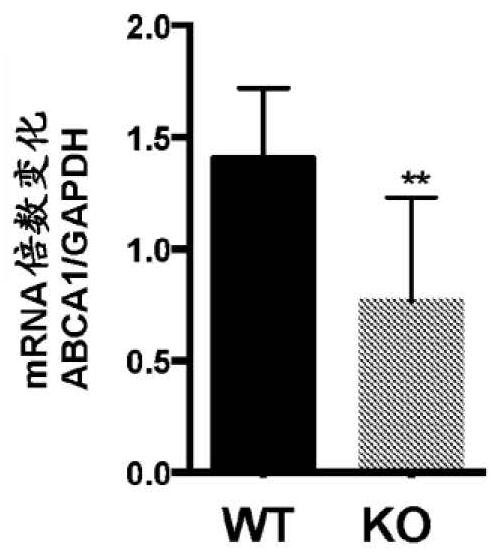
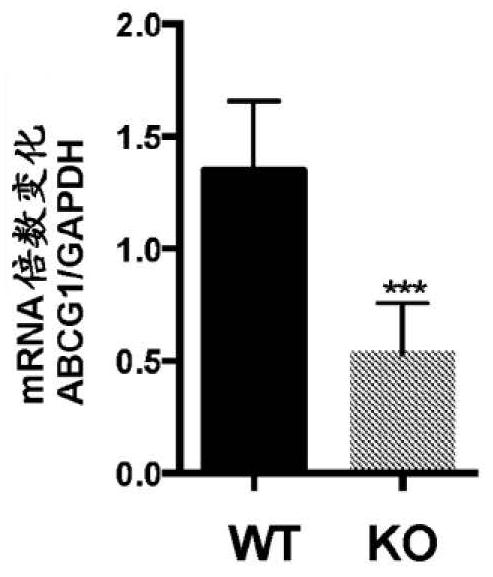
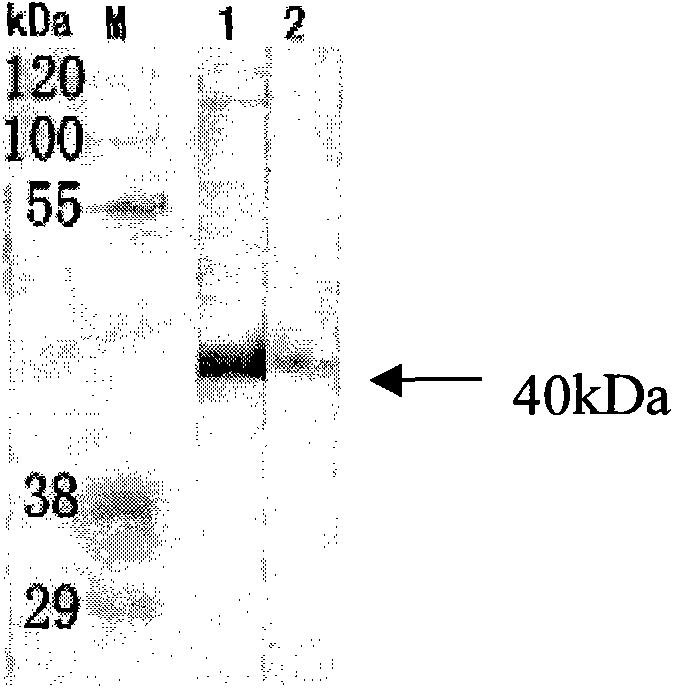
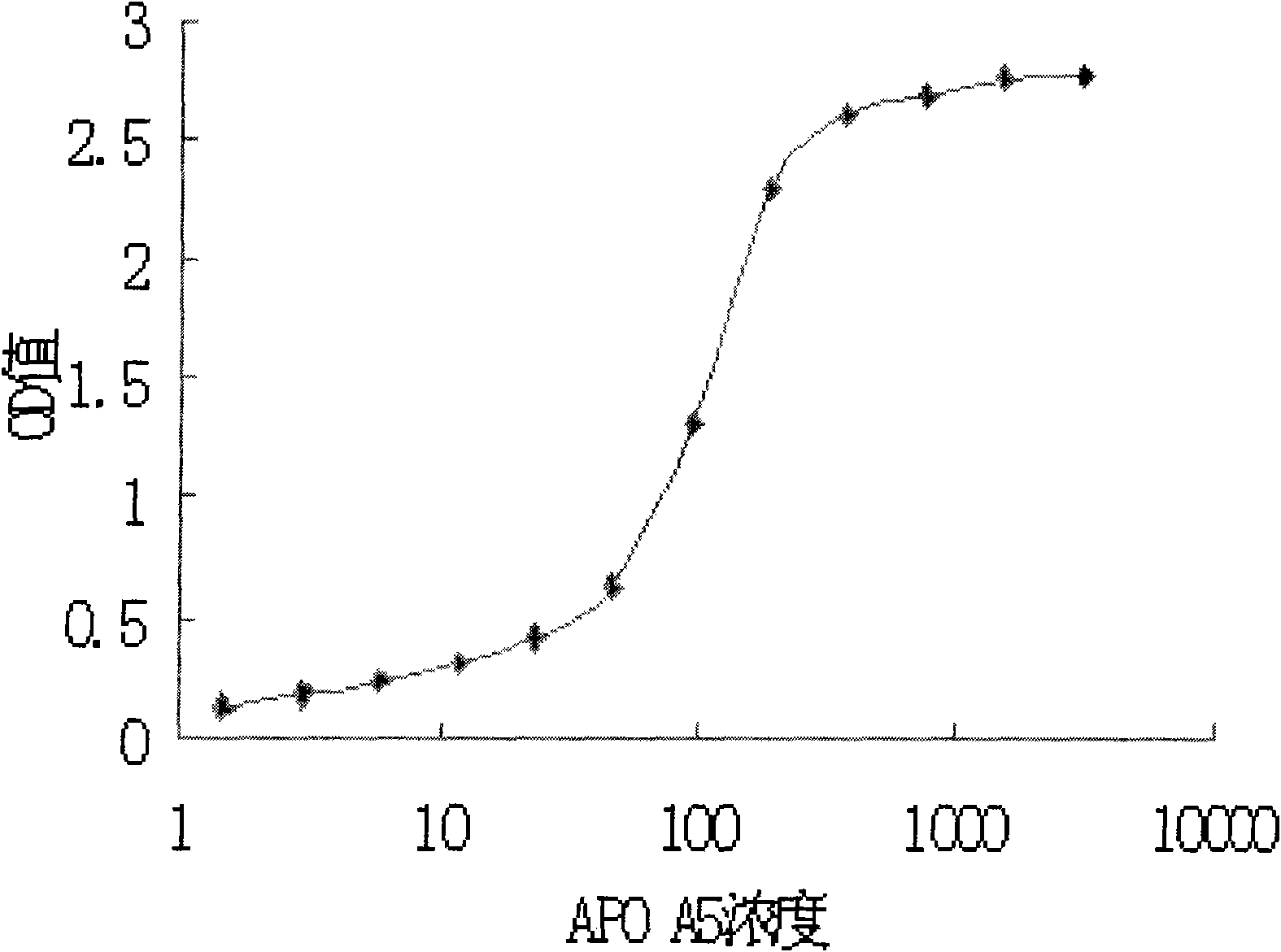
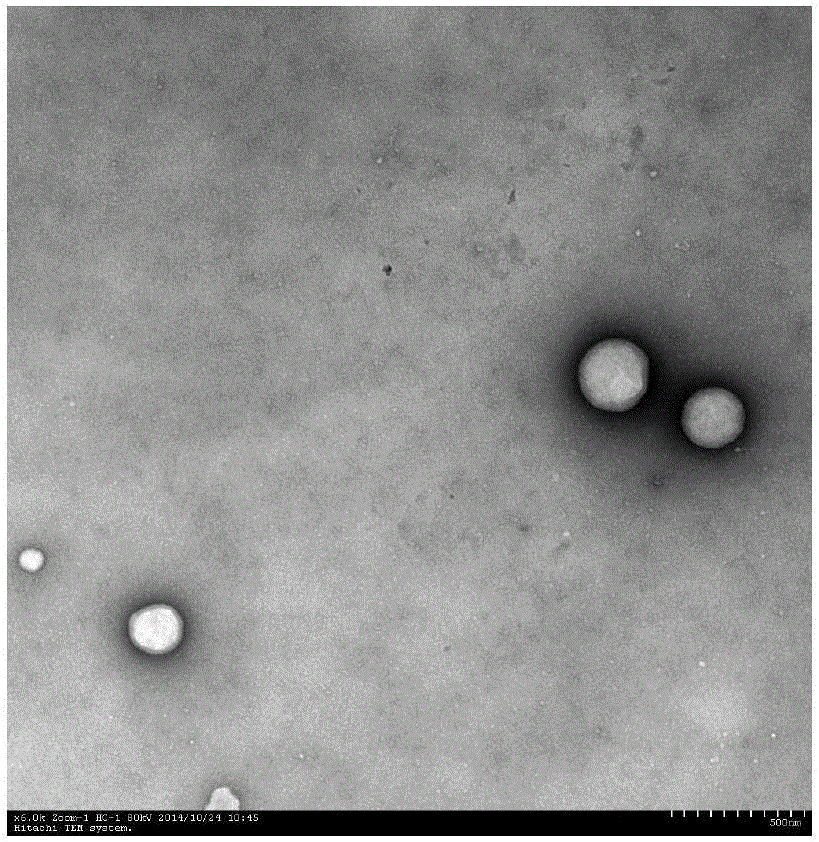
Patents
Literature
63 results about "Human apolipoprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human blood fat (serum/plasma) quality management reference kit and preparation method
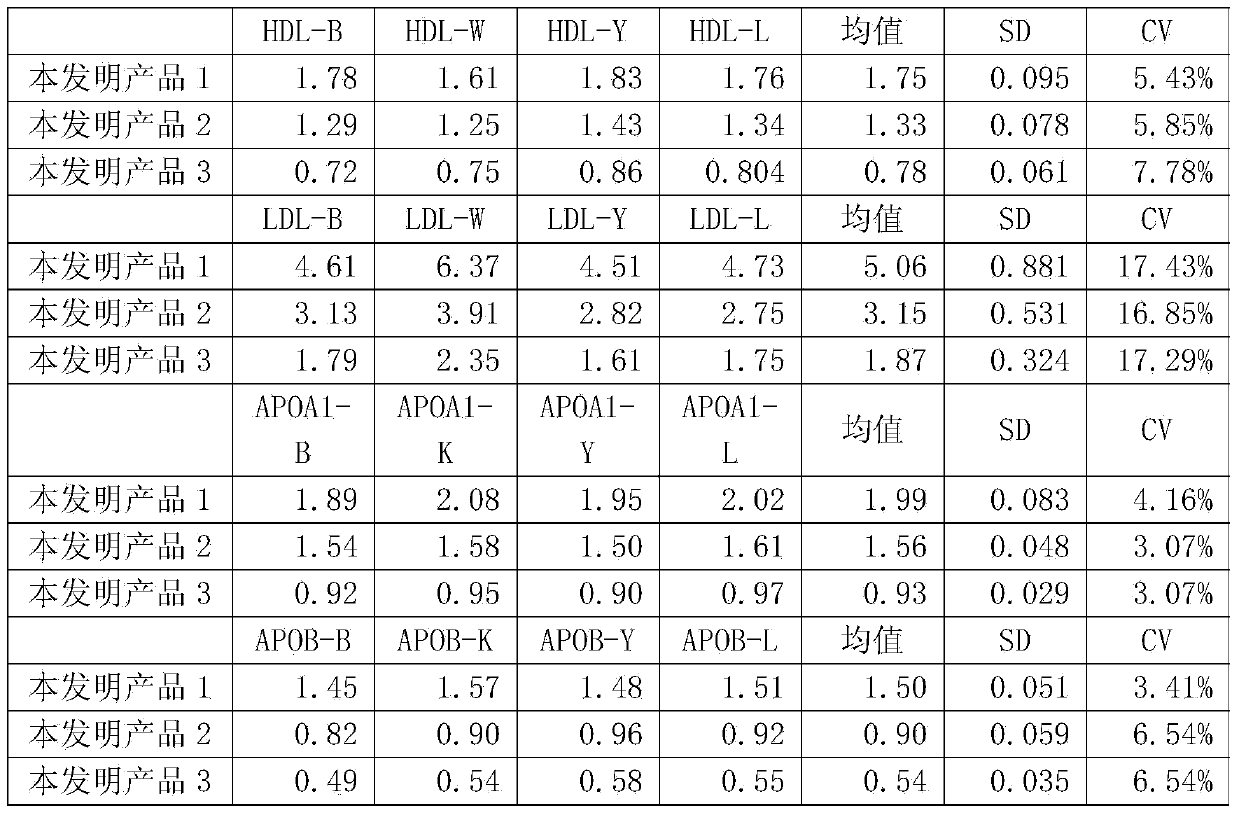
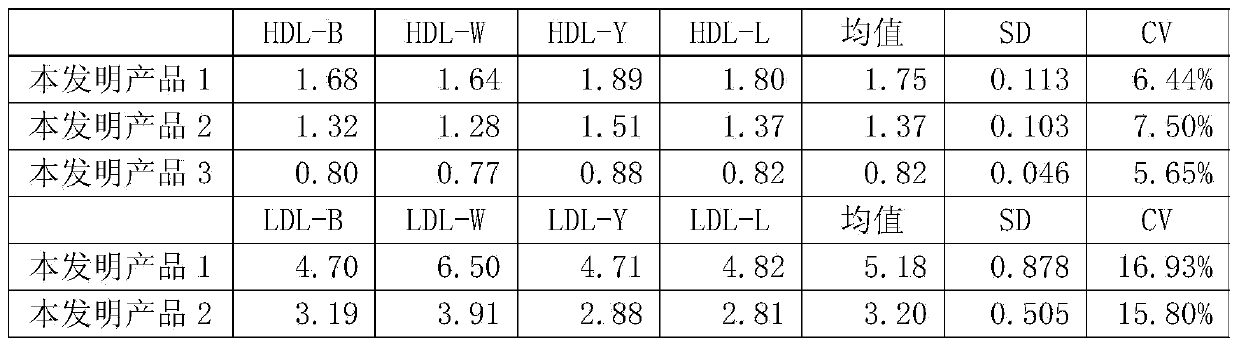
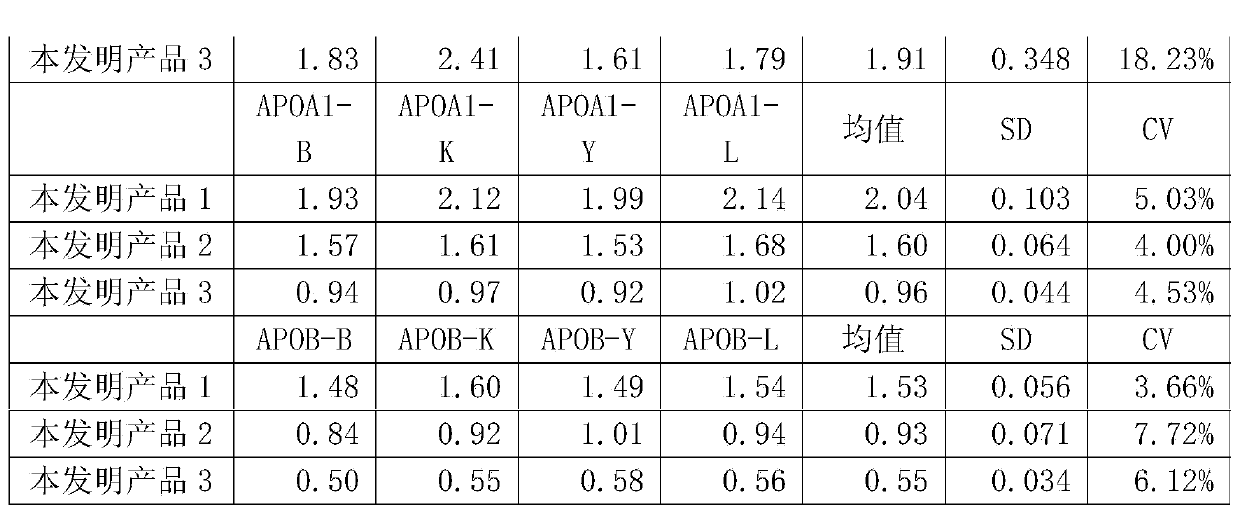
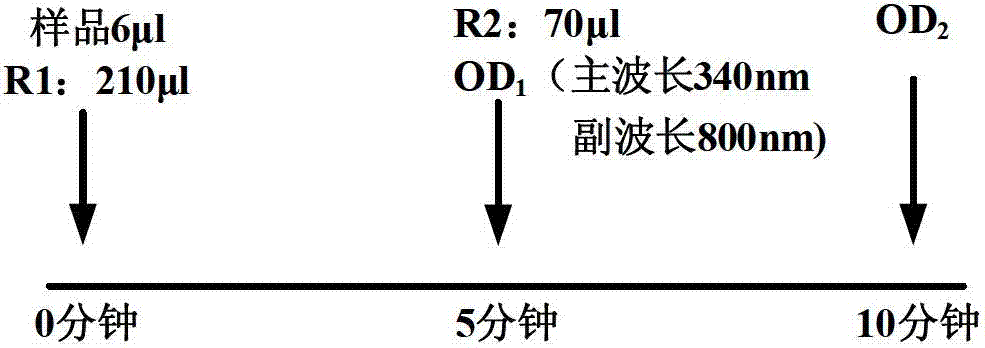
ActiveCN103472240ANo medium effect interferenceSolve medium effect interferenceBiological testingAntigenLow density lipoprotein cholesterol
The invention provides a human blood fat (serum / plasma) quality management reference kit. The kit takes human serum (plasma) as a matrix; a human blood fat component (human apolipoprotein A1 antigen, apolipoprotein B antigen, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and the like), a stable system and an anticorrosion system are added to the matrix; the stable system is any combination of a surfactant of Triton series, Tween series, EDTA (ethylene diamine tetraacetic acid) series and the like, a polymer of polyvinyl alcohol series, polyvinylpyrrolidone series, polyethylene glycol series and the like, and a phosphate buffer solution; and the anticorrosion system is any combination of sodium azide, gentamicin, amphomycin and ProClin series. The invention further provides a preparation method of the kit. The kit is free from medium effect interference, is used for quality control and evaluation between the detection systems, and more really reflects actual quality conditions of human serum (plasma) samples, which are detected by detection systems.
Owner:上海北加生化试剂有限公司
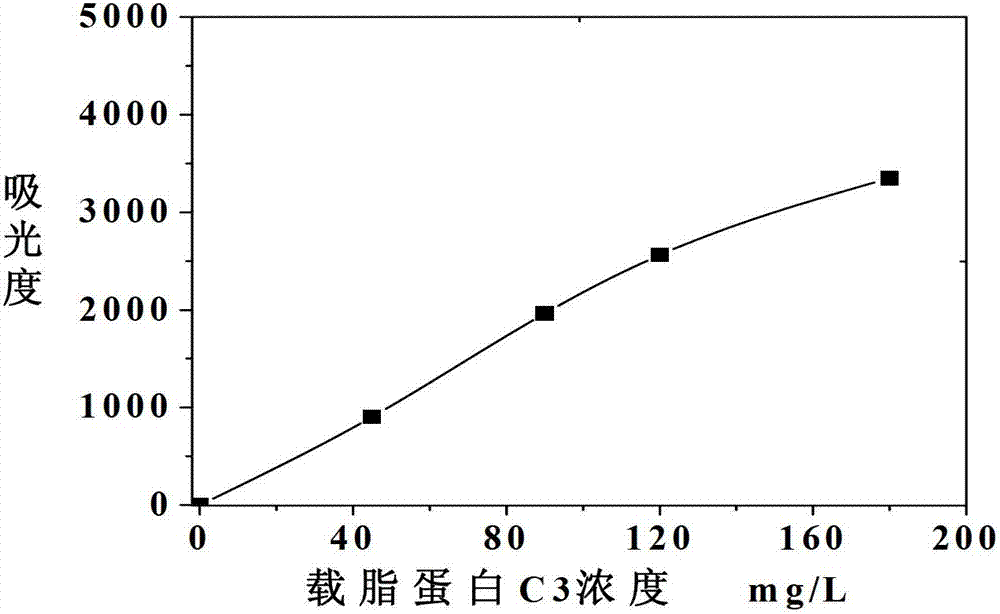
Apolipoprotein C3 detecting kit and detecting method for apolipoprotein C3 by adopting same
The invention discloses an apolipoprotein C3 detecting kit, comprising a reagent R1 consisting of a buffer solution, a surfactant, a stabilizer, electrolyte, a macromolecular promoter and a preservative, with the pH of 6-9, a reagent R2 consisting of a buffer solution, an anti-human apolipoprotein C3, a stabilizer, electrolyte and a preservative, with the pH of 6-9, and a calibrator consisting of a buffer solution, an apolipoprotein C3 antigen, a stabilizer, a preservative and antioxidant. The invention further discloses method for detecting an apolipoprotein C3 by using the apolipoprotein C3 detecting kit. The apolipoprotein C3 detecting kit is easy to operate, high in detection speed and wide in detection range; requirements on quick and high-throughput sample detection in clinic can be met; and the detection efficiency is obviously improved.
Owner:潍坊三维生物工程集团有限公司
Gene base editor
Owner:SHANGHAI TECH UNIV
Lipoprotein (a) detection kit
ActiveCN103149370AImprove stabilityImprove bindingBiological testingHuman apolipoproteinA lipoprotein
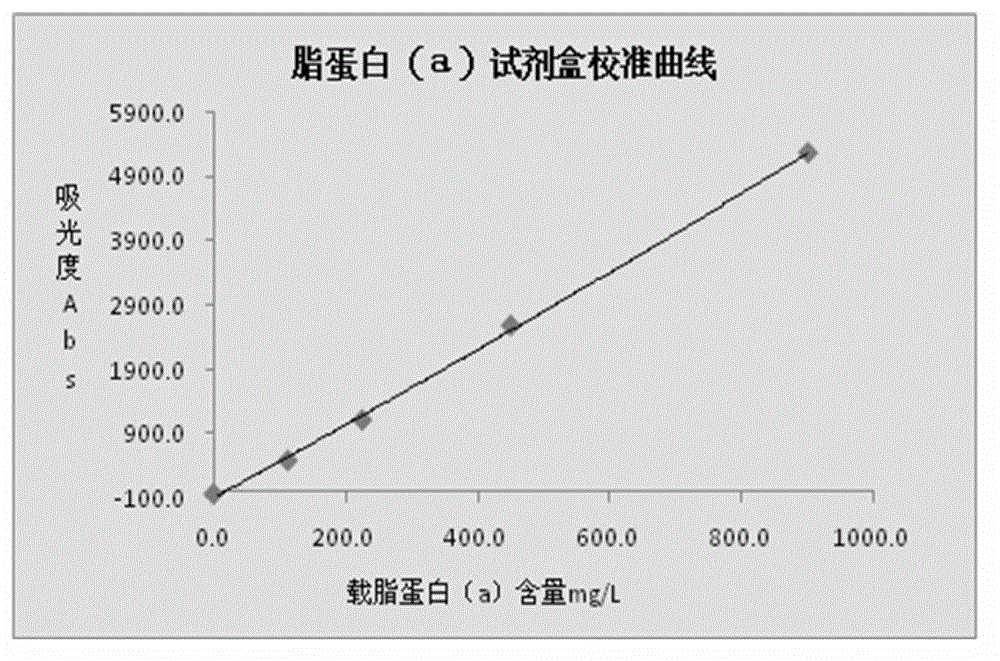
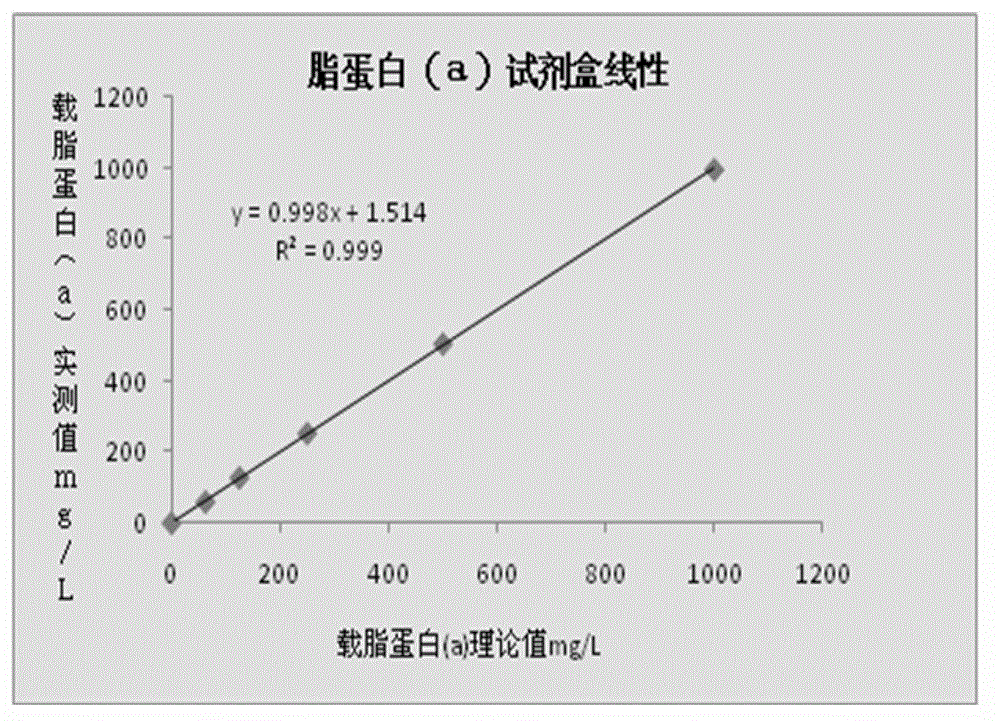
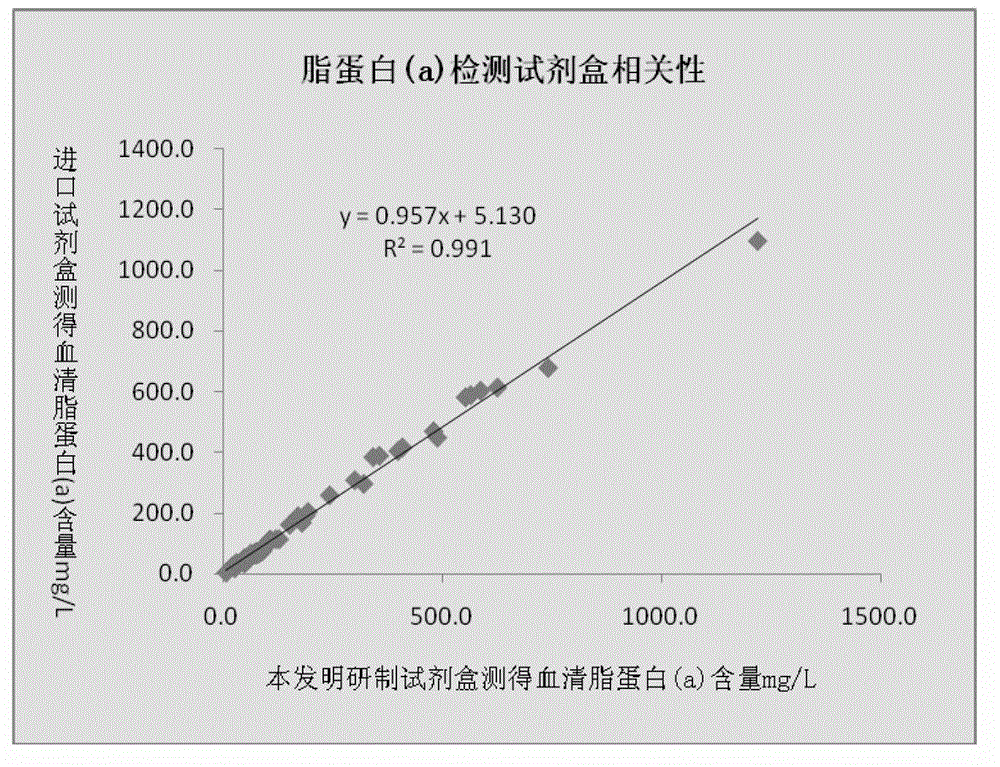
The invention relates to a lipoprotein (a) detection kit which comprises a reagent R1, a reagent R2 and a reference substance, wherein the reagent R1 is an HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer system which comprises 0.5-10 g / L HEPES, 2-20 g / L sodium chloride, 0.05-1.0 ml / L Tween-20, 0.1-2 g / L bovine serum albumin, 5-25 g / L polyethyleneglycol-6000, 1-5 g / L EDTA (ethylene diamine tetraacetic acid) and 0.1-2 g / L Proclin 300; the reagent R2 comprises a polystyrene latex particle mixture which is prepared by a chemical crosslinking method, coated with an anti-human-apolipoprotein (a) polyclonal antibody and provided with carboxylic groups on the surface, a 0.5-10 g / L HEPES buffer solution and aspartame, wherein the weight-to-volume ratio of the aspartame to the reagent R2 is (0.01-0.5):100 (g / L); and the reference substance is a human serum or serum-matrix-like liquid with human recombinant apolipoprotein (a). The kit provided by the invention has the advantages of high detection sensitivity, high accuracy, favorable precision, favorable linearity within detection range, high stability, low production cost and low blank absorbance, and has anti-interference performance.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Apolipoprotein E ELISA reagent box and method of producing the same
The invention discloses an apolipoprotein E4 ELISA kit and a preparation method thereof, the invention applies the purified human apolipoprotein E4 to prepare an anti-human ApoE4 antibody, and an ELISA plate is coated after the compatible purification. The kit composition includes the ELISA plate coated by the anti-human ApoE4 monoclonal antibody, an enzyme-labeled antibody, ApoE4 standard frozen powder, sample dilution solution, TMB substrate color developing solution, concentration washing liquid and reaction termination liquid. The ApoE4 in the antibody capture standard solution or the sample solution which is fixed on the microporous surface of the ELISA plate is identified by and combined with the enzyme-labeled antibody, so as to form the antibody-ApoE4-antibody-enzyme compound, the absorbance is measured after the reaction and color development of the enzyme and the substrate and the concentration of the ApoE4 in the sample can be calculated by the standard curve. The kit can detect the consistency of the apolipoprotein E4 in human serum, plasma or cerebrospinal fluid, the invention has the advantages of sensitivity, rapidness, simpleness and accuracy, so the invention provides the effective means for in vitro diagnosis and determination of ApoE4 gene type and the gene dosage.
Owner:杭州浙大生科生物技术有限公司
Administering antisense oligonucleotides complementary to human apolipoprotein b
InactiveUS20100297105A1Reduced risk of coronary heart diseaseSlowing and stopping progressionOrganic active ingredientsSpecial deliveryHuman apolipoproteinLipid level
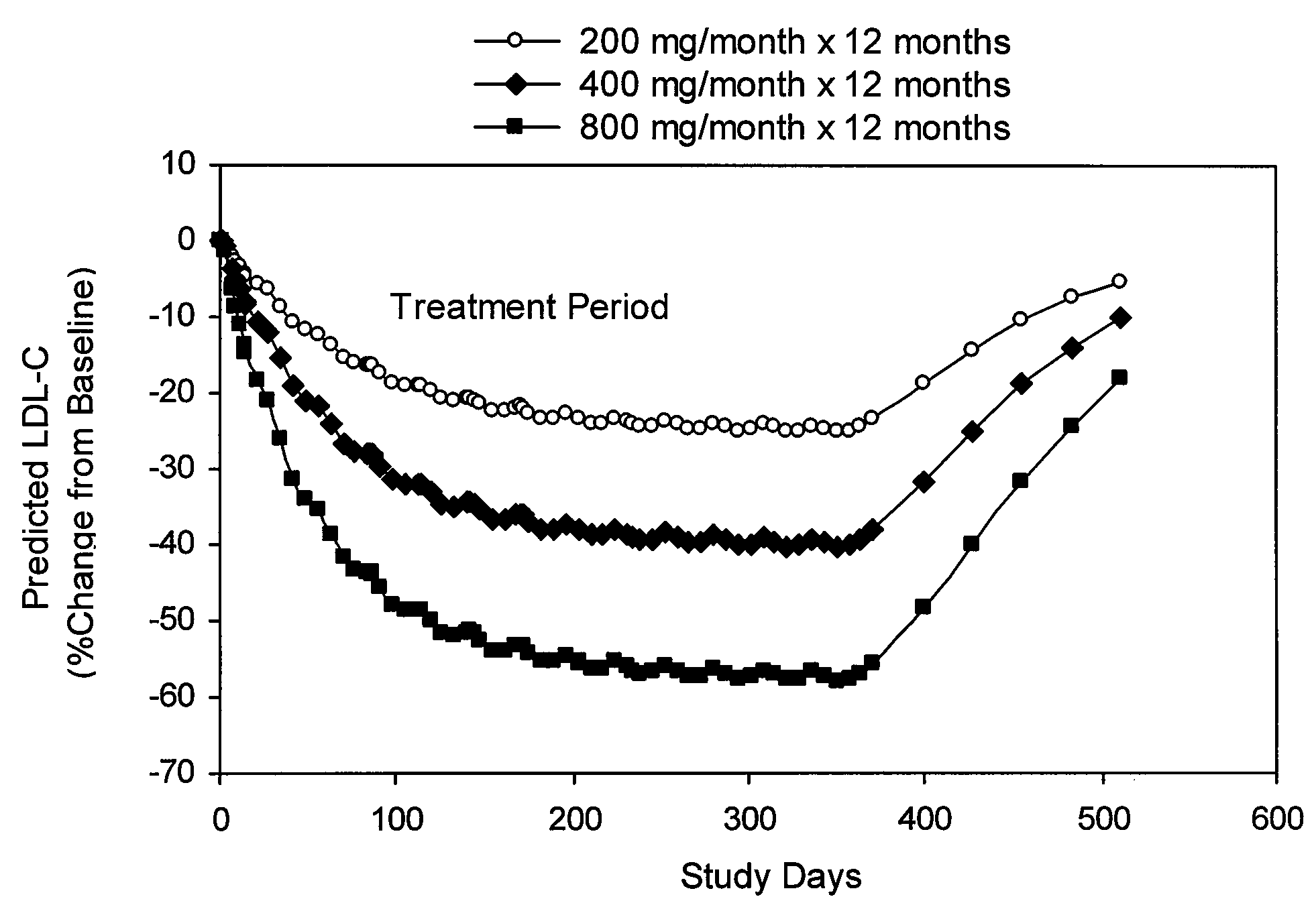
Methods for long-term lowering of lipid levels in human subjects and for the treatment of conditions associated with elevated LDL-cholesterol and elevated ApoB are provided.
Owner:KASTLE THERAPEUTICS LLC
Immunoturbidimetric assay apolipoprotein E detection kit
InactiveCN103399160ASimple and fast operationHigh sensitivityColor/spectral properties measurementsBiological testingHuman apolipoproteinAntiserum
The invention relates to an immunoturbidimetric assay apolipoprotein E detection kit, and relates to the technical field of kit. The kit is composed of three parts which are an R1 reagent, an R2 reagent, and a calibrator. The R1 reagent is composed of 40-50mmol / L of a buffer solution with a pH value of 7, 70-150mmol / L of a surfactant, 15-30mmol / L of disodium ethylenediamine tetraacetate, and 0.1-1% of a preservative. The R2 reagent is prepared by adding anti-human-apolipoprotein-E antiserum with a volume ratio of 30-50% into a certain amount of the R1 reagent. The calibrator is 80-200g / L ApoE antigen, and also comprises 0.2-15% of a preservative, 1-4% of a stabilizing agent, and 1-10% of a surfactant, wherein the percentages are human serum matrix volume amount percentages. The kit has the advantages of simple operation, high sensitivity, good specificity, fast determination, and accurate and reliable result.
Owner:上海睿康生物科技有限公司
Apolipoprotein l- i variants and their use
InactiveUS20120128682A1Reduced blood cholesterol levelCholesterol-reduced fatOrganic active ingredientsPeptide/protein ingredientsHuman apolipoproteinWild type
An isolated human Apolipoprotein L-I corresponding to a wild type human Apolipoprotein sequence is modified by a deletion at its C-terminal end.
Owner:UNIV LIBRE DE BRUXELIES
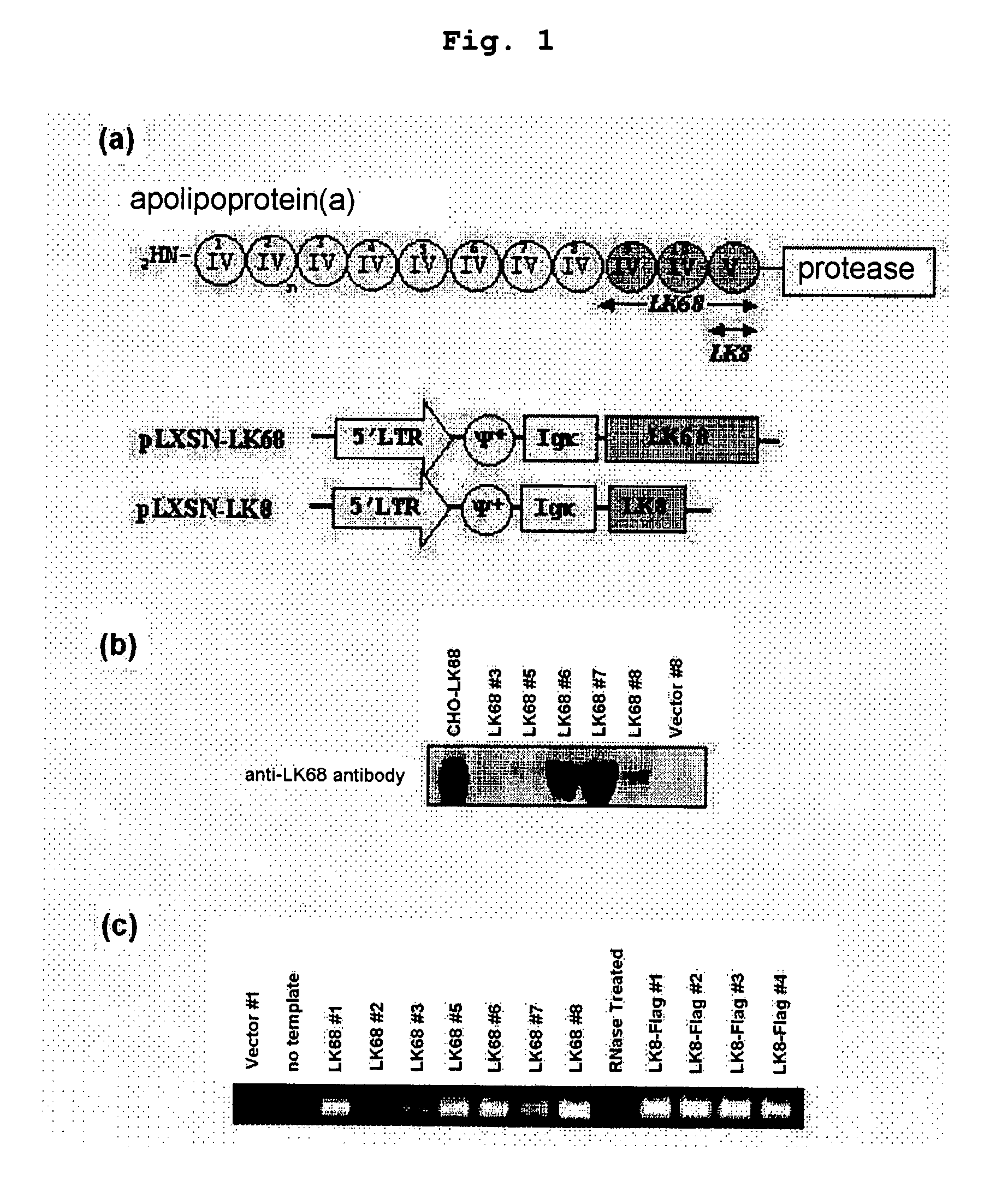
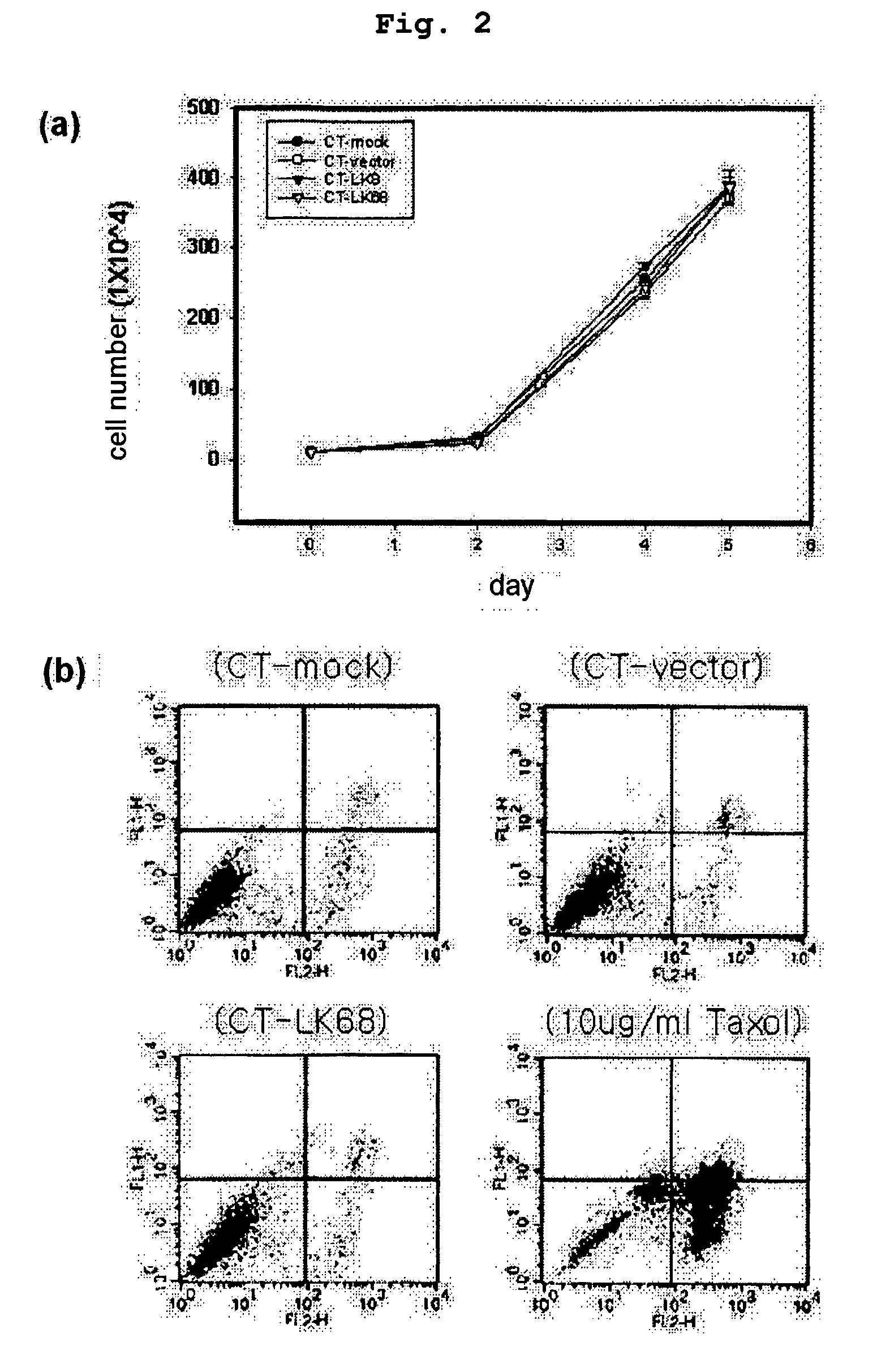
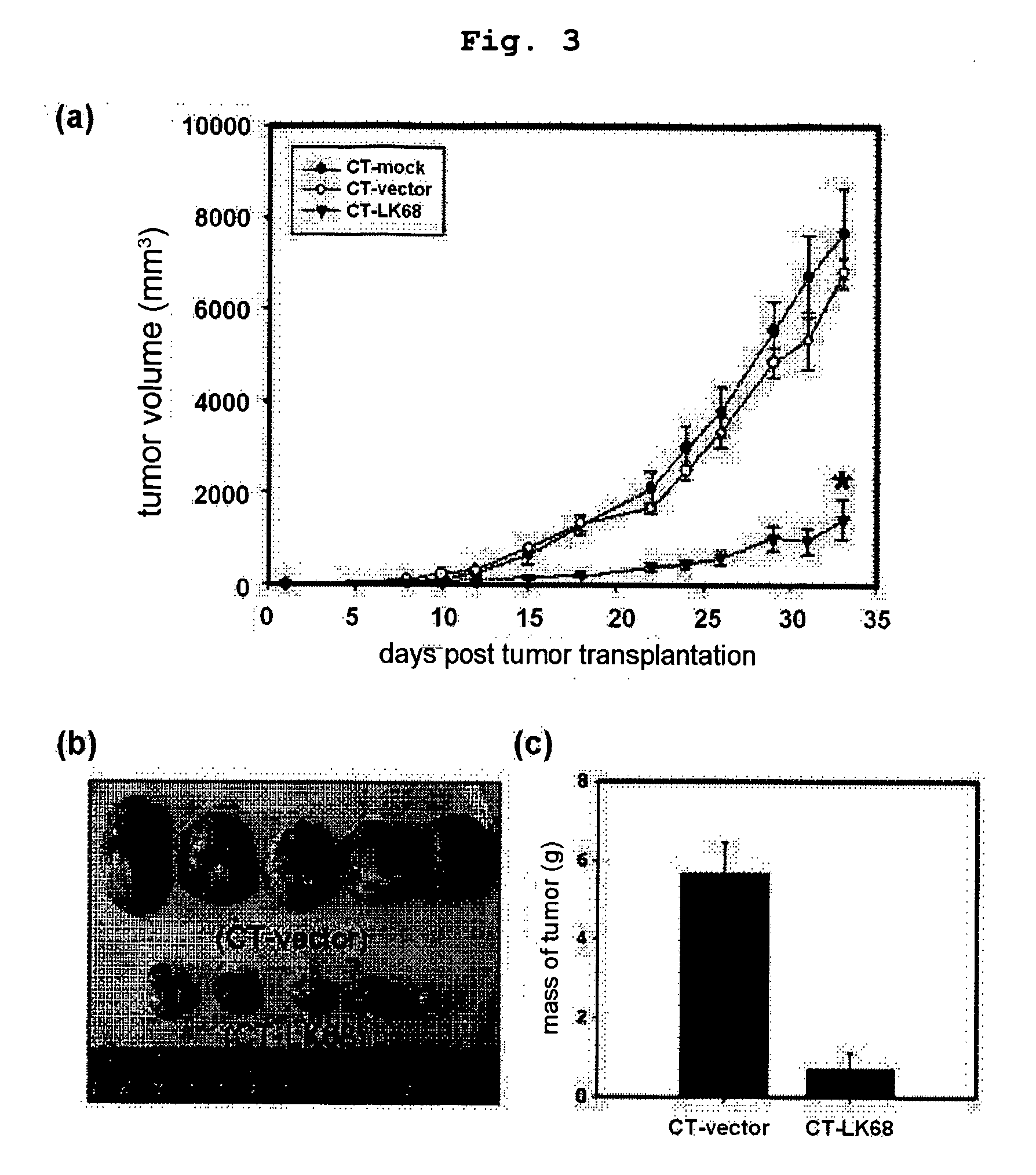
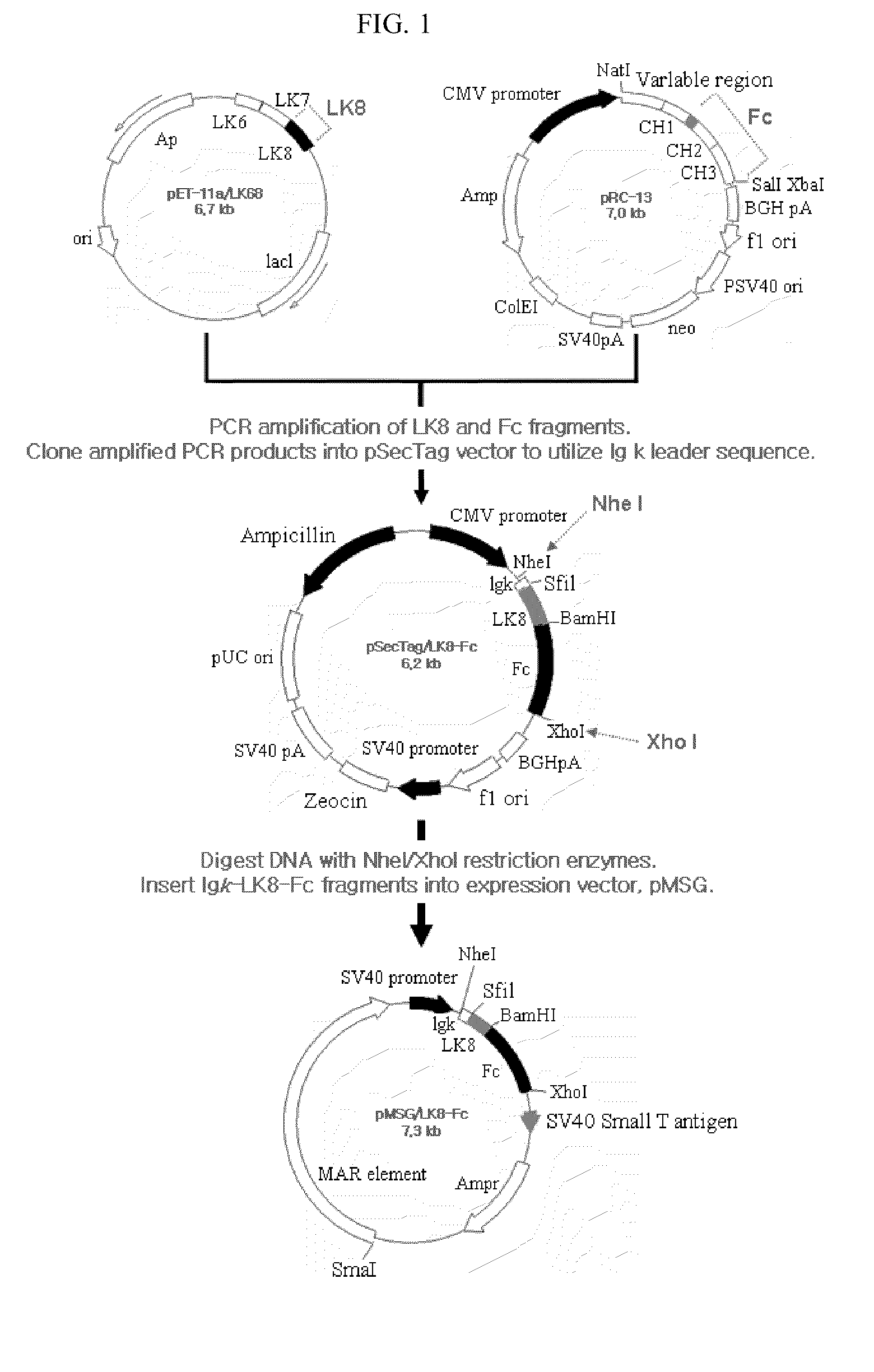
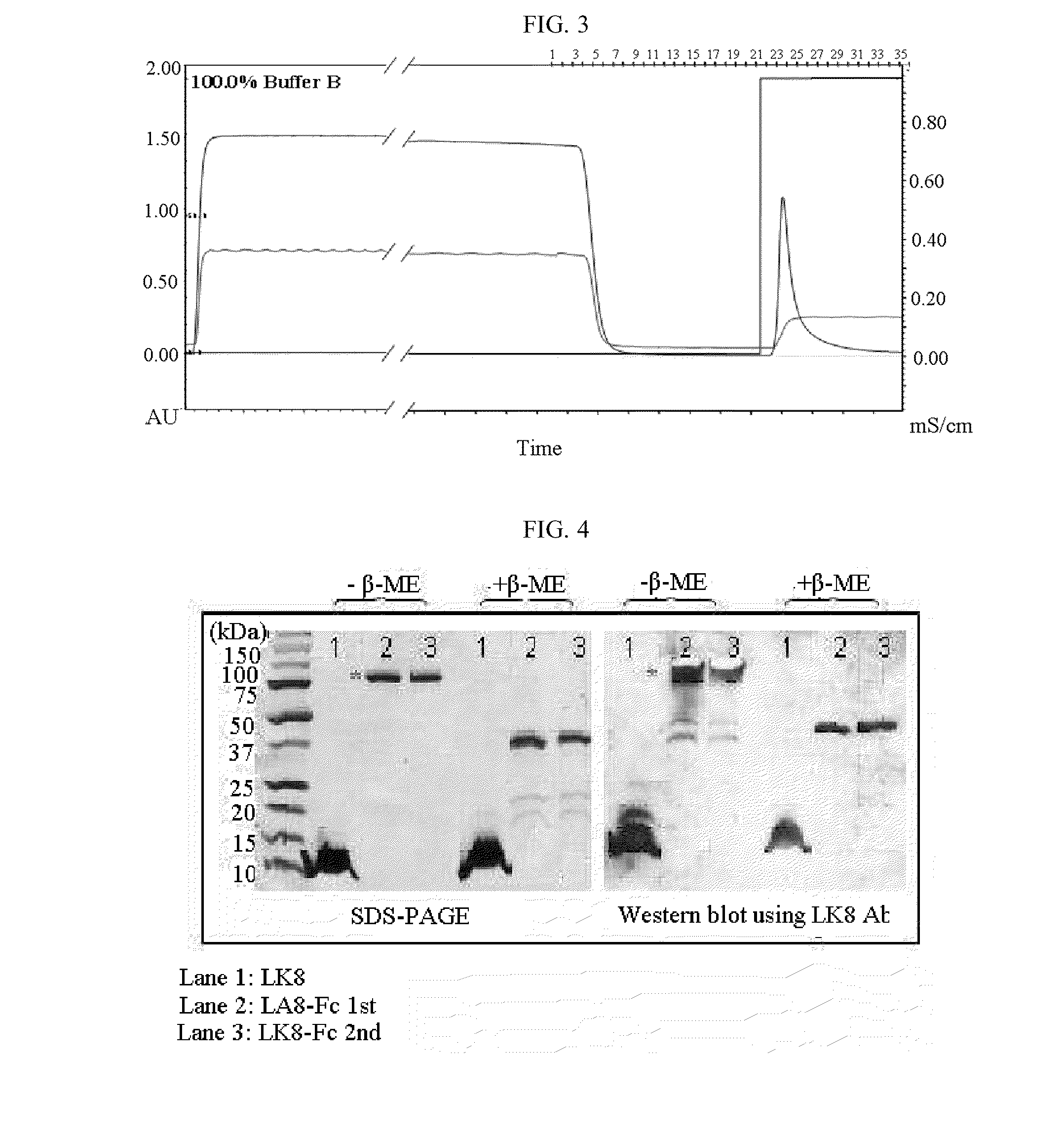
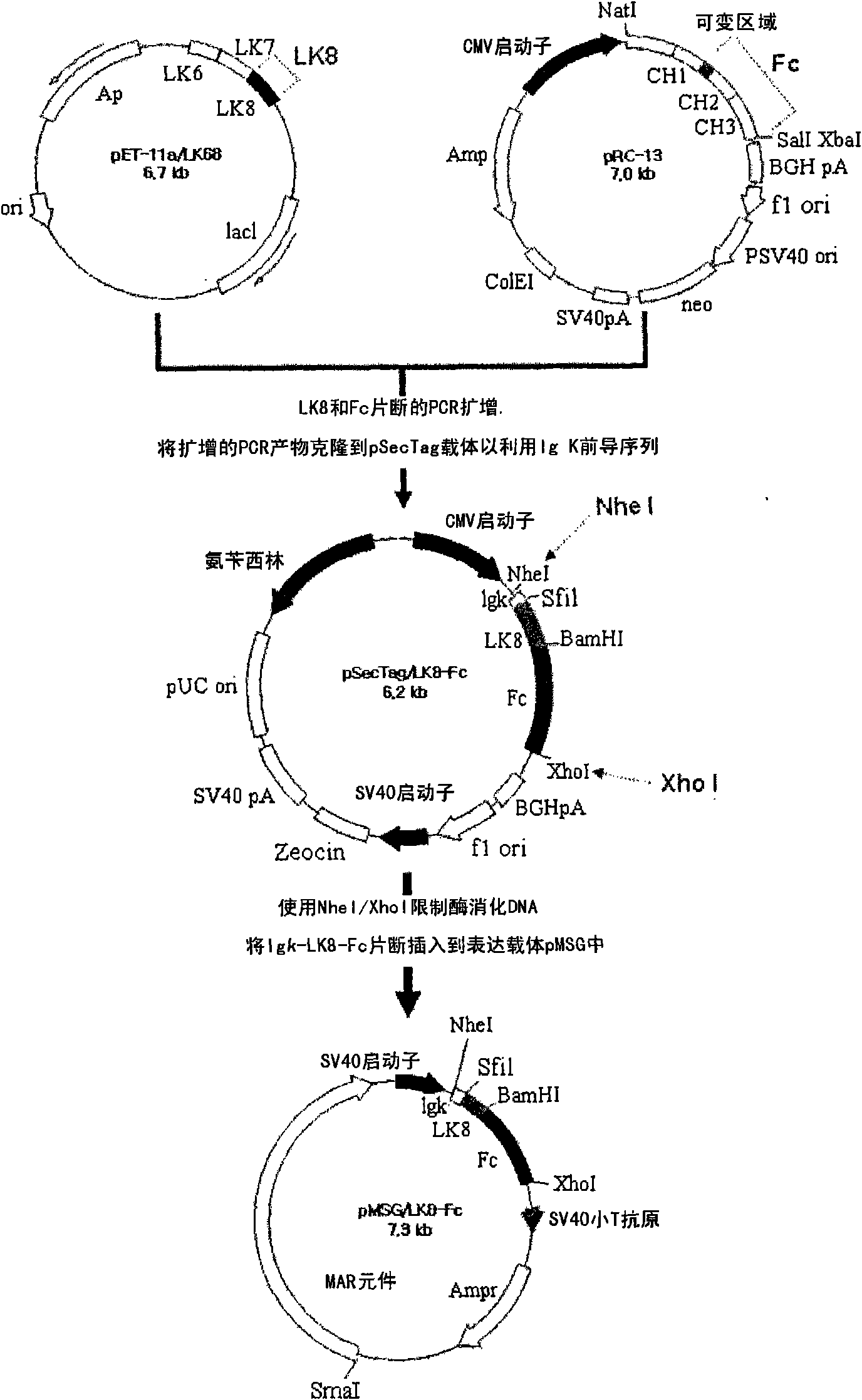
Therapeutic agent for treatment of cancer comprising human apolipoprotein (a) kringles lk68 or lk8 genes as effective ingredient, and method for treating cancer using the same
The present invention relates to an anticancer or an anti-metastatic agent for gene therapy, more precisely, an anticancer or an anti-metastatic agent for gene therapy containing a gene carrier or cells harboring human apolipoprotein (a) kringle KIV9-KIV10-KV (LK68) or KV (LK8) gene as an effective ingredient, and a treatment method for cancer using the same. The agent for gene therapy of the present invention has an inhibiting effect on the growth and the metastasis of a tumor, so it can be effectively used for the prevention and the treatment of various solid tumors as a metastasis inhibitor or a therapeutic agent for primary tumors.
Owner:MOGAM BIOTECH RES INST
Kit for measurement of apolipoprotein C3 by latex turbidimetric inhibition immuno assay
InactiveCN109613265ANo reconstitution requiredImprove stabilityBiological testingAntigenHuman apolipoprotein
The invention discloses a kit for measuring apolipoprotein C3. The kit comprises a reagent R1, a reagent R2 and a calibrator. The R1 comprises a buffer solution, an electrolyte, a surfactant, a reaction enhancer, a stabilizer, and a preservative. The R2 comprises a buffer solution, sheep anti-human apolipoprotein C3 antibody latex particles, an electrolyte, a surfactant, a stabilizer, and a preservative. The calibrator comprises a buffer solution, recombinant apolipoprotein C3, bovine serum albumin, mannitol, and sodium azide. The apolipoprotein C3 kit is a liquid double-reagent, reconstitution preparation is not required, and therefore the reagents can be directly used when taken out from a bottle. Stability of the obtained latex particles is high. Accuracy of detecting result is greatlyimproved. The sugar residues of the Fc end of the apolipoprotein C3 antibody are oxidized to aldehyde by sodium periodate. The binding rate and stability of the antibody are improved. The active region of antibody binding to antigen is effectively protected. Sensitivity and stability of the detecting reagents are improved.
Owner:中拓生物有限公司
Fusion protein of immunoglobulin fc and human apolipoprotein(a) kringle fragment
InactiveUS20100196370A1Improve bioavailabilitySenses disorderAntipyreticHuman apolipoproteinLymphatic Spread
The present invention relates to an LK8-Fc fusion protein, which has increased angiogenesis inhibitory activity and in vivo stability. More specifically, relates to an LK8-Fc fusion protein in which an LK8 protein having angiogenesis inhibitory activity is fused with the Fc region of human immunoglobulin IgG1, as well as a composition for treating cancer, which contains the fusion protein. The LK8-Fc fusion protein has not only angiogenesis inhibitory activity leading to anticancer and metastasis inhibitory activities, but also a very long in vivo half-life, and thus can be used as a more efficient and economic cancer therapeutic agent or cancer inhibitor.
Owner:MOGAM BIOTECH RES INST
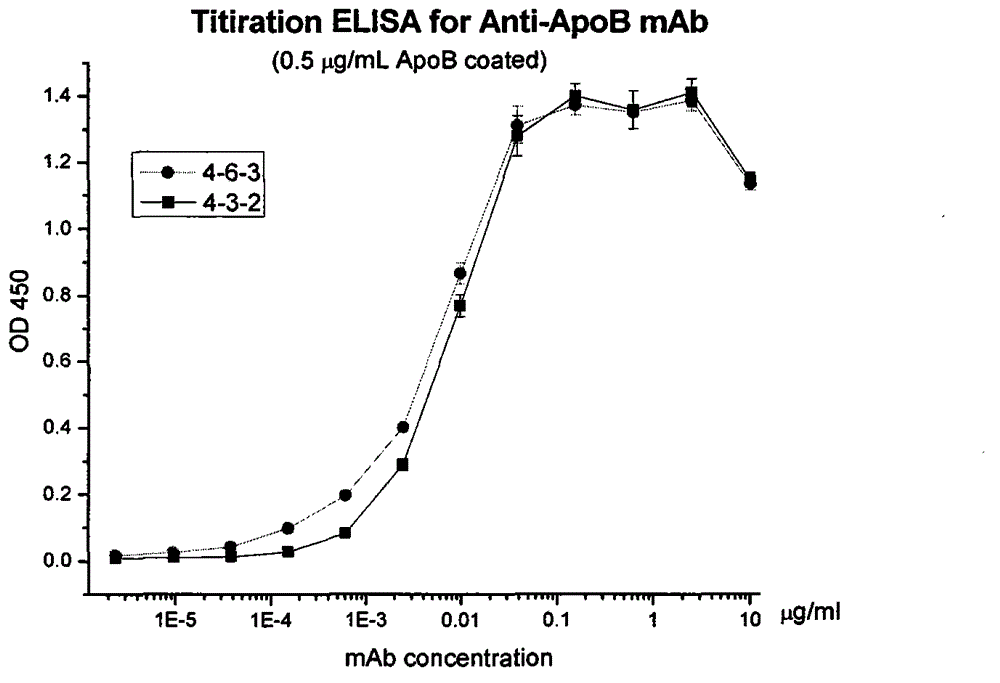
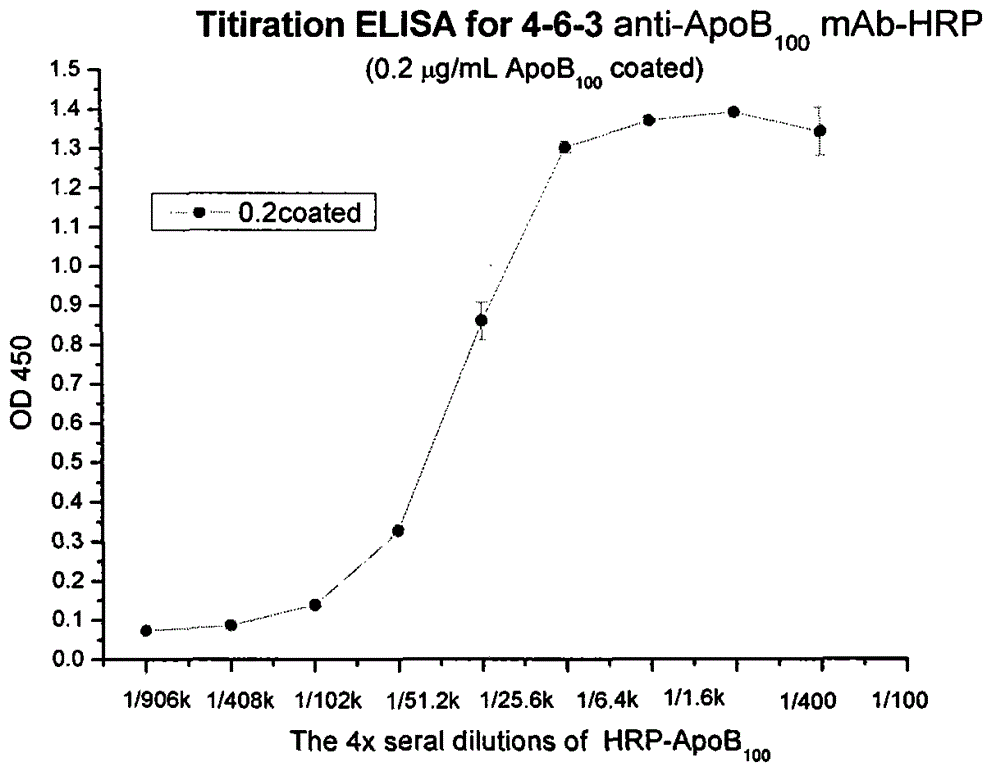
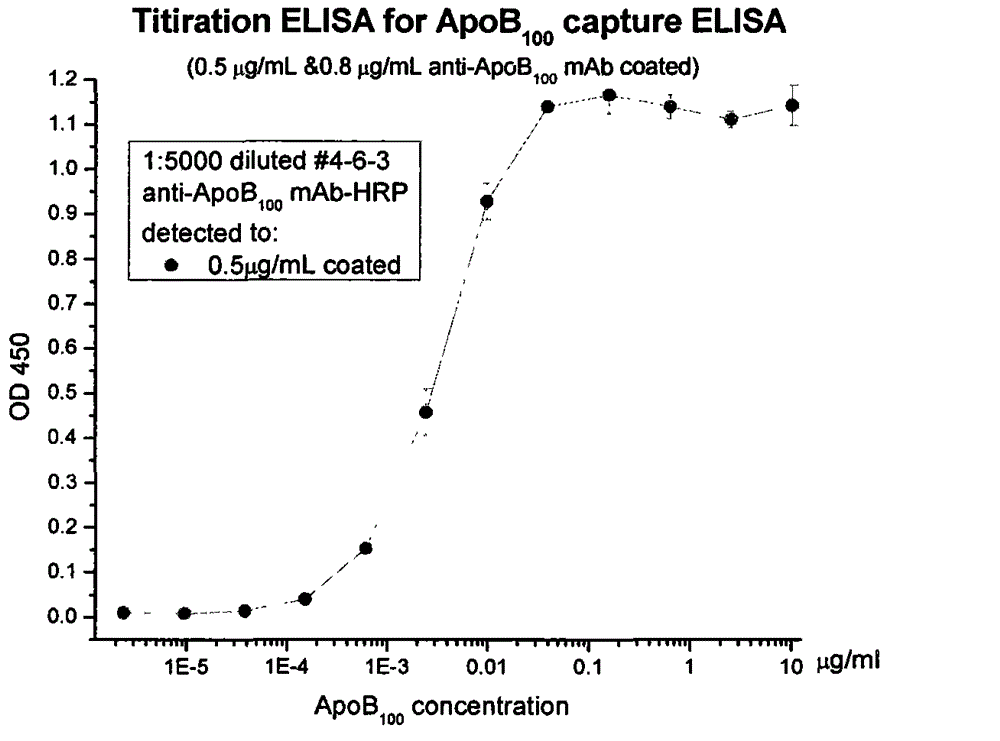
Human apolipoprotein B100 (ApoB100) monoclonal antibody and chemiluminescence immune assay determination kit adopting the human ApoB100 monoclonal antibody
ActiveCN102943066AHigh purityStrong specificityImmunoglobulins against animals/humansMicroorganism based processesHuman apolipoproteinMonoclonal antibody
The invention provides a chemiluminescence immune assay determination kit adopting a human apolipoprotein B100 (ApoB100) monoclonal antibody and a preparation method thereof. The invention also provides the human ApoB100 monoclonal antibody which can specifically recognize human ApoB100, a hybrid tumor for producing the human ApoB100 monoclonal antibody, and a method for high-sensitivity detection of human ApoB100 by the human ApoB100 monoclonal antibody. The chemiluminescence immune assay determination kit has high sensitivity, a wide detection scope and a low cost and is convenient for operation.
Owner:大庆麦伯康生物技术有限公司 +2
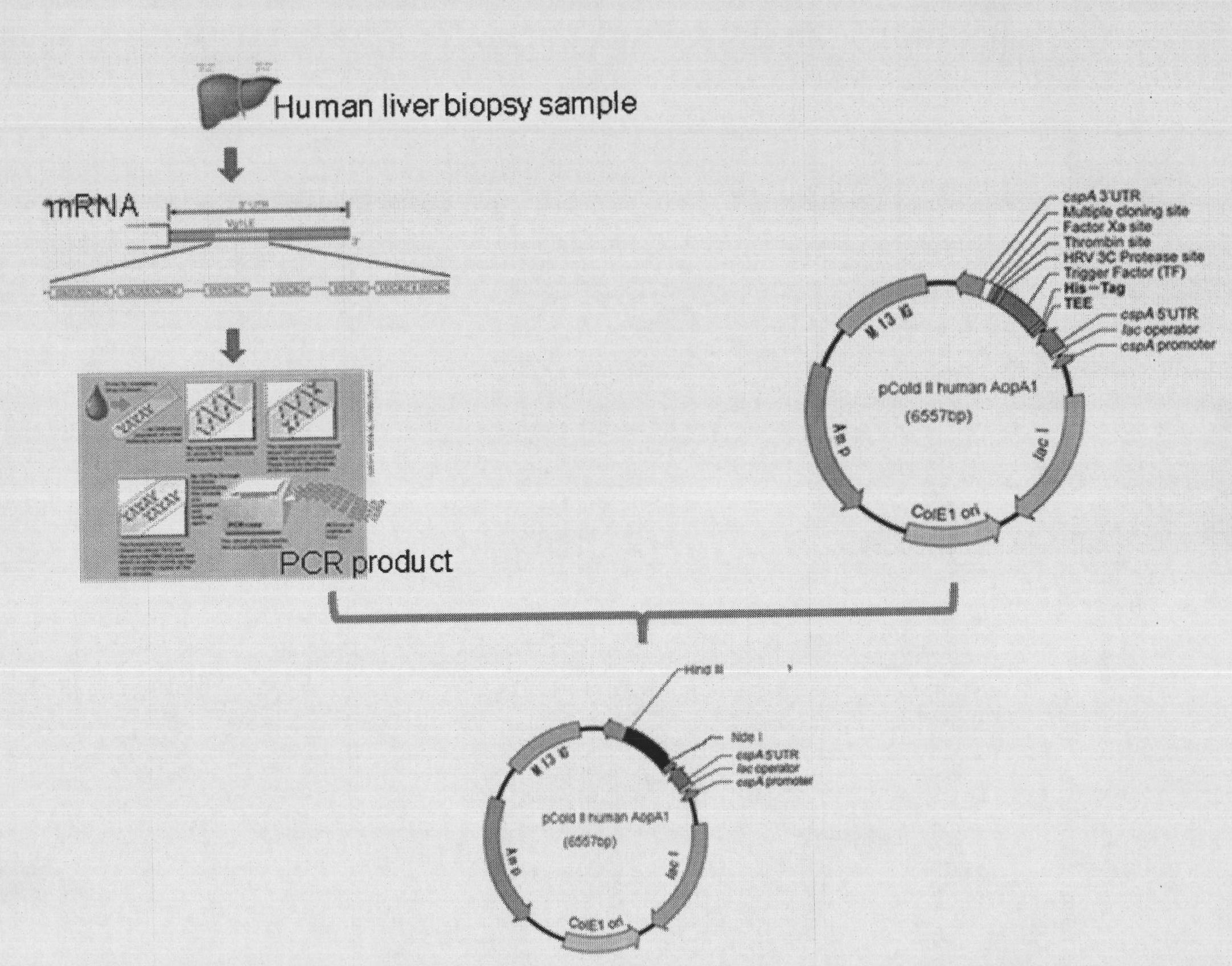
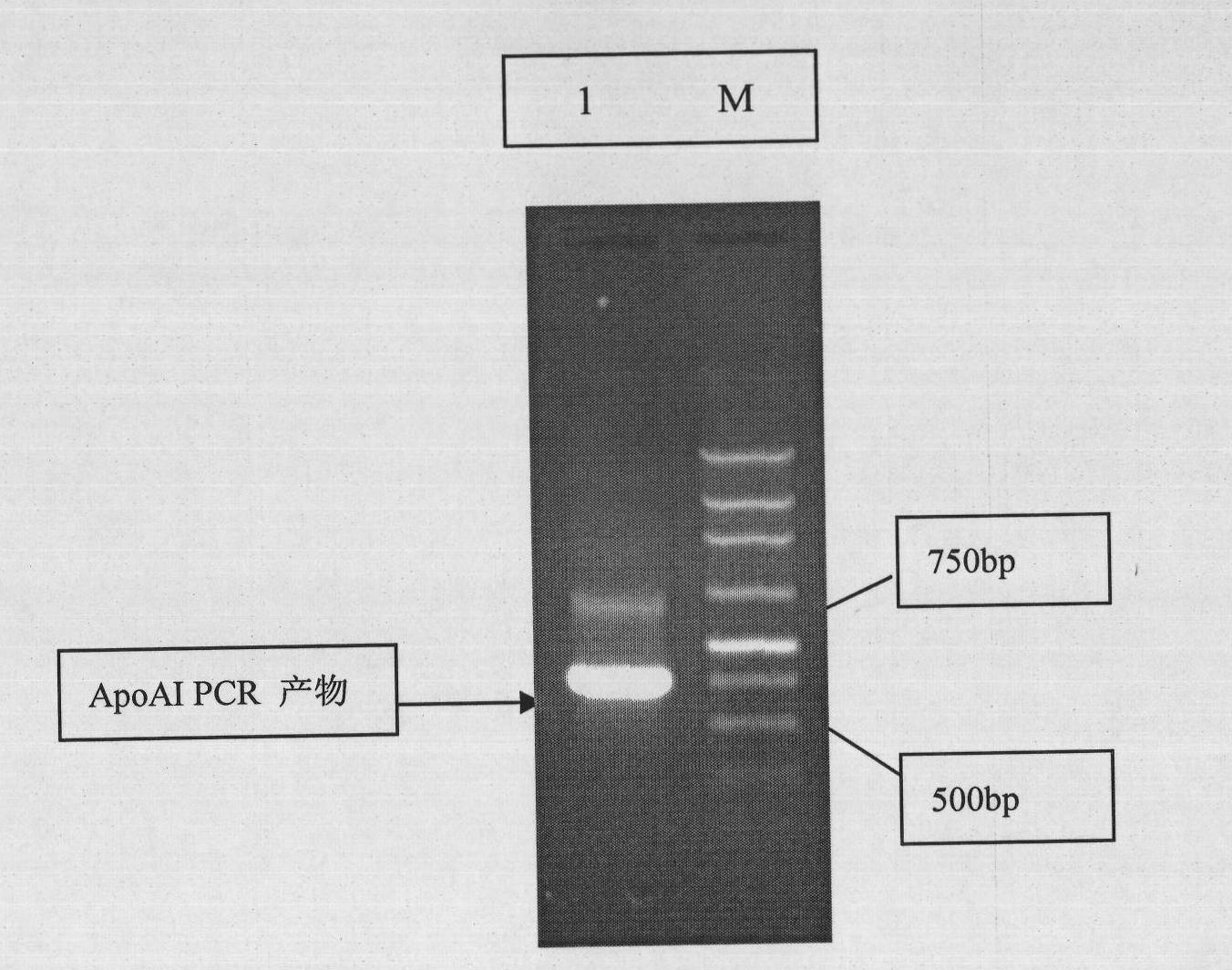
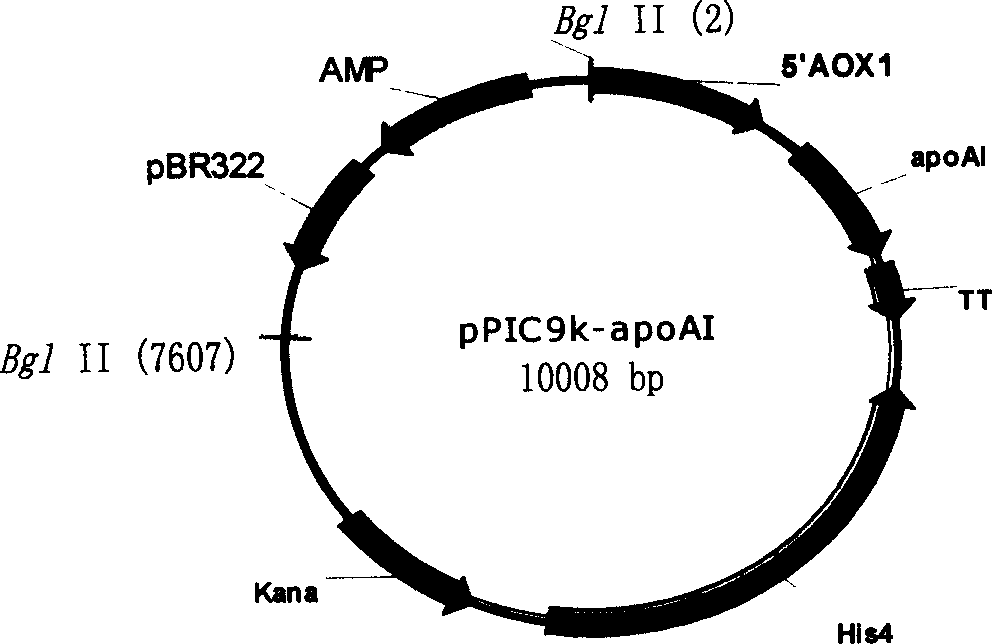
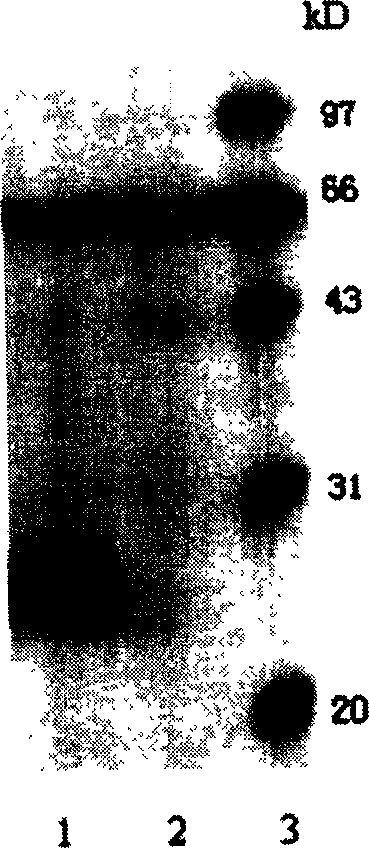
Human apolipoprotein AI genetic engineering preparation method and expression vector and engineering bacteria thereof
InactiveCN101921793AIncrease productionOvercome the problem of being easily degradedApolipeptidesBacteriaEscherichia coliProtein target
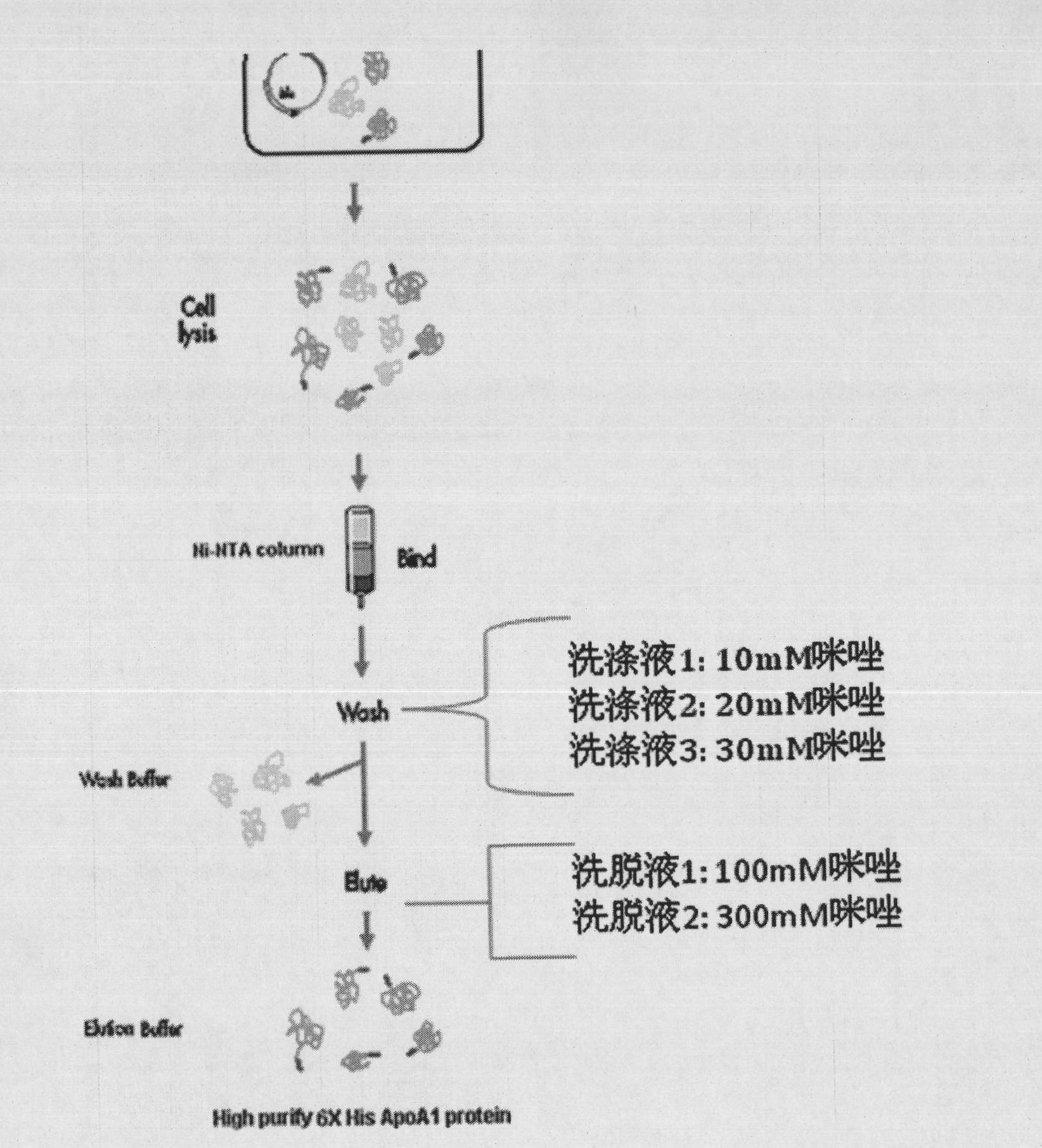
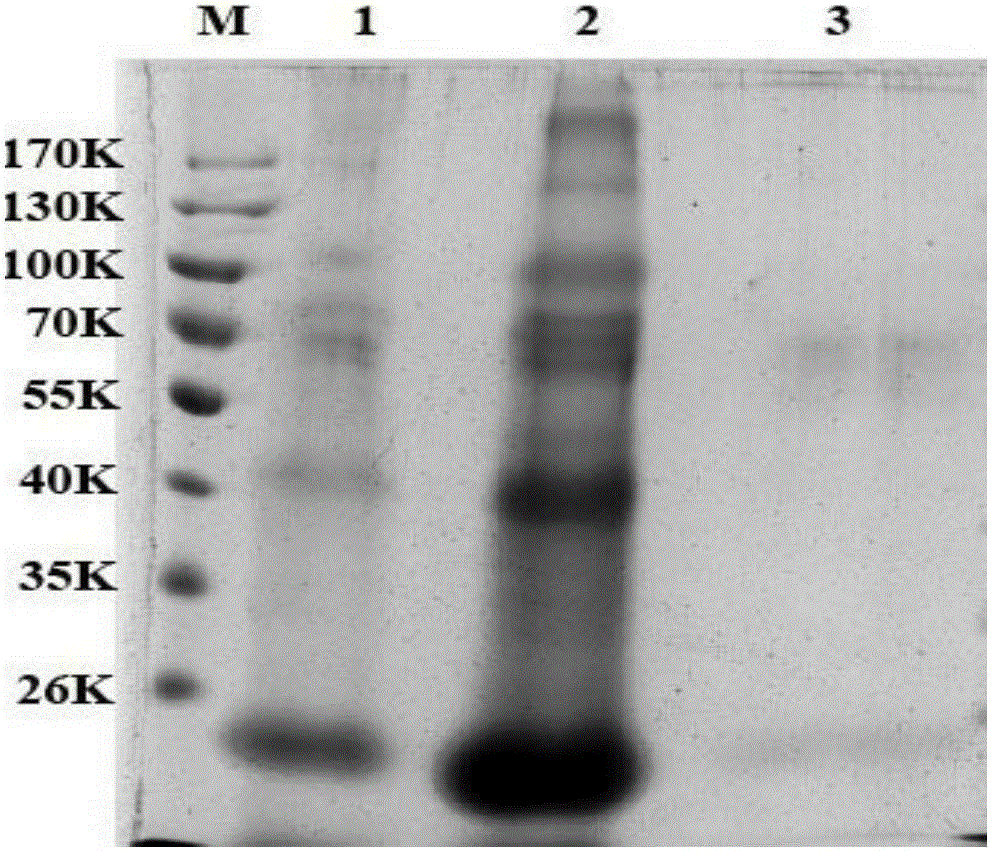
The invention provides a high efficiency soluble human apolipoprotein AI genetic engineering preparation method and expression vector and engineering bacteria thereof. Plasmid pCold containing recombinant human apolipoprotein AI gene sequence is selected as fusion expression vector, ApoA1 coding sequence is arranged between Nde I and Hind III endonuclease site on pCold vector. The engineering bacteria is escherichia coli, and the escherichia coli is converted into pCold-ApoA1 / Rosetta-gami by virtue of the expression vector plasmid pCold of the invention, and low temperature culture is carried out at 10-20 DEG C, thus inducing expression of soluble target protein. The invention adopts nickel crosslinking affinity column one-step method, operation is simple, yield is high, time is short, and the purity of the obtained target protein is higher than 90%, thus being applicable to industrialized mass production.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
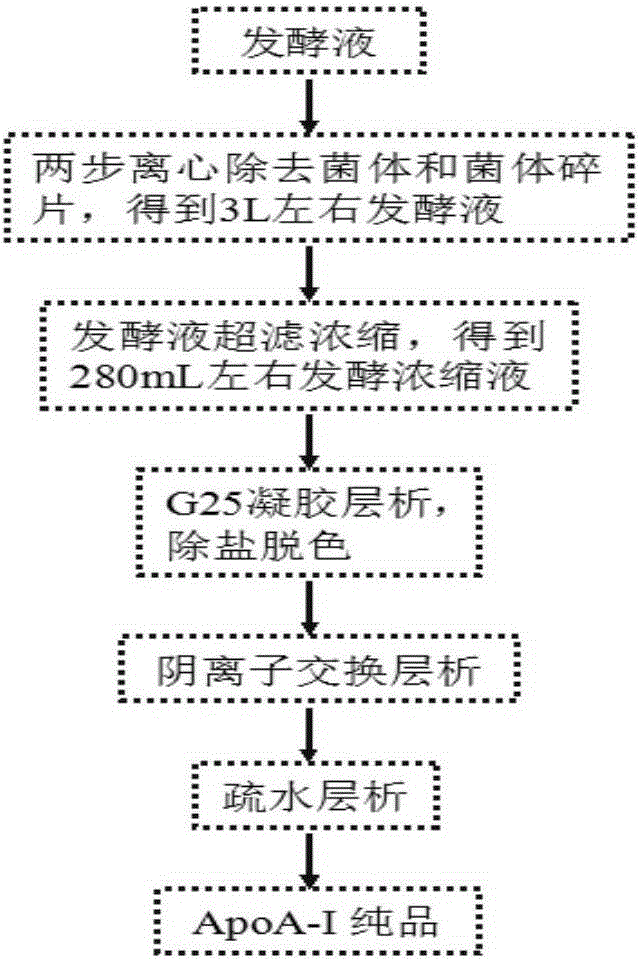
Method for large-scale purification of recombinant human apolipoprotein Apoa-I
InactiveCN106543266AHigh recovery rateEasy to operatePeptide preparation methodsProtein targetHuman apolipoprotein
Belonging to the technical field of genetic engineering recombinant protein purification, the invention relates to a method for purification of recombinant protein. The method includes: 1) centrifuging a fermentation broth to obtain a supernatant, and performing ultrafiltration and concentration on the supernatant; 2) subjecting the ultrafiltered concentrated solution to gel filtration chromatography to remove pigment and salt; 3) conducting anion chromatography on the obtained liquid, performing high salt gradient elution, and collecting a target protein peak; and 4) subjecting the collected liquid to hydrophobic chromatography to obtain pure recombinant human apolipoprotein ApoA-I. The method provided by the invention is employed to purify recombinant human apolipoprotein Apoa-I, the fermentation supernatant is subjected to ultrafiltration and concentration, further gel chromatography and hydrophobic chromatography are adopted, the final eluent is water, and can be directly subjected to freeze drying without desalination, the obtained recombinant human ApoA-I has purity of more than 96%, the recovery rate is higher than 82%, and the treatment capacity for each time is 3L. The method has the advantages of simplicity and convenience, stable process and easy amplification, can solve the scale problem in industrialization production of recombinant human apolipoprotein ApoA-I, and is suitable for separation and purification of production.
Owner:FUDAN UNIV
New preparation method of high-density lipoprotein
InactiveCN101928739AReduce sizeSpeed up entryPeptide/protein ingredientsMetabolism disorderDiseaseHuman apolipoprotein
The invention relates to a new preparation method of high-density lipoprotein (rhHDL), in particular to a function of regulating blood fat metabolism through the rhHDL and application of the rhHDL to preparation of a medicament for preventing and treating blood fat metabolism abnormal diseases. The rhHDL is mainly characterized in that protein components comprise recombinant human apolipoprotein A-I, recombinant human apolipoprotein A-II, recombinant human apolipoprotein C-I, recombinant human apolipoprotein C-II and recombinant human apolipoprotein E which are prepared from methyl nutritional yeast; and the biological activity of the rhHDL is similar to that of natural high-density lipoprotein.
Owner:吉林圣元科技有限责任公司
Vascular aging-predicting factor and utilization of the same
InactiveCN101365718AApolipeptidesImmunoglobulins against animals/humansHuman apolipoproteinVascular ageing
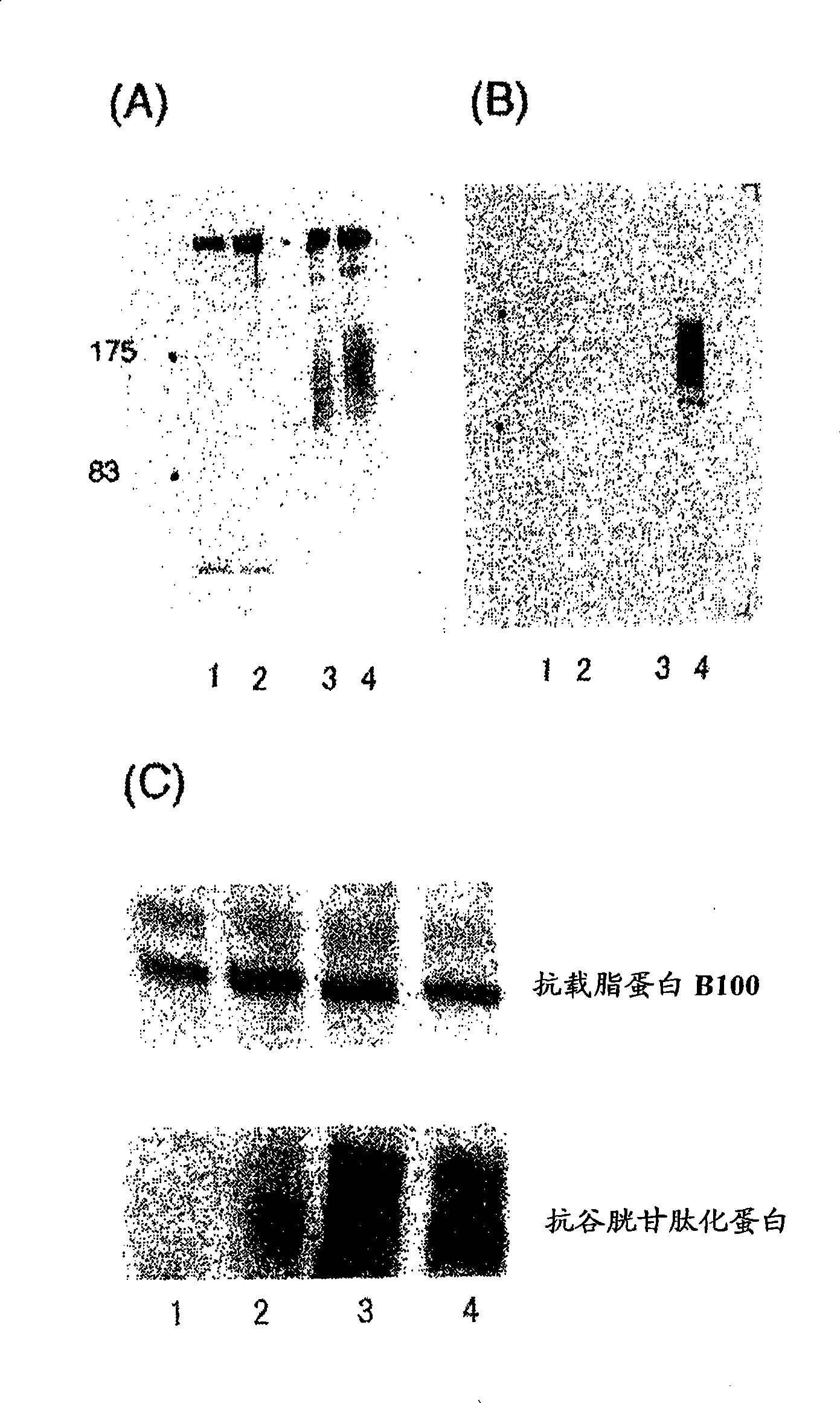
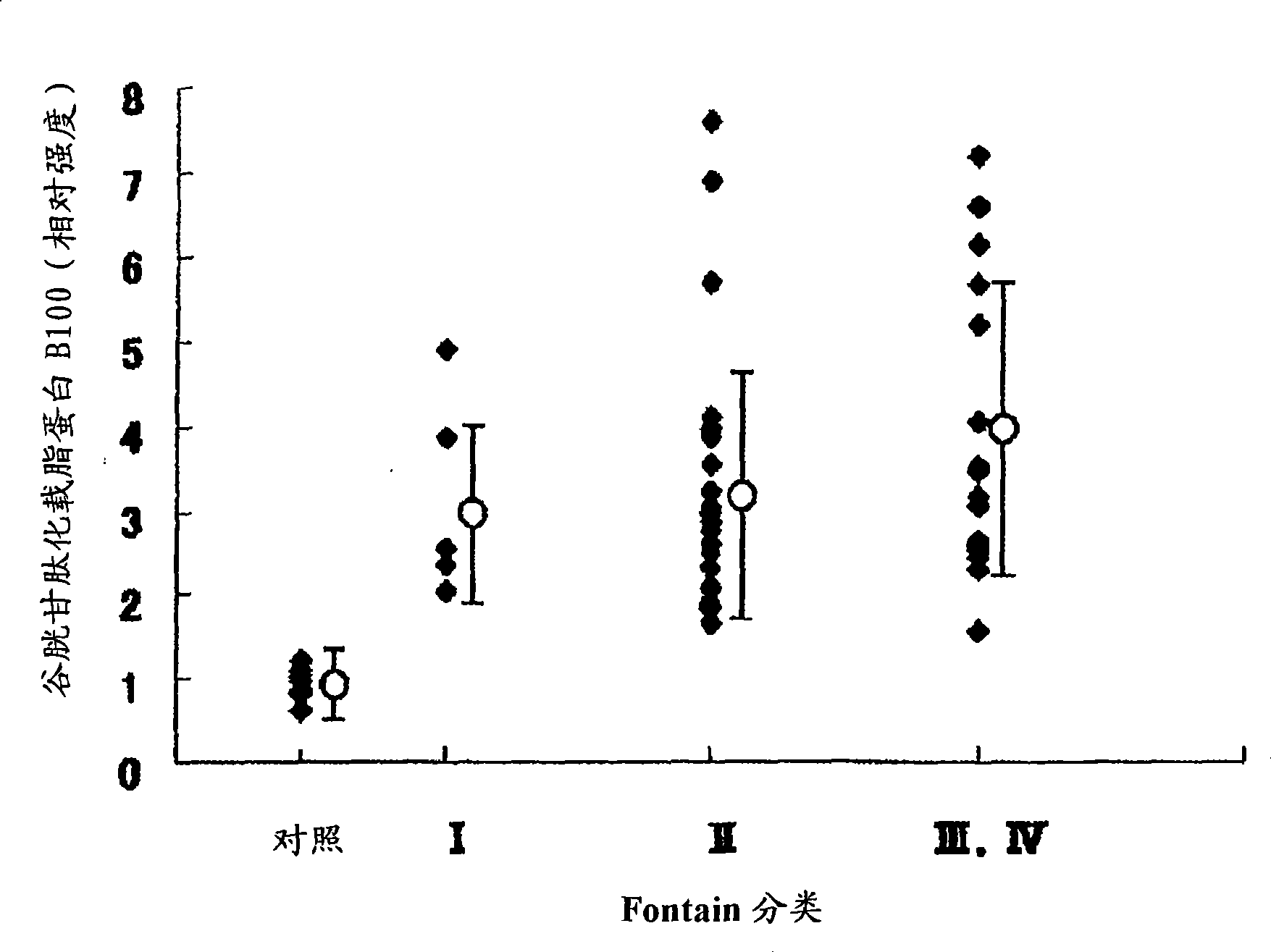
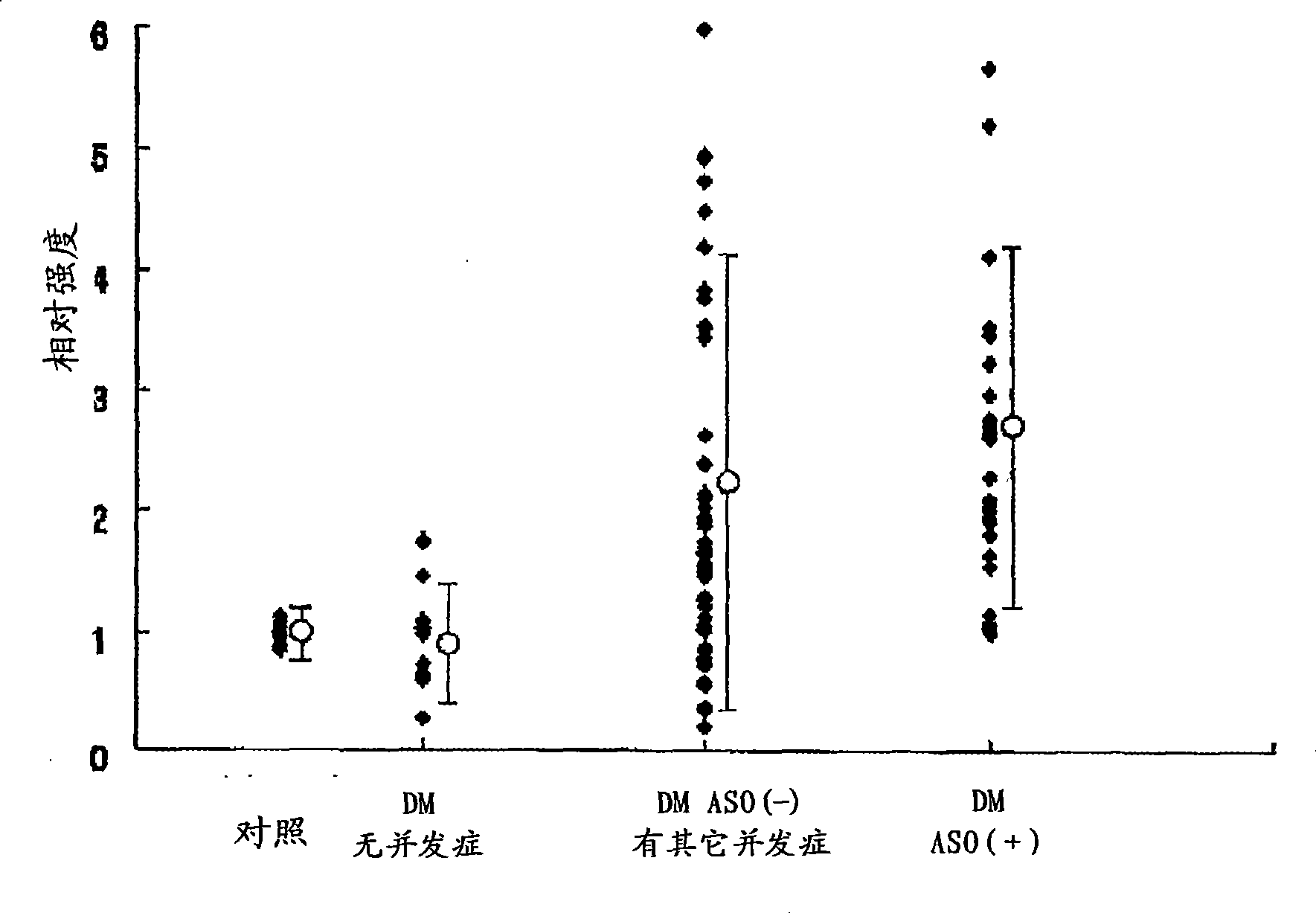
It is intended to provide a factor which predicts vascular aging and a method of examining an early lesion caused by vascular aging. More specifically speaking, it is intended to provide: a factor predicting vascular aging which comprises human apolipoprotein B100 having a glutathionated thiol group; a method of examining a lesion caused by vascular aging which comprises measuring human apolipoprotein B100 having a glutathionated thiol group in a blood sample; an antibody capable of specifically recognizing apolipoprotein B100 having a glutathionated thiol group; and a diagnostic or a diagnostic kit for an early lesion caused by vascular aging which contains an antibody capable of recognizing human apolipoprotein B100 having a glutathionated thiol group.
Owner:NAGASAKI UNIVERSITY
Serum/plasma protein molecular marker related to auxiliary diagnosis of intrahepatic cholestasis in gestation period and application thereof
ActiveCN111239416AImprove featuresIncreased sensitivityDisease diagnosisBiological testingHuman apolipoproteinProtein molecules
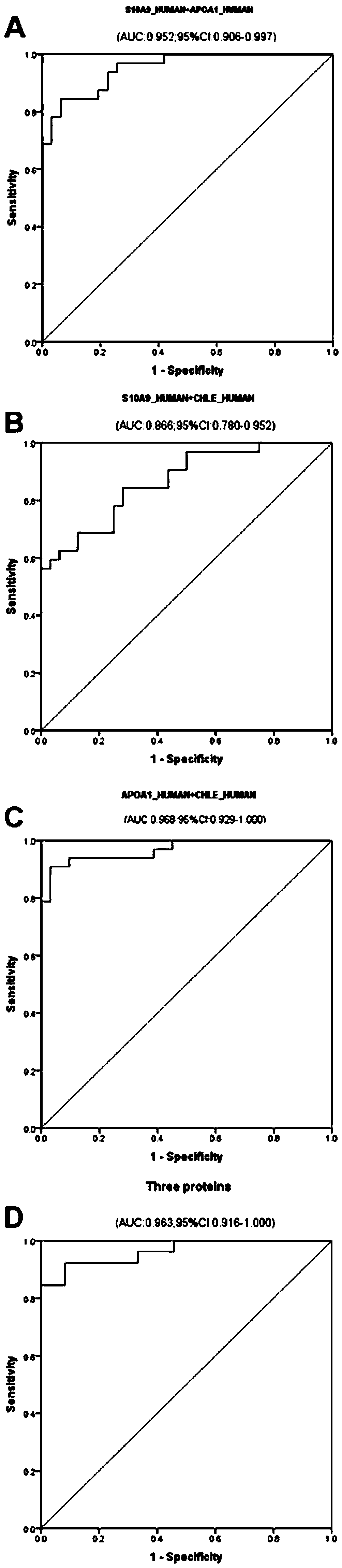
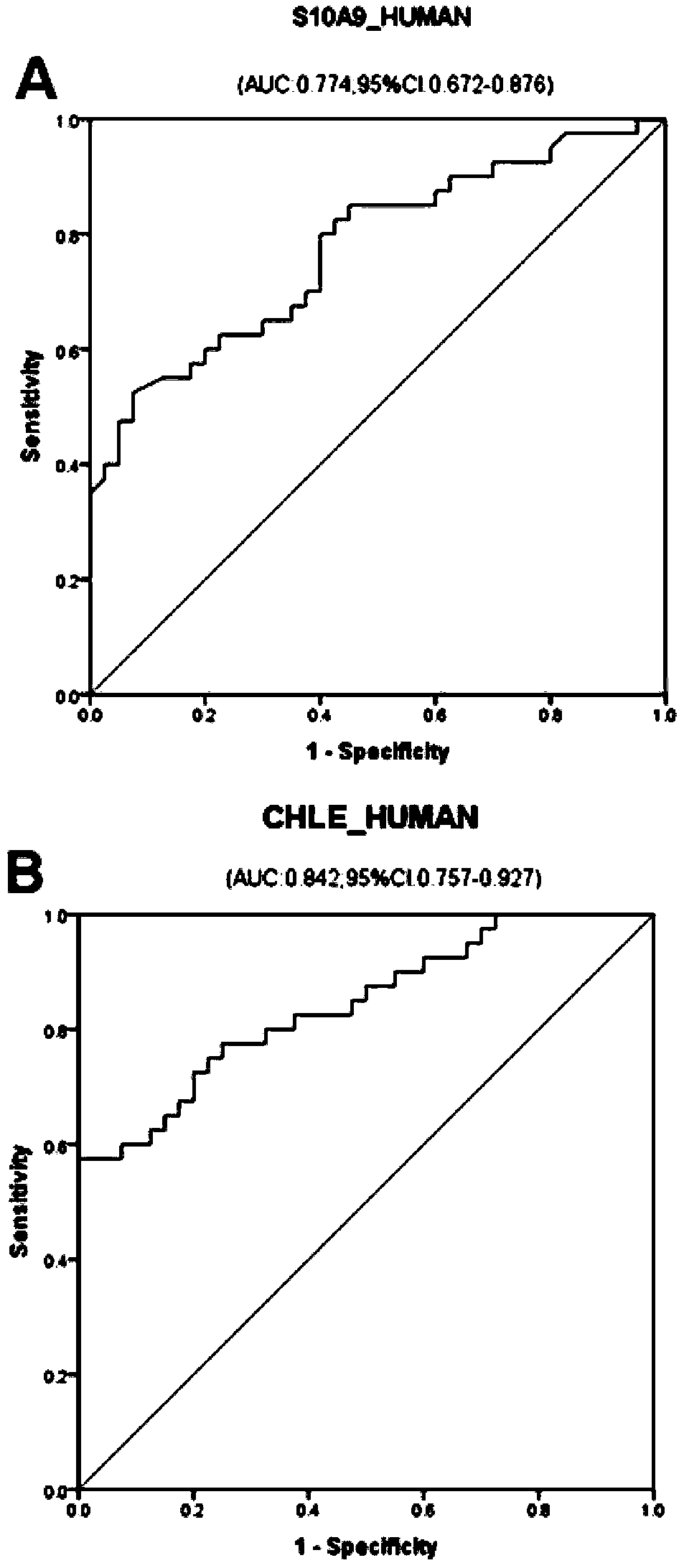
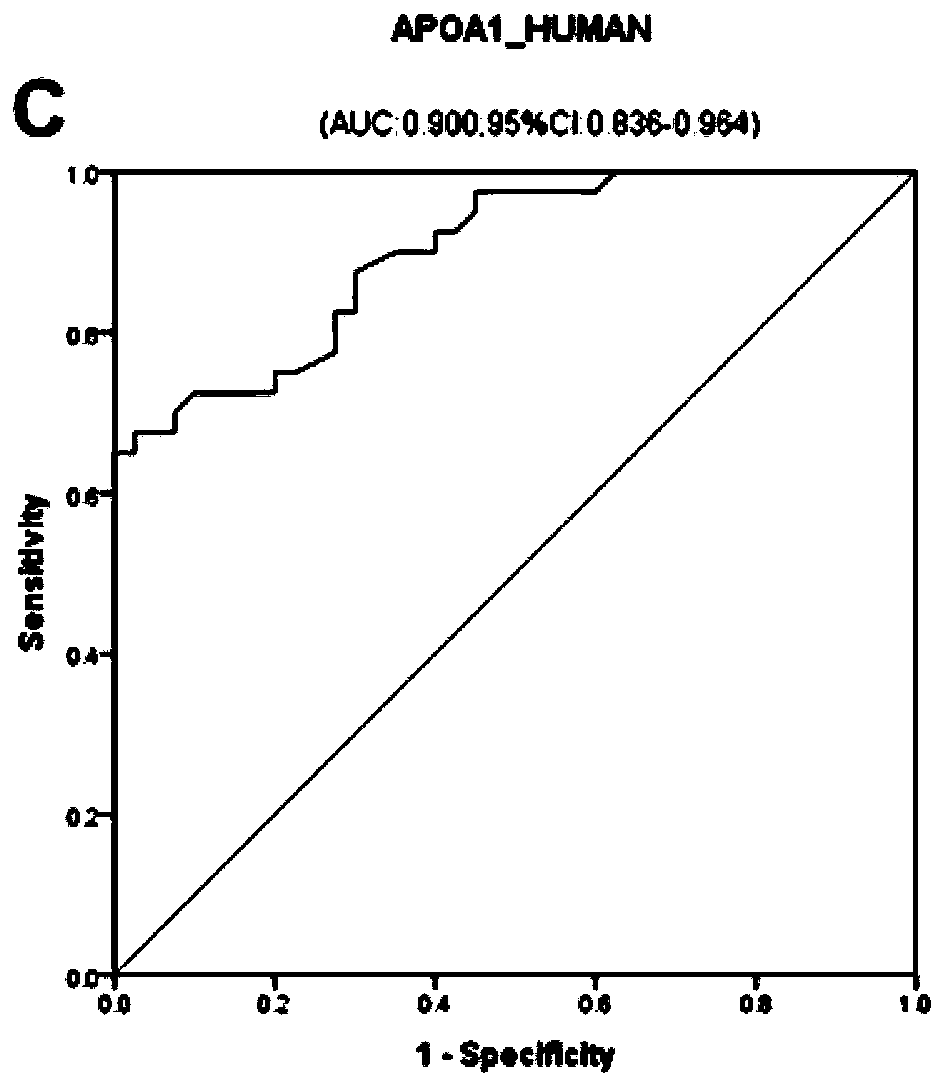
The invention discloses a serum / plasma protein molecular marker related to auxiliary diagnosis of intrahepatic cholestasis in a gestation period and an application thereof. The invention relates to aserum / plasma protein molecular marker related to ICP assisted diagnosis. The serum / plasma protein molecular marker is composed of S10A9_HUMAN (human S100 calcium binding protein A9), CHLE_HUMAN (humancholinesterase) and APOA1_HUMAN (human apolipoprotein A1). The inventor separates and studies ICP cases of initial birth and single pregnancy and protein molecular markers in control serum / plasma ofhealthy pregnant women matched with the ICP cases in age; a group of high-specificity and high-sensitivity protein molecular markers highly related to ICP morbidity are searched, and laboratory support is provided for ICP screening and diagnosis treatment.
Owner:WUXI MATERNAL & CHILD HEALTH HOSPITAL
Process for preparing human apolipoprotein A-I
InactiveCN1594582AMaintain binding activityHighly secreted expressionFungiPeptide preparation methodsPichia pastorisFractional Precipitation
The invention concerns bioengineering technology field. The invention relates to a method for preparing human apoprotein A-I(ApoA-I), especially a highly effective method of obtaining human apoprotein A-I(ApoA-I) through using Pichia pastoris system, constructing recombinant engineered bacterium, expressing ,separating and purifying. The invention recombines natural apoA-I gene to P.pastoris host bacterium by gene engineering approach. Expressed product amount is highly to 200mg / L. The expressed products of electrophoresis grade purity ApoA-I protein is obtained through cold acetone fractional precipitation and sepharose separation and purification. The expressed protein are identical with human blood derived ApoA-I protein after western-blot identification, protein spectrum detection, N-terminal amino acid sequencing and cell associativity determination.
Owner:FUDAN UNIV
Anti-human apoa1 monoclonal antibody and its preparation method and application
ActiveCN108659126BImprove the consistency rateImprove the detection rateImmunoglobulins against animals/humansMicroorganism based processesDiseaseHuman apolipoprotein
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
Method for treating kidney disorders
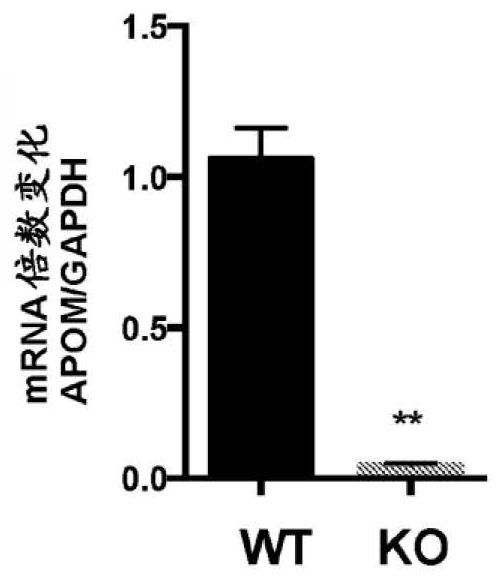
Disclosed herein is a method of treating or preventing renal disease in a subject suffering from Alport Syndrome, as well a method of treating or preventing chronic kidney disease. The method comprises administering to a subject in need thereof an effective amount of human apolipoprotein M.
Owner:UNIV OF MIAMI
Fusion protein of immunoglobulin fc and human apolipoprotein(a) kringle fragment
The present invention relates to an LK8-Fc fusion protein, which has increased angiogenesis inhibitory activity and in vivo stability. More specifically, relates to an LK8-Fc fusion protein in which an LK8 protein having angiogenesis inhibitory activity is fused with the Fc region of human immunoglobulin IgGl, as well as a composition for treating cancer, which contains the fusion protein. The LK8-Fc fusion protein has not only angiogenesis inhibitory activity leading to anticancer and metastasis inhibitory activitivies, but also a very long in vivo half-life, and thus can be used as a more efficient and economic cancer therapeutic agent or cancer inhibitor.
Owner:MOGAM BIOTECH RES INST
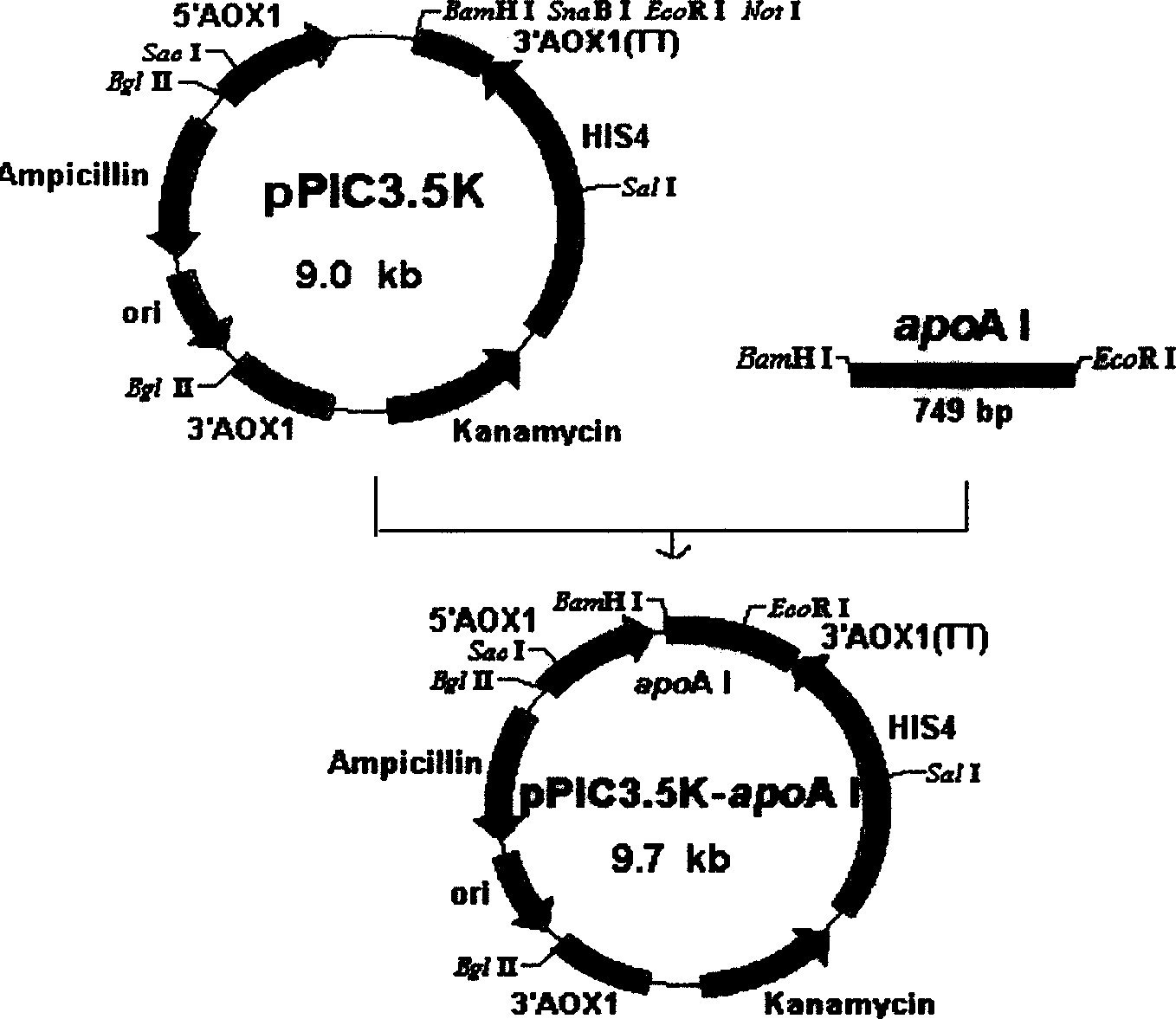
Method of expressing human apolipoprotein ApoA I inside Pichia yeast cell
InactiveCN1637145AImprove expression levelReduce manufacturing costFungiOther foreign material introduction processesBiotechnologyHuman apolipoprotein
The present invention belongs to the field of biological engineering technology, and is especially method of expressing human apoolipoprotein ApoA I inside Pichia yeast cell. Artificially synthesized ApoA I gene is inserted into cell to express plasmid pPIC3.5k, Pichia yeast strain GS115 is electric shock introduced, and after shake flask fermentation and methanol induction, there is obvious rApoA I protein expression inside yeast cell with the molecular weight identical with that of ApoA I extracted from human blood plasma.
Owner:FUDAN UNIV
Human apolipoprotein AV monoclonal antibody and application thereof
InactiveCN101575375AImprove stabilityTimely preventionImmunoglobulins against animals/humansTissue cultureSerum igeHuman apolipoprotein
The invention provides a human apolipoprotein AV monoclonal antibody and an application thereof. The human apolipoprotein AV monoclonal antibody is obtained by syncretizing a splenic cell and a myeloma cell of a human apolipoprotein AV immunized mouse and then cultivating the syncretized cell. The monoclonal antibody can be used for detecting human apolipoprotein AV in human serum. The Apo AV monoclonal antibody can be used for detecting Apo AV in human serum. When the Apo AV monoclonal antibody is used for detecting the Apo AV, the linear detection scope is wide, ApoAI, ApoB and Lp(a) in the serum do not influence a detection result, and the detection result has good stability, therefore, the Apo AV monoclonal antibody can be used for preparing corresponding detection products so as to detect the Apo AV concentration in the human serum, determine the high risk group of coronary heart disease as early as possible and prevent coronary heart disease in time and cure the coronary heart disease in the early period.
Owner:湖南远泰生物技术有限公司 +1
Applications of recombinant high-density lipoprotein in treating hypertensive disorders in pregnancy
ActiveCN106511970AGood dispersionSpeed up entryOrganic active ingredientsPeptide/protein ingredientsDiseaseYeast
The invention relates to applications of recombinant high-density lipoprotein in treating hypertensive disorders in pregnancy, and in particular relates to the preparation of recombinant high-density lipoprotein, and applications of the recombinant high-density lipoprotein in producing the medicines for preventing and treating hypertensive disorders in pregnancy. The recombinant high-density lipoprotein has the main features that the protein components comprise two kinds of apolipoprotein, namely, recombinant human apolipoprotein A-I and recombinant human apolipoprotein E which are produced by adopting methylotrophic yeast, and vitamin E replaces cholesteryl ester in natural high-density lipoprotein, and is taken as the core part of the recombinant high-density lipoprotein, so that the recombinant high-density lipoprotein has the strong antioxidant activity.
Owner:JILIN UNIV
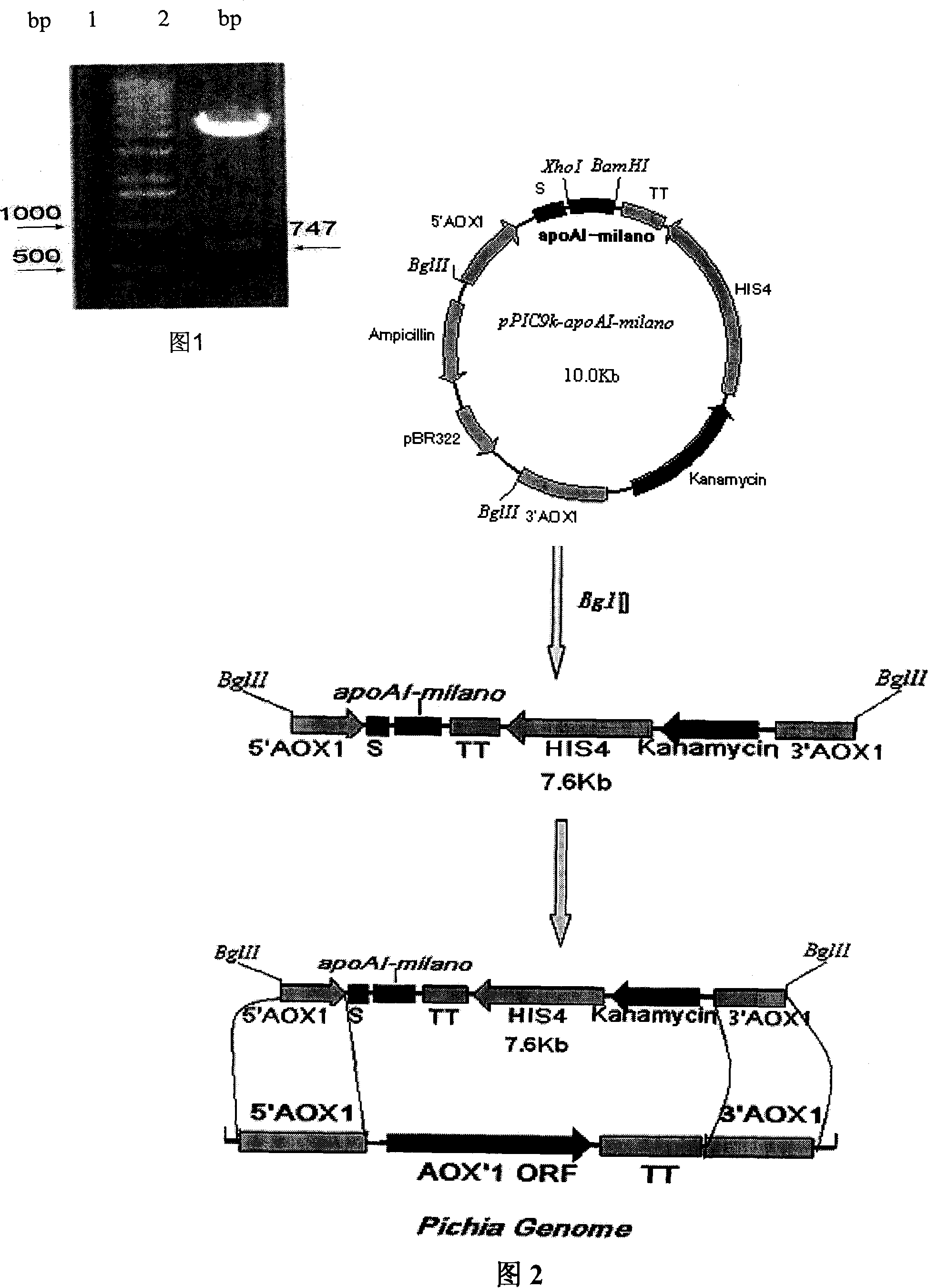
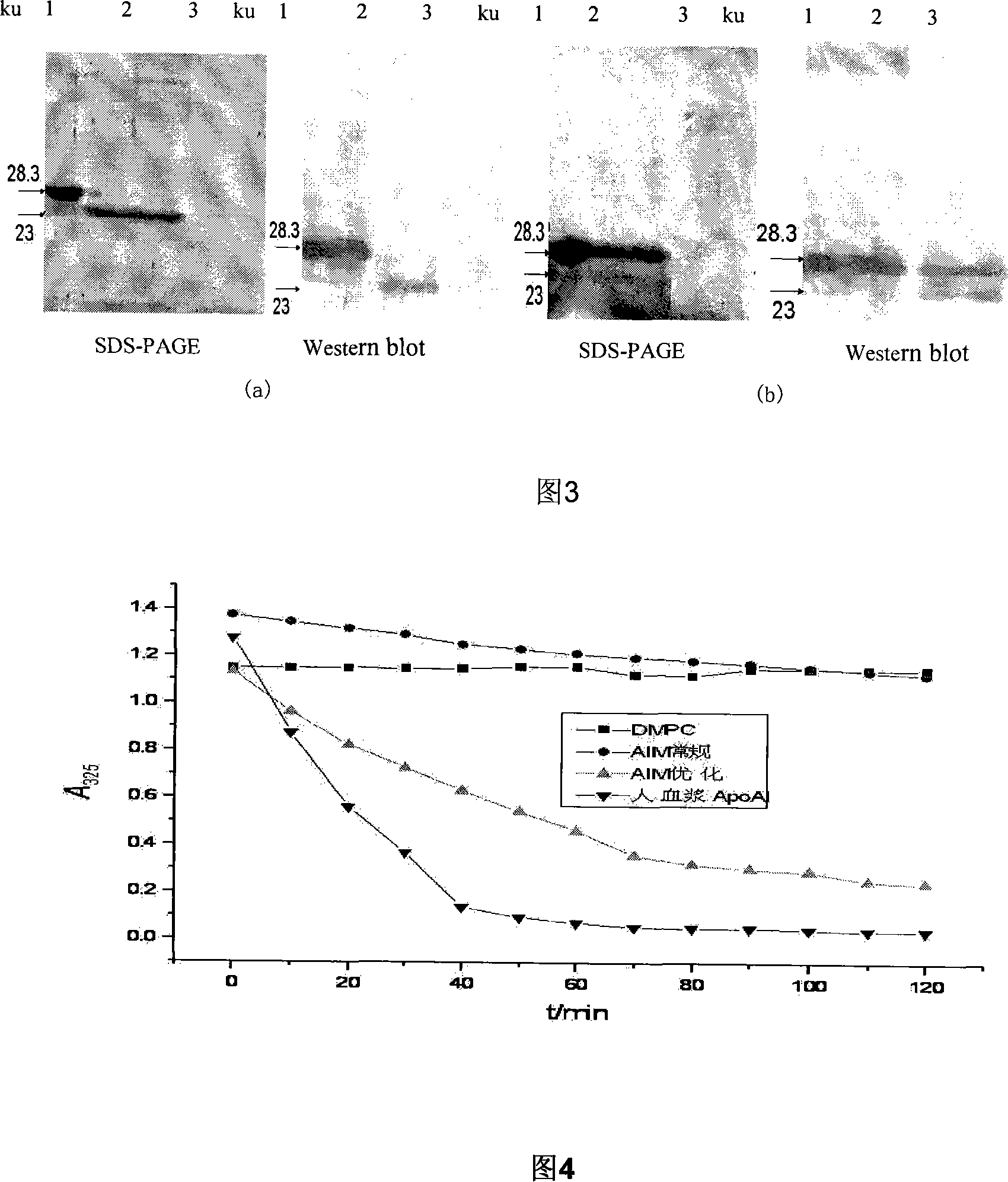
Expression method for recombination human apolipoprotein AI Milano variant in pichia pastoris
The invention pertains to the technical field of bio-engineering, in particular to an expression method of a recombinant human apolipoprotein A-I-Milano variant excreted in Pichia yeast. The mutated apoAI-milano cDNA is inserted into expression plasmid pPIC9K, and induced to Pichia yeast GS115 by electric shock, and by the optimization of substrate in a shake flask and culture conditions, the fermented suspentant liquid has obvious rAIM expression with a mocular weight identical to the ApoAi extracted from human blood plasma. The purified rAIM has phospholipid-binding activity.
Owner:FUDAN UNIV
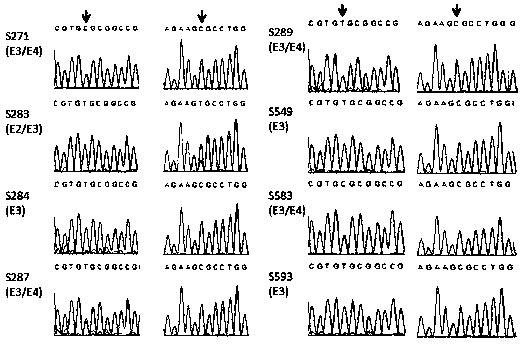
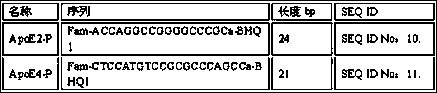
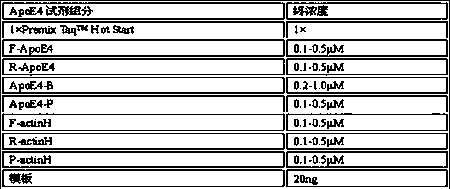
Primer and probe combination for human apolipoprotein E (ApoE) gene typing, and using method of primer and probe combination
InactiveCN110499363AEasy to operateReduce use costMicrobiological testing/measurementDNA/RNA fragmentationDiseaseHuman apolipoprotein
The invention belongs to the technical field of genes, and provides a primer and probe combination for human apolipoprotein E (ApoE) gene typing, and a using method of the primer and probe combination. An allele specific primer is designed for an ApoE gene point mutation gene sequence, a closed primer is designed for a wild-type sequence, a mutation-rich amplification reaction condition is adopted, and thus ApoE genes can be subjected to typing detection. The method has the characteristics of being quick in detection, easy and convenient to operate, high in sensitivity, high in specificity andlow in cost, result interpretation is easy and clear, the sample gene type is judged only according to the Ct value, the primer and probe combination and the using method thereof can be widely used for ApoE typing detection of various DNA samples of humans, and research work of the relation between ApoE gene polymorphism and related diseases is facilitated.
Owner:SUZHOU RES INST OF TONGJI UNIV
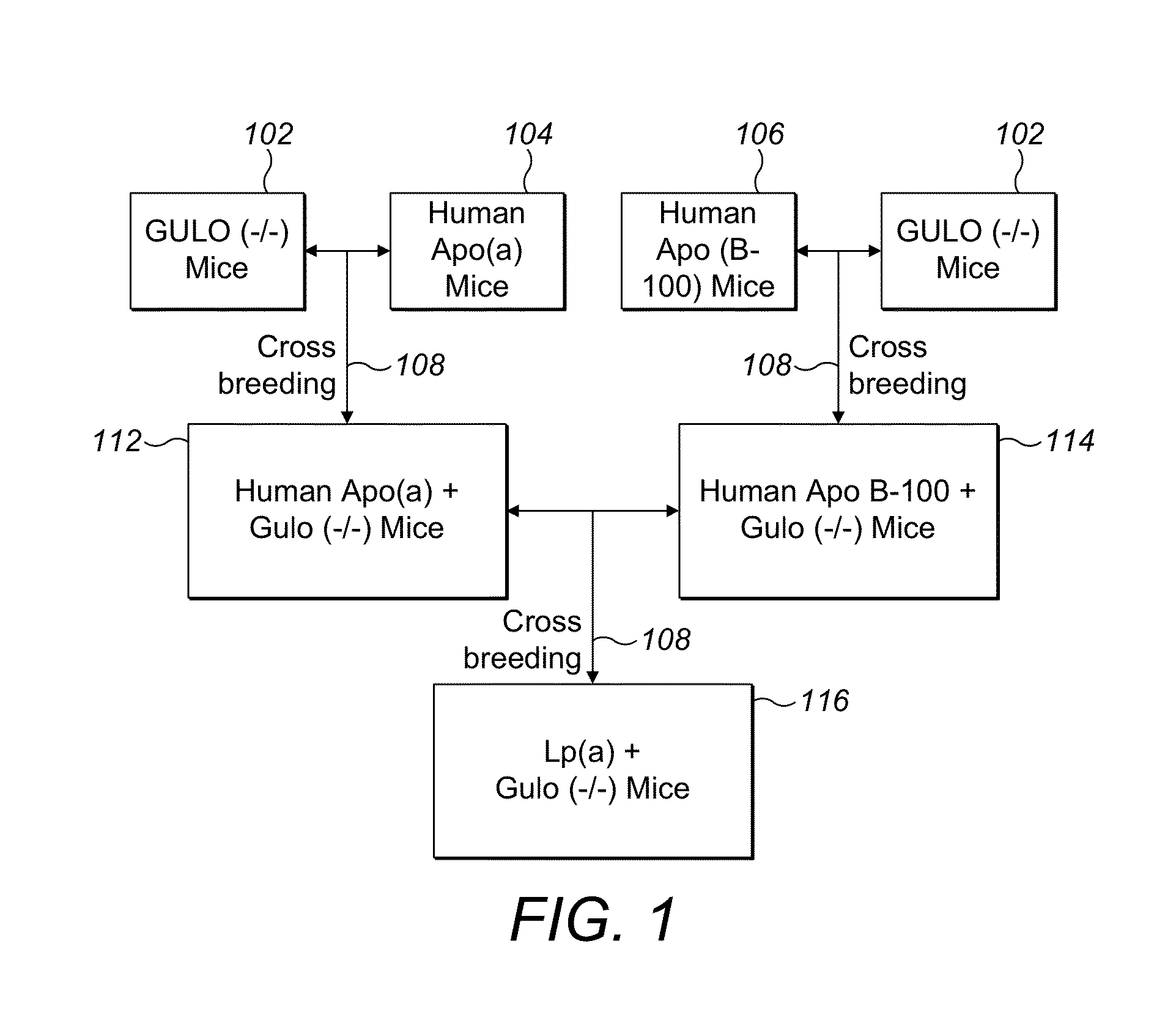
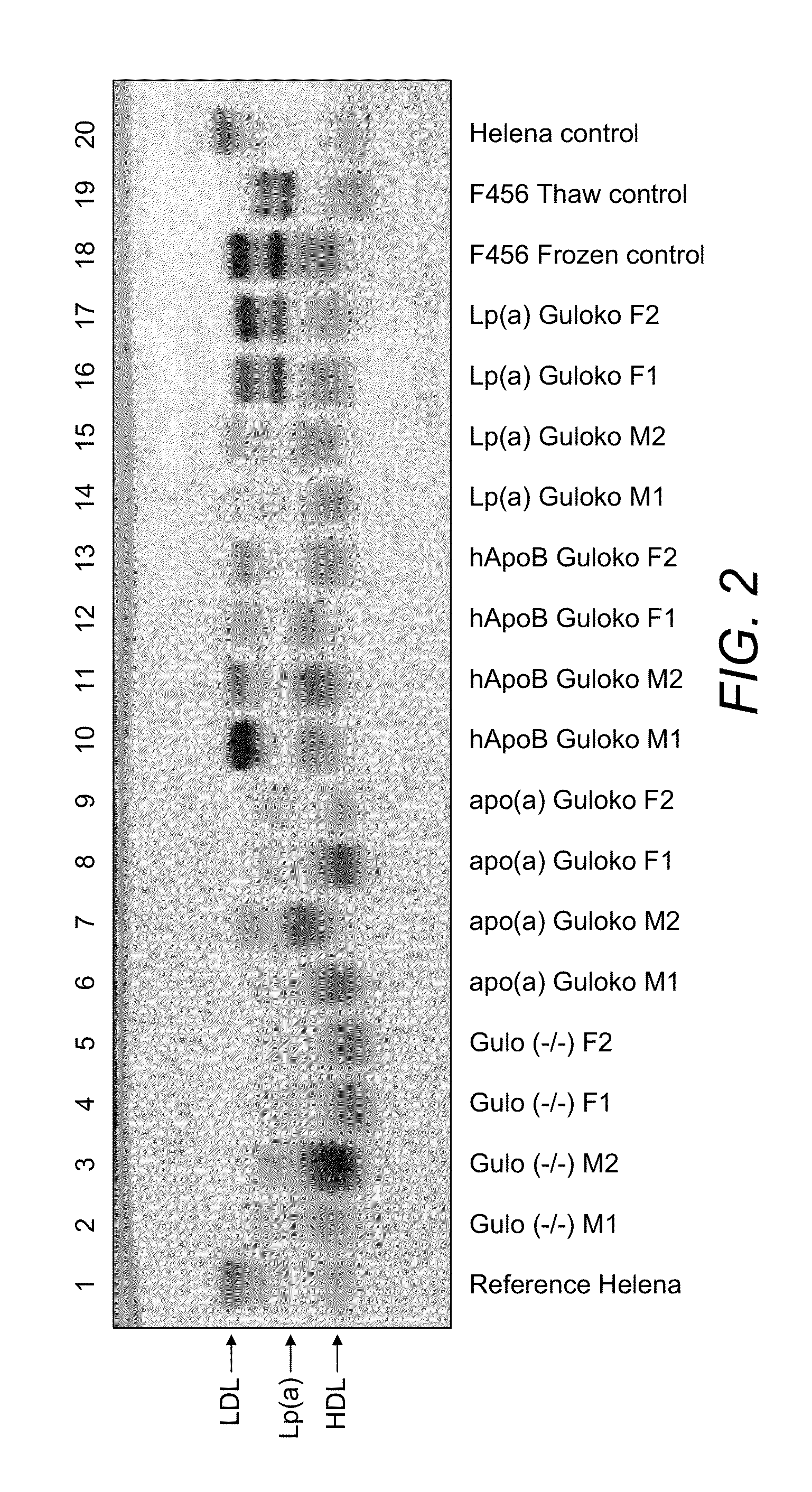
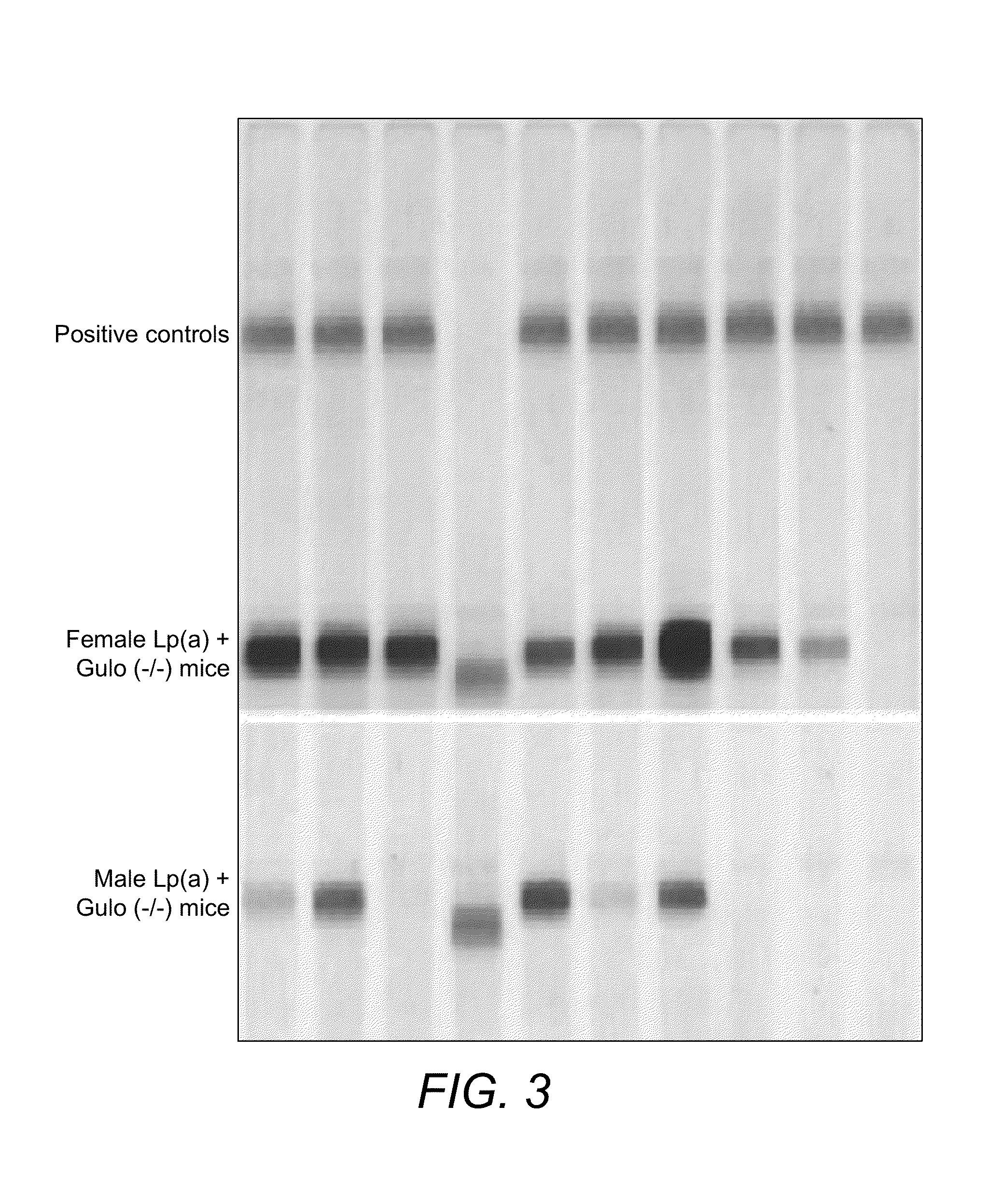
Transgenic mouse expressing human apo(a) and human apo(B-100) with disabled vitamin C gene produces human Lp(a)
The invention discloses novel model of transgenic mammal, a method of crossbreeding transgenic mammal and the use of the transgenic mammal for assessing prevention and / or treatment methods for cardiovascular and other diseases related to lipoprotein(a). The transgenic mammal expresses human apolipoprotein (a) (apo(a)) and human apolipoprotein B-100 (apo B-100) genes and produces human lipoprotein (a), apo (a) and apo B-100 and produces no vitamin C. This novel dual transgenic mammal is the ideal model for testing pharmaceutical compounds for efficacy and usefulness in the prevention and / or treatment of human diseases.
Owner:RATH MATTHIAS W
Nucleic acid encoding angiogenesis inhibitor
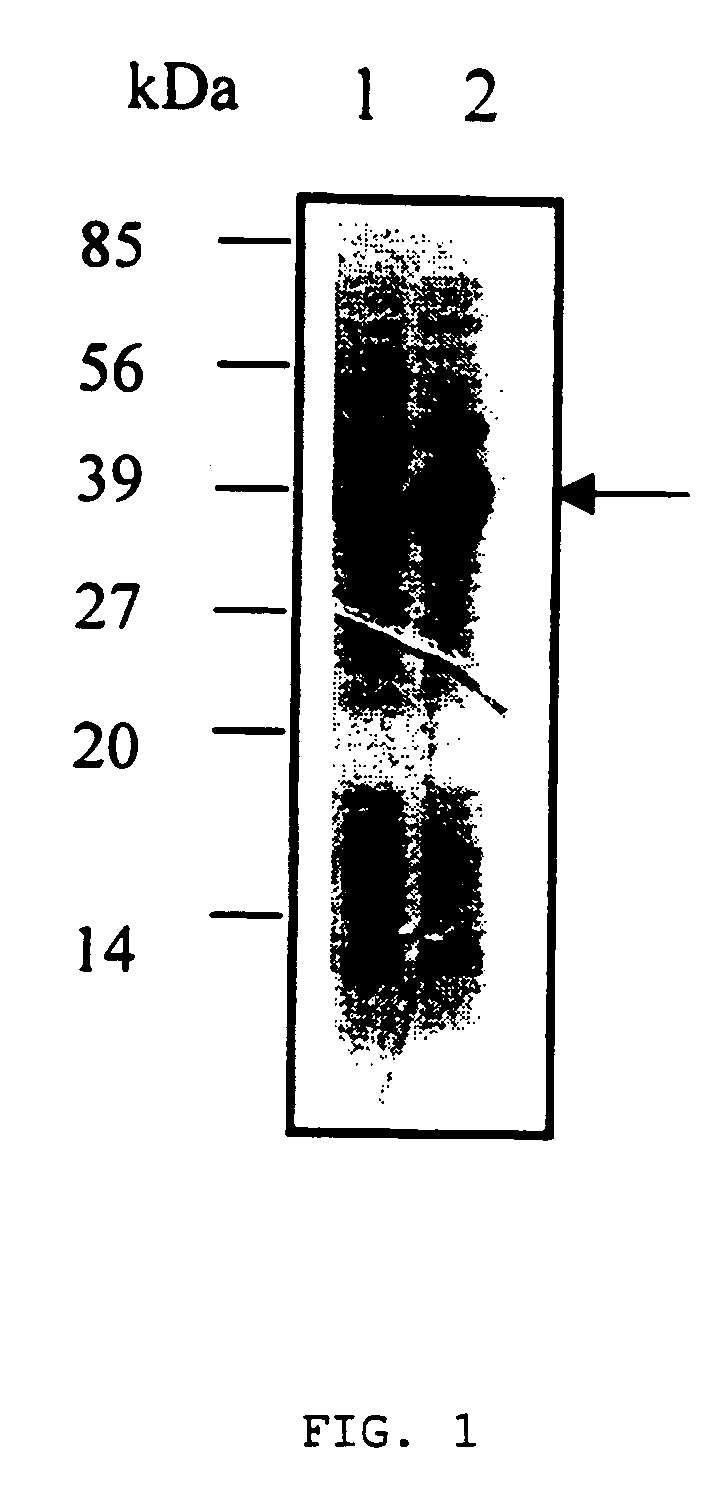
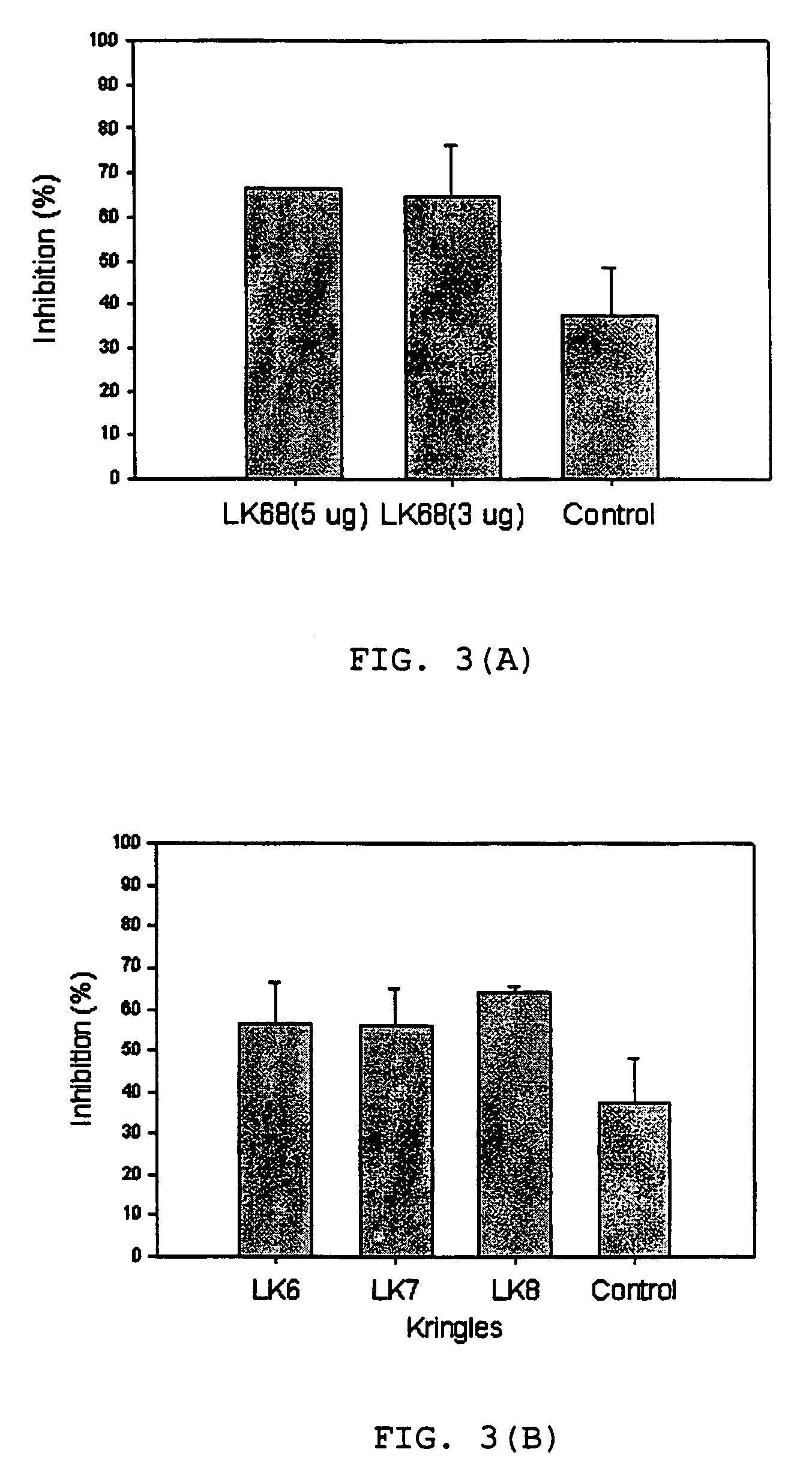
The present invention provides a novel angiogenesis inhibitor, LK68 whose amino acid sequence is identical with the human apolipoprotein (a) kringle domains IV36, IV37 and V38, a cDNA sequence encoding the LK68, a recombinant expression vector comprising the cDNA, a recombinant microorganism transformed with the recombinant expression vector and a novel use of the LK68 as an anticancer agent and a method for treating angiogenesis-mediated disease. LK68, LK6, LK7 and LK8 exhibit inhibitory activities on the cultured endothelial cell proliferation as well as on the endothelial cell migration. LK68 and its single kringles also inhibit the normal development of capillaries in the chick embryo chorioallantoic membrane (CAM). It was also showed that systemic administration of LK68 causes the inhibition of primary tumor growth, which is correlated with a suppression of tumor-induced angiogenesis. Accordingly, LK68 protein, its single kringles or their functional equivalents may be applied for the development of a potent anti-cancer agent, which is highly effective for angiogenesis-mediated diseases covering cancer, rheumatoid arthritis, psoriasis, ocular angiogenic disease, etc.
Owner:MOGAM BIOTECH RES INST
Hyperlipemia transgenic miniature pig and preparation method thereof
InactiveCN102086454AReduced activityInhibitory activityFermentationVector-based foreign material introductionFiberHuman apolipoprotein
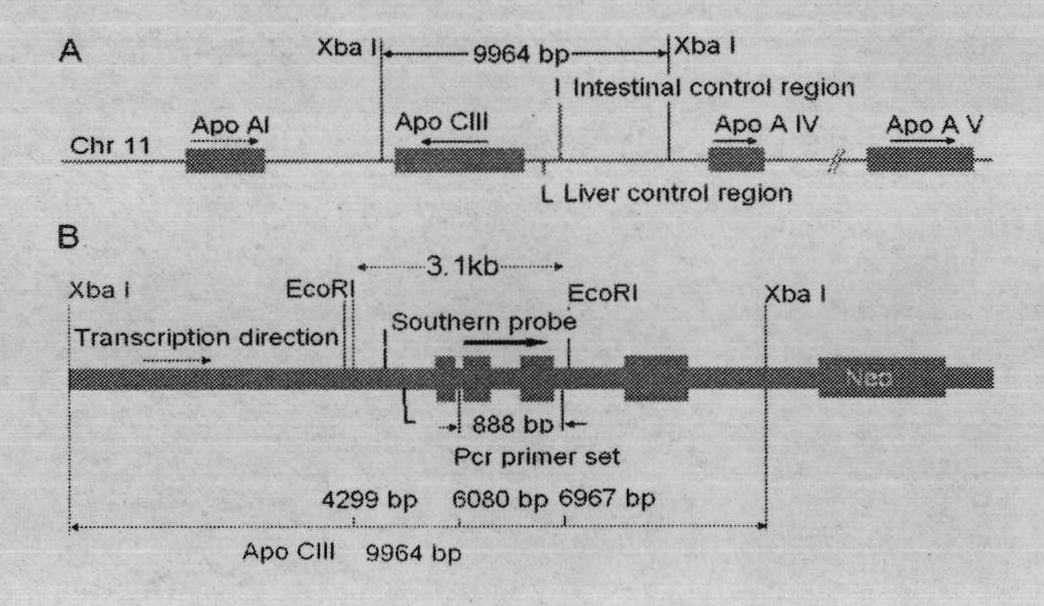
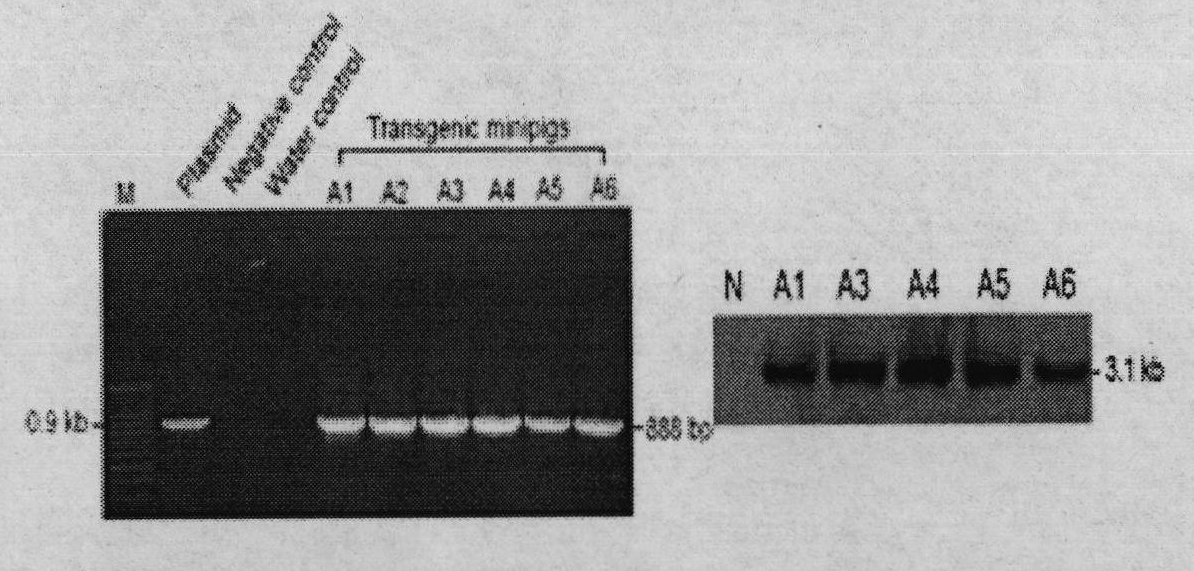
The invention provides a transgenic miniature pig for preparing a human hyperlipemia disease animal model and a preparation method thereof. A human apolipoprotein CIII expression vector pcDNA-CIII is constructed and expressed by using a gene engineering method; a miniature pig fibroblast capable of expressing the human apolipoprotein CIII is screened out by using cell transfection and cell screening technologies; and the miniature pig is cloned by using a somatic cell nuclear transfer technology. The cloned miniature pig can be used for research into pathogenesis of human hyperlipemia related diseases, and screening and preclinical pretesting of medicine intervention and treatment of related diseases.
Owner:JILIN UNIV
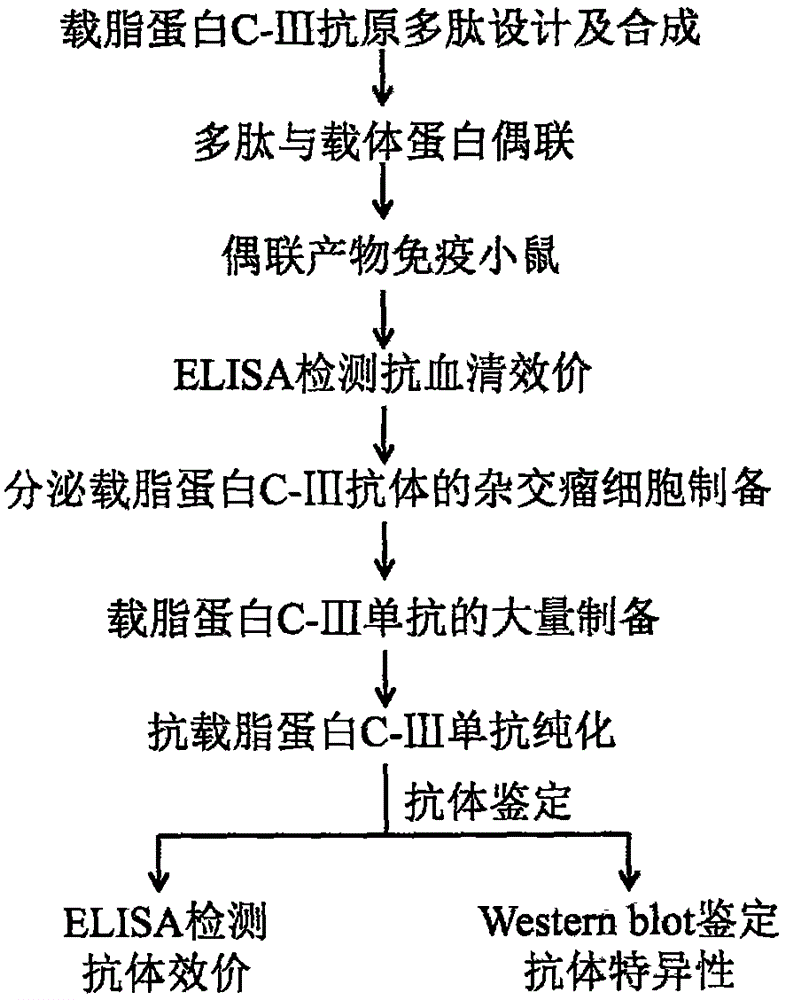
Preparation and application of apolipoprotein C-III monoclonal antibody
The invention discloses preparation and application of a mice anti human apolipoprotein C-III monoclonal antibody. Specifically, the invention provides an amino acid sequence of apolipoprotein C-III antigen polypeptide, a mice hybridoma cell strain WLC001 with a preservation number of CCTCC No. C2015191, and an apolipoprotein C-III monoclonal antibody generated by the hybridoma cell strain. The invention also relates to application of the apolipoprotein C-III monoclonal antibody in detecting immunoblotting of the apolipoprotein C-III.
Owner:西藏自治区人民医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com