Human apolipoprotein B100 (ApoB100) monoclonal antibody and chemiluminescence immune assay determination kit adopting the human ApoB100 monoclonal antibody
A monoclonal antibody, apolipoprotein technology, applied in the biological field, can solve the problems of false positives, cannot be stored for a long time, false negatives, etc., and achieve the effects of wide detection range, improved sensitivity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of hybridoma
[0041] 1. Immunization of mice: The immunized animals were 8-week-old male Balb / C mice, mixed with human ApoB100 with a protein content of 50ug and complete Freund's adjuvant, and after fully emulsified, take multiple points on the back, and underarms, Inguinal immunization; 15 days later, the same dose was mixed with Freund's incomplete adjuvant for the second time; 15 days later, the same dose was mixed with Freund's complete adjuvant for the third time; 10 days later, blood was collected from the tail and measured by indirect ELISA method For serum titer, boost immunization with the same dose of pure antigen without adjuvant; take splenocytes for fusion after 3 days;
[0042] 2. Cell fusion: Sp2 / 0 cells in the logarithmic growth phase were mixed with splenocytes at a ratio of 1:10, and hybridoma cells were obtained by the polyethylene glycol (PEG) method, named 4-3-2 and 4-6 -3. The obtained hybridoma cells were suspende...
Embodiment 2
[0043] Example 2: Screening of Monoclonal Antibodies
[0044] The supernatant of the culture medium was recovered from the wells in which the hybridoma cells obtained in Example 1 were cultured, and the monoclonal antibody reacting with the antigen polypeptide in the ELISA method was selected.
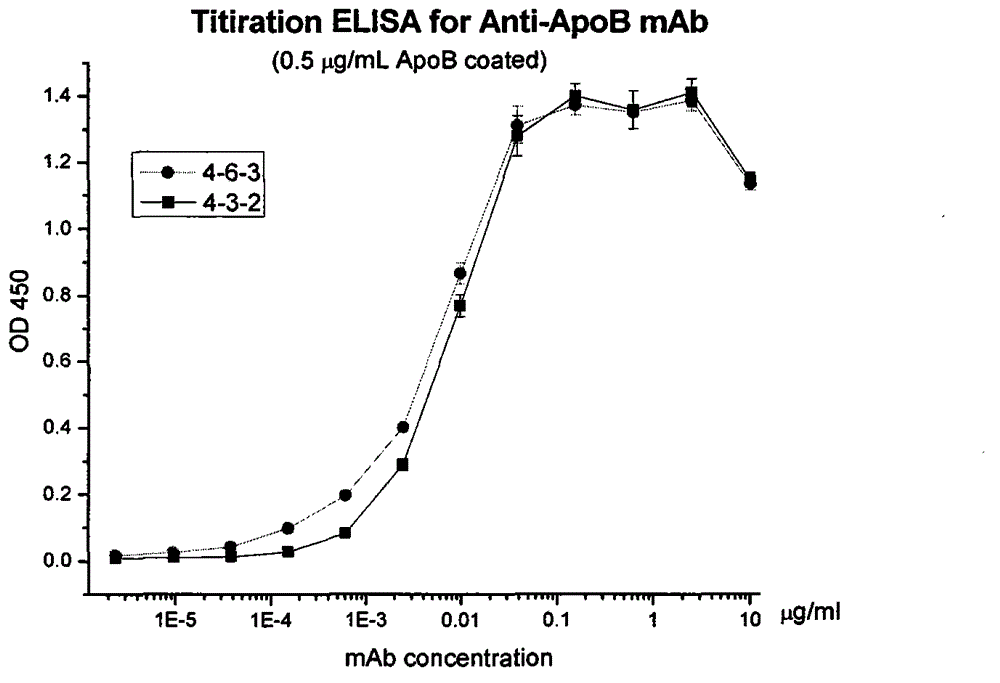
[0045] First, 100uL of human ApoB at a concentration of 0.2ug / ml 100 Put them into each well of a 96-well plate, fix them on the solid phase after overnight at 4°C, and then block with 150uL bovine serum albumin at a concentration of 1% for 2 hours. Add 100uL hybridoma culture medium supernatant to each well, react at 37°C for 2 hours, then add horseradish peroxidase-conjugated anti-mouse antibody diluted 10,000 times and react at 37°C 1 hour. Color development was performed using tetramethylbenzidine microperoxidase substrate (TMB) as substrate. After adding 50 uL of 0.2N sulfuric acid to terminate the reaction, measure the absorbance at 450, select 2C6 and 3E8 with absorbance abou...
Embodiment 3
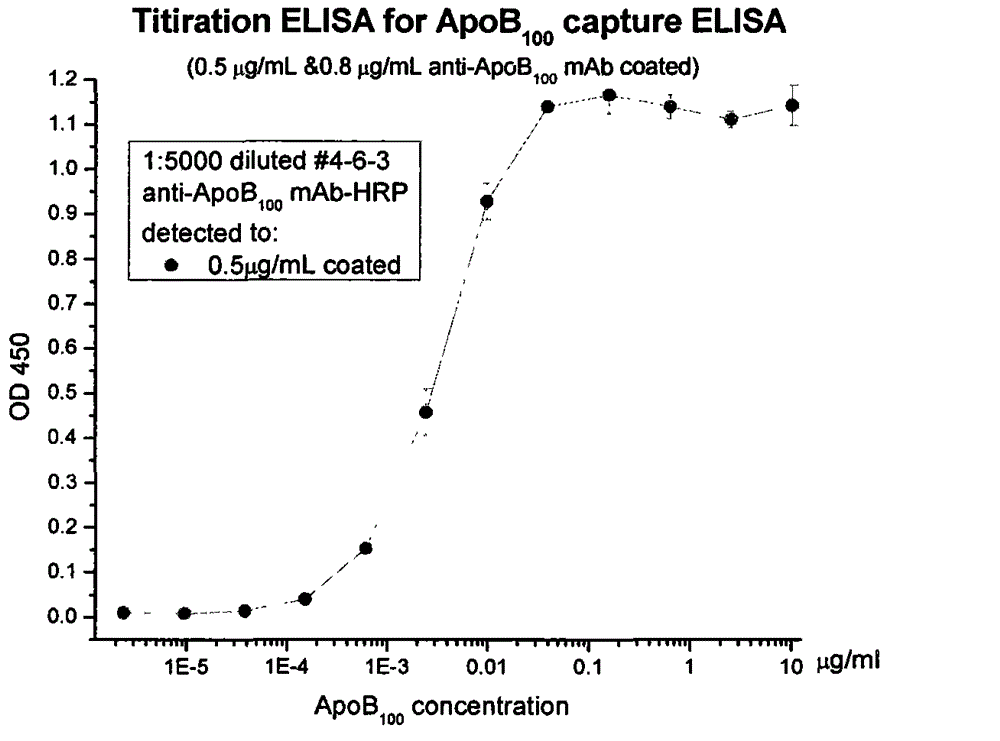
[0048] Example 3: Labeling of Monoclonal Antibodies
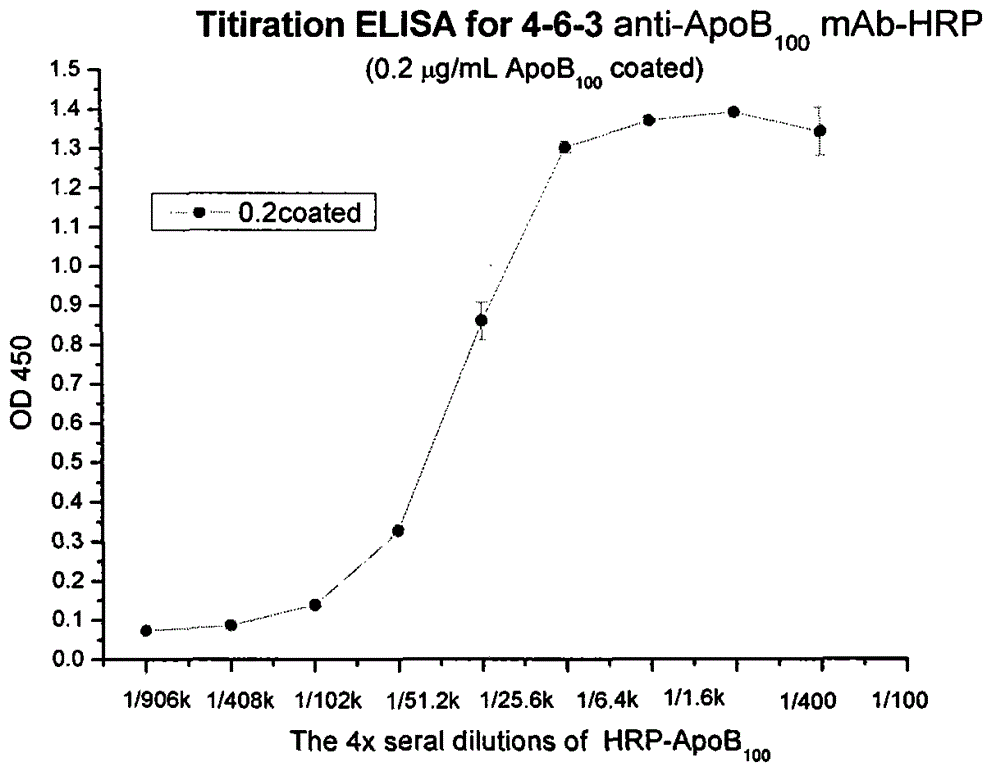
[0049] Monoclonal antibody 4-6-3 was used for HRP labeling according to conventional methods. The titer of the labeled monoclonal antibody was determined by the following method. Human ApoB at a concentration of 0.2ug / ml 100 Immobilized on a 96-well microplate (100uL / well). Use 1% bovine serum albumin to block for 2 hours, add labeled monoclonal antibody (diluted 100 times from the first well), make 4-fold dilution from the second well, and react at room temperature for 2 hours. After addition of TMB, the reaction was carried out at room temperature for 20 minutes and quenched with 0.2N sulfuric acid. The absorbance at 450 nm was measured, and the titer against the antigen immobilized in the solid phase was obtained in the manner in Example 2. The results show that there is an effective titer ( figure 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com