Process for preparing human apolipoprotein A-I
An apolipoprotein and protein technology, applied in the field of bioengineering, can solve problems such as difficulty in separation and purification, and achieve the effect of high-efficiency secretion and expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
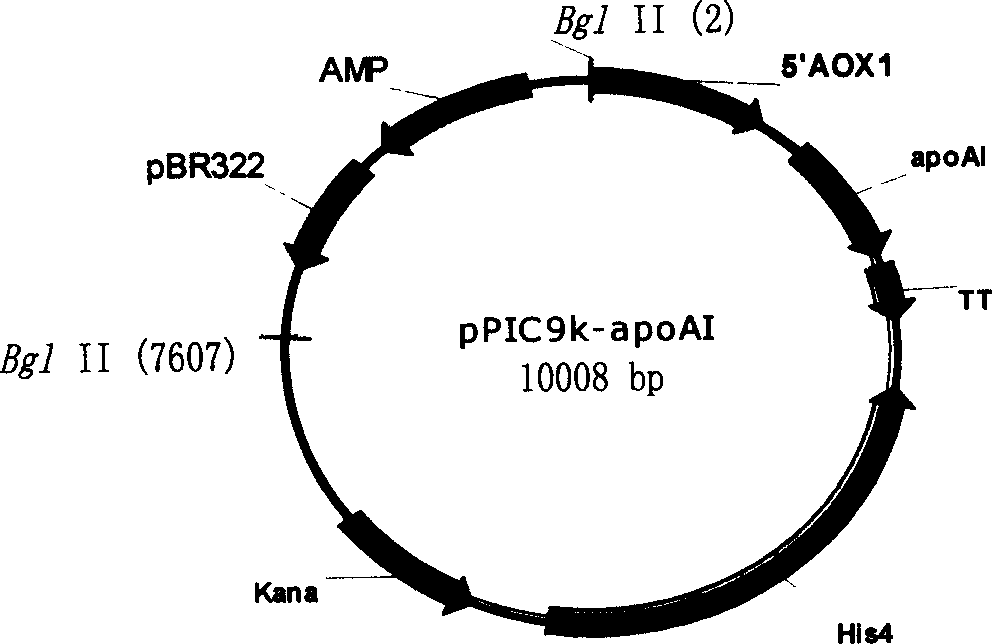
[0030] Example 1 Obtaining recombinant apoA-I expression plasmid
[0031] According to the apoA-I gene sequence, the expression plasmid DNA of the insect expression system was used as a template to amplify the natural fragment by PCR, and Xhol I and EcoR I restriction sites were set at the N-terminus and C-terminus respectively, and the above-mentioned apoA-I gene fragment The expression plasmid was obtained by inserting into the P. pastoris secreted expression vector through Xhol I and EcoRI double digestion.
Embodiment 2
[0032] Example 2 Expression plasmid electrotransformation Pichia pastoris GS115
[0033]The constructed expression plasmid pPIC9k-apoA-I was digested with BglII and linearized. The host bacteria P.pastorisGS115 (his mut+) was cultured to an OD of 1.6-1.8 to prepare electrocompetent cells, mixed with the above plasmids, and then electrotransformed with GIBCOL BRL electrotransformer CELL-PORATOR, where the voltage was 1500V, the capacitance was 50μF, and the resistance was 4kΩ , add 0.5ml 1mol / L cold sorbitol to the transformation cup, take 200μl and smear it on the MD plate, culture at 30℃ until a single colony appears, and more than 1000 transformants are obtained as a result.
Embodiment 3
[0034] Example 3 Screening of highly resistant Pichia recombinant strains
[0035] Spread more than 1000 transformants on YPD plates containing G418 at 1mg / ml, 2mg / ml, 3mg / ml, and 4mg / ml in turn, and culture them at 28-30°C. As a result, 22 transformants were obtained on plates containing 4mg / mlG418 Turn.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com