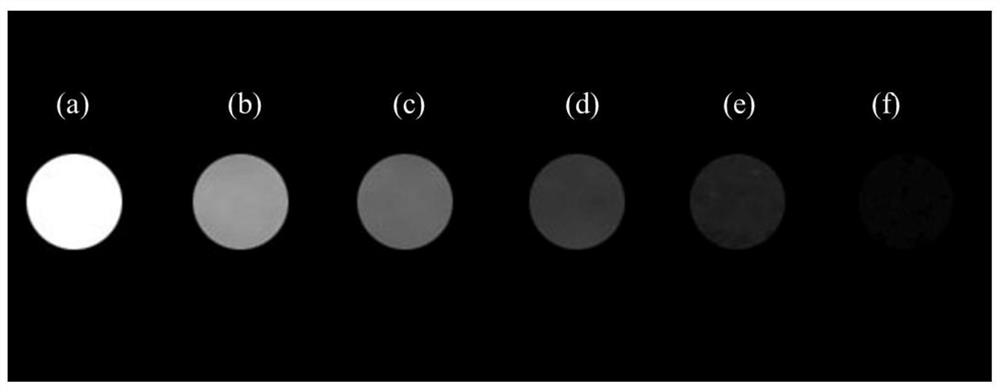
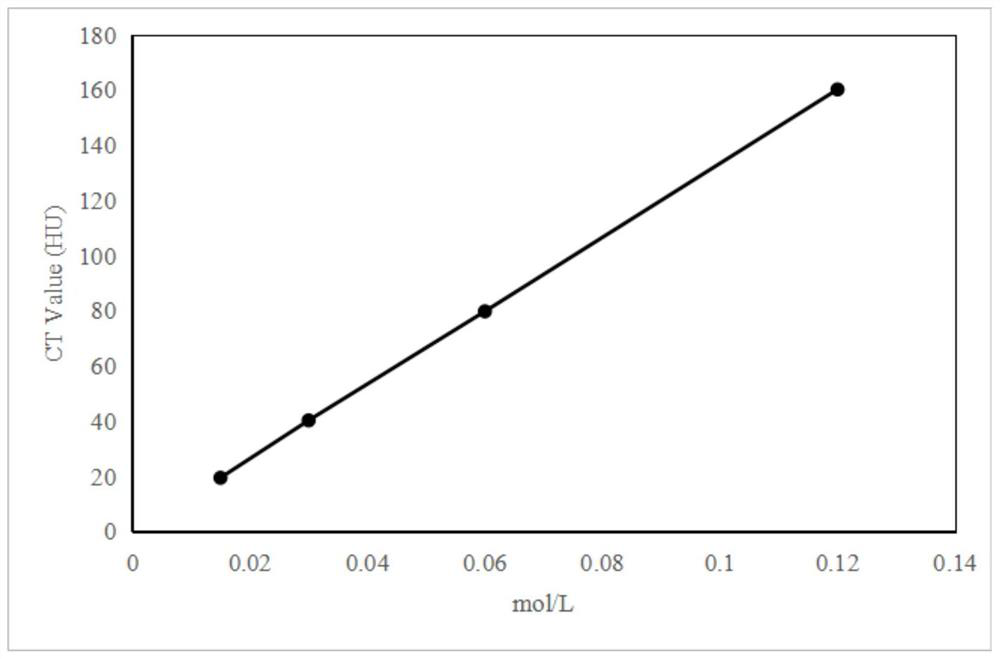
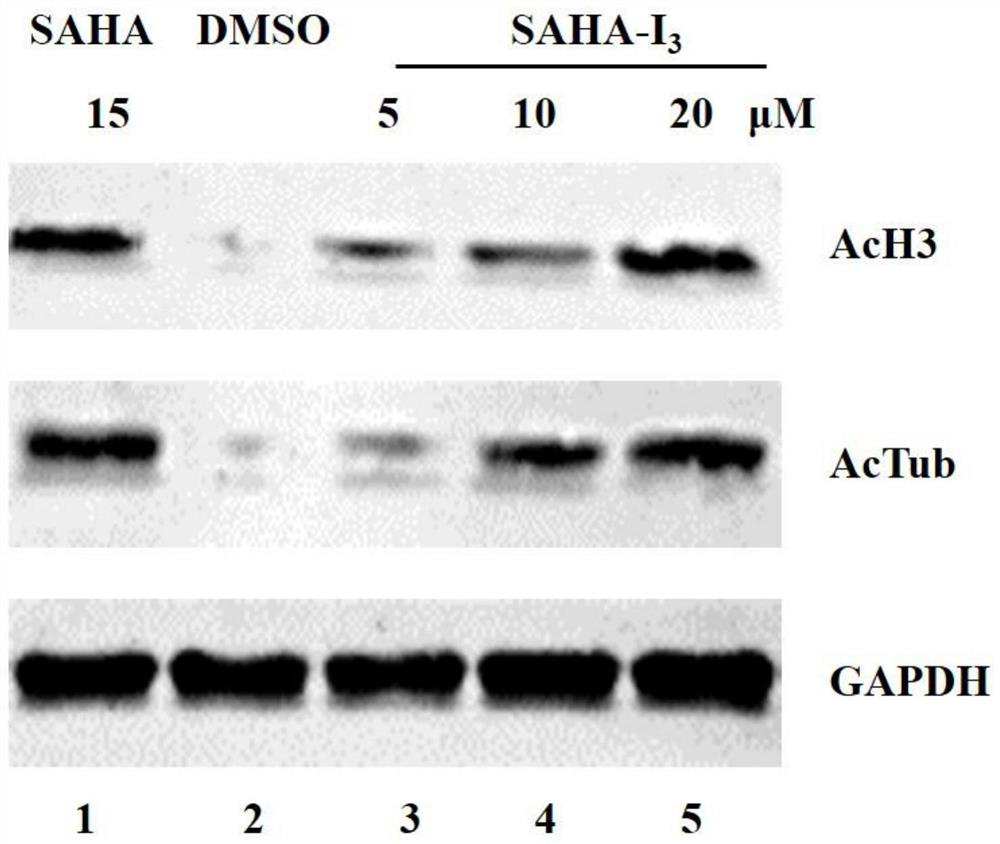
Diagnosis and treatment integrated compound as well as preparation method and application thereof
One compound, one technology, applied in the field of medicine, can solve problems such as undeveloped cancer treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In the second aspect, the embodiment of the present invention also proposes a method for preparing the integrated compound of diagnosis and treatment as described above, including the following steps: synthesizing Boc-2,3,5-triiodobenzoic acid: combining 2,3,5-triiodobenzene Formic acid was dissolved in t-BuOH, then DPPA and TEA were added, and the mixture was stirred at 85-95 °C for 1.5-2.5 h under a nitrogen atmosphere, then the mixture was desolvated under reduced pressure, the residue was dissolved in DCM, washed with saturated NaHCO 3 The organic phase was obtained by washing, and then the organic phase was washed with anhydrous Na 2 SO 4 After drying, the organic phase was concentrated, and the residue was purified by silica gel column chromatography, eluting with PE / EA, to obtain Boc-2,3,5-triiodobenzoic acid; to synthesize 2,3,5-triiodoaniline: Boc -2,3,5-Triiodobenzoic acid dissolved in CH 2 Cl 2 In solution, at -5-5°C to CH of Boc-2,3,5-triiodobenzoic acid ...
Embodiment 1
[0052] The preparation method of the integrated compound of diagnosis and treatment comprises the following steps: synthesizing Boc-2,3,5-triiodobenzoic acid: dissolving 2,3,5-triiodobenzoic acid in t-BuOH. Then DPPA and TEA were added, and the mixture was stirred at 85 °C under nitrogen atmosphere for 1.5 h, then the mixture was desolvated under reduced pressure, the residue was dissolved in DCM, washed with saturated NaHCO 3 The organic phase was obtained by washing, and then the organic phase was washed with anhydrous Na 2 SO 4 After drying, the organic phase was concentrated, and the residue was purified by silica gel column chromatography, eluting with PE / EA (V:V=10:1), to obtain Boc-2,3,5-triiodobenzoic acid;
[0053] Synthesis of 2,3,5-triiodoaniline: Dissolving Boc-2,3,5-triiodobenzoic acid in CH 2 Cl 2 In solution, at -5°C to CH of Boc-2,3,5-triiodobenzoic acid 2 Cl 2 TFA was added to the solution, and after consumption of the starting material, the mixture was e...
Embodiment 2
[0057] The preparation method of the integrated compound of diagnosis and treatment comprises the following steps: synthesizing Boc-2,3,5-triiodobenzoic acid: dissolving 2,3,5-triiodobenzoic acid in t-BuOH. Then DPPA and TEA were added, and the mixture was stirred at 95°C under nitrogen atmosphere for 2.5 hours, then the mixture was desolvated under reduced pressure, the residue was dissolved in DCM, washed with saturated NaHCO 3 The organic phase was obtained by washing, and then the organic phase was washed with anhydrous Na 2 SO 4 After drying, the organic phase was concentrated, and the residue was purified by silica gel column chromatography, eluting with PE / EA (V:V=10:1), to obtain Boc-2,3,5-triiodobenzoic acid;
[0058] Synthesis of 2,3,5-triiodoaniline: Dissolving Boc-2,3,5-triiodobenzoic acid in CH 2 Cl 2 In solution, at 5°C to CH of Boc-2,3,5-triiodobenzoic acid 2 Cl 2 TFA was added to the solution, and after consumption of the starting material, the mixture was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com