Patents
Literature
36 results about "O-iodoaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
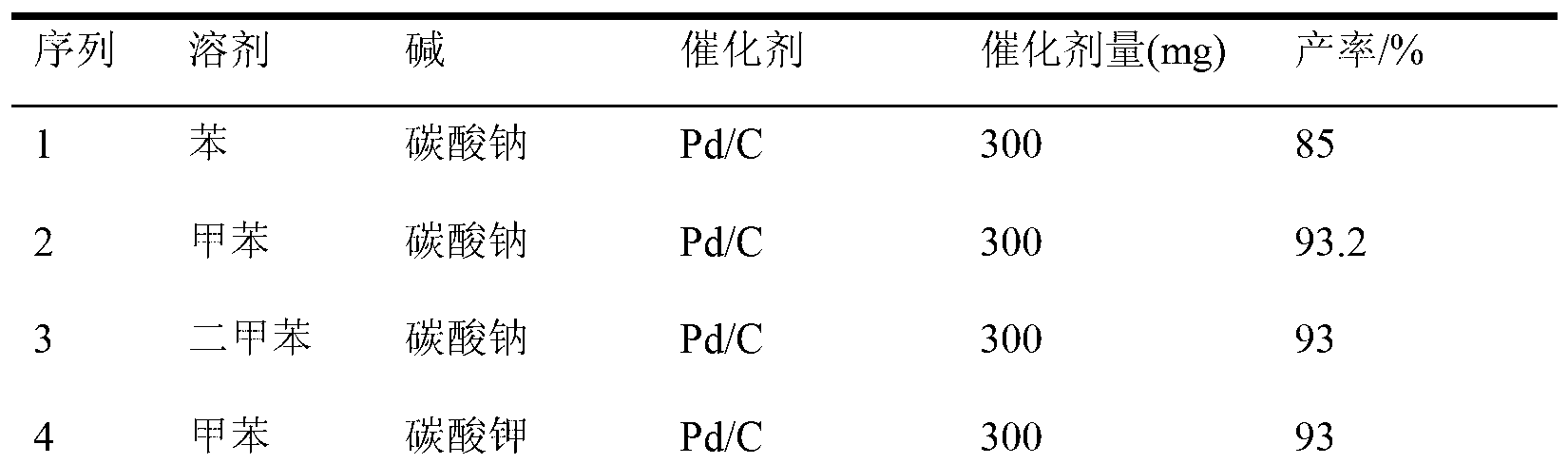
Preparation method of Boscalid
InactiveCN103073489AReduce manufacturing costHigh yieldAmino preparation from aminesAlkaline waterChlorobenzene
The invention discloses a preparation method of Boscalid, which comprises the following steps that Suzuki coupling reaction occurs between o-iodoaniline and 4-chlorobenzene boronic acid to produce an intermediate product 4'-chlorinated biphenyl-2-amine under the catalytic effect of Pd / C by using methylbenzene and alkaline water as mixed solvent; and condensation reaction occurs between the 4'-chlorinated biphenyl-2-amine and 2-chloronicotinyl chloride to produce the Boscalid. The preparation method of the Boscalid has the advantages that the production cost is reduced, the yield of the intermediate product 4'-chlorinated biphenyl-2-amine is improved, the total yield of the two steps exceeds 84 percent and the industrial production is expected to be realized.
Owner:LIMIN CHEM CO LTD
Preparation method of 2-trifluoromethyl substituted quinazolinone derivative
PendingCN112125856AStrong designabilityImprove reaction efficiencyOrganic chemistrySolid carbonPtru catalyst
The invention discloses a preparation method of a 2-trifluoromethyl substituted quinazolinone derivative, which comprises the following steps of: adding a palladium catalyst, a ligand, a carbon monoxide substitute, an additive, trifluoroethyl imine acyl chloride and o-iodobenzidine into an organic solvent, reacting at 90 DEG C for 16-30 hours, and after the reaction is completed, carrying out post-treatment to obtain the 2-trifluoromethyl substituted quinazolinone derivative. The preparation method has the advantages of simple operation, cheap and easily available reaction starting raw materials, compatibility with a plurality of substituents, good substrate applicability, realization of synthesis of trifluoromethyl quinazolinone derivatives substituted by different groups through substrate design, convenience in operation, and higher practicality. According to the method, 1, 3, 5-triformic acid phenol ester is used as a solid carbon monoxide substitute, so that the use of toxic and colorless gas carbon monoxide is avoided.
Owner:ZHEJIANG SCI-TECH UNIV
Preparation method of boscalid
ActiveCN104016915ALow costEasy to recycleOrganic compound preparationCarboxylic acid amides preparationPalladium catalystSolvent
The invention relates to the field of compound preparation, particularly a preparation method of a novel bactericide boscalid. The method comprises the following steps: in a polar aprotic solvent or high boiling solvent, carrying out Suzuki reaction on a palladium catalyst p-chlorophenylboric acid and o-acetaminobrombenzene in the presence of alkali and quaternary ammonium salt to obtain an intermediate product 4'-chloro-2-acetaminobiphenyl; and deprotecting the intermediate product in the presence of concentrated hydrochloric acid, and condensing with 2-chloronicotinoyl chloride. According to the method, the Pd(OH)2 / C is used as the catalyst to perform the Suzuki coupling reaction, and is recovered and reutilized many times; and thus, compared with the prior art, the method provided by the invention greatly saves the catalyst cost, is convenient for recovering the catalyst, and avoids using the high-cost raw material o-iodoaniline.
Owner:CHONGQING TECH & BUSINESS UNIV +1
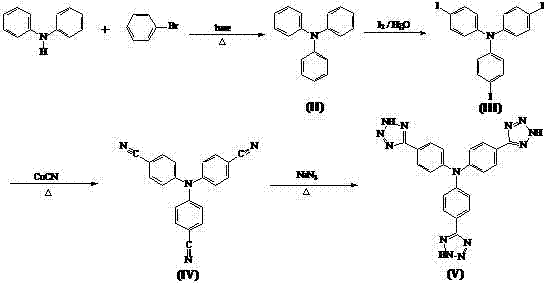
Synthesis method of tris-(4-tetrazolyl-phenyl)amine
The invention relates to a synthesis method of tris-(4-tetrazolyl-phenyl)amine, belonging to the field of material chemistry. The technical scheme of the invention is as follows: reacting diphenylamine with halo-benzene under a basic condition to obtain triphenylamine, carrying out substitution reaction on triphenylamine with iodine to generate tris-(4-iodophenyl)amine, then reacting tris-(4-iodophenyl)amine with CuCN to generate tris-(4-cyanophenyl)amine, and finally reacting tris-(4-cyanophenyl)amine with sodium azide to generate tris-(4-tetrazolyl-phenyl)amine. The porous material built by reacting obtained tris-(4-tetrazolyl-phenyl)amine with a metal has the stability of carboxylic acid porous frames, achieves intra-pore functionalization, and has pores in a mesoporous size. The adsorption capacity with respect to specific sulfur-containing small molecules shows that the porous material has potential application value in developing high-performance petrochemical products.
Owner:扬州三友合成化工有限公司
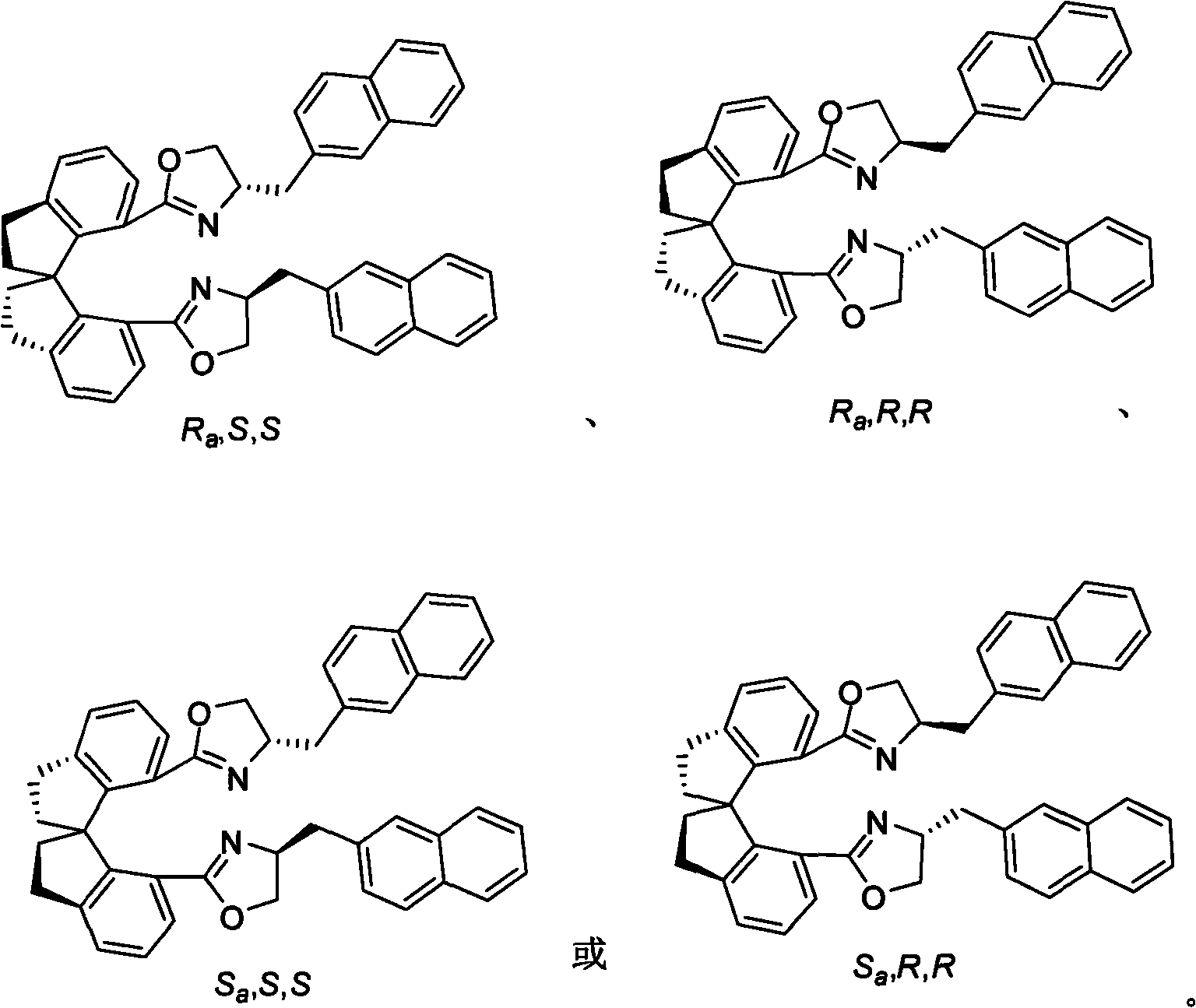
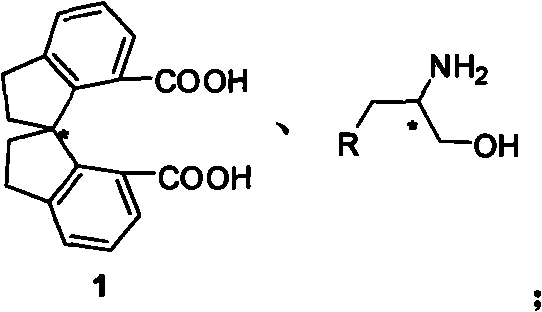
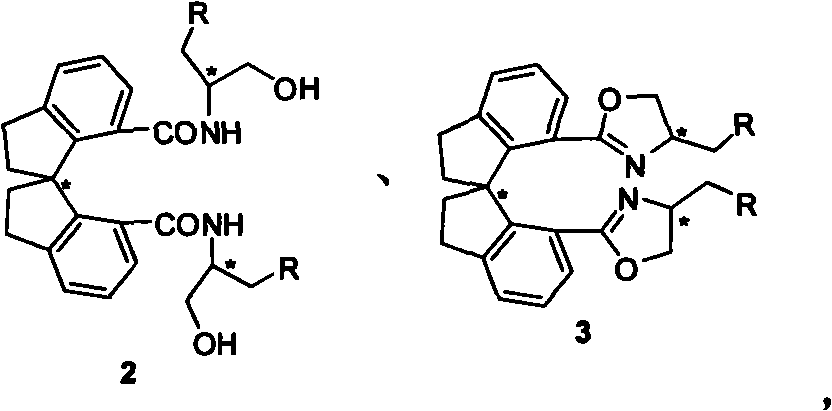
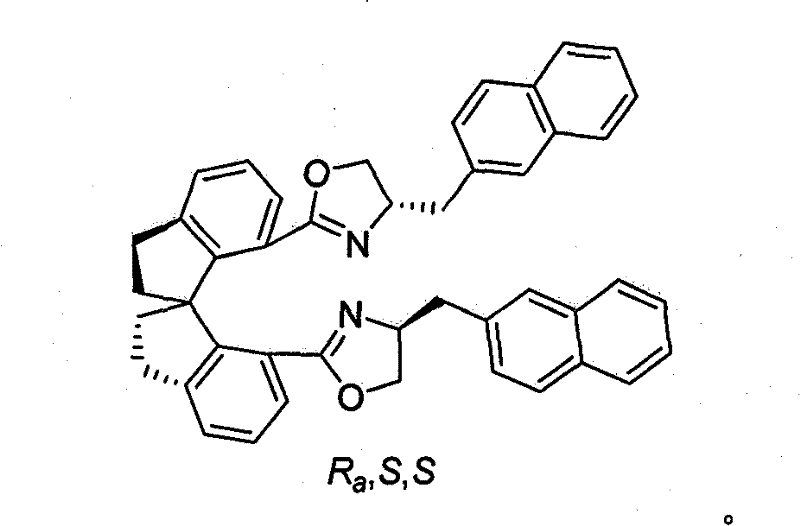
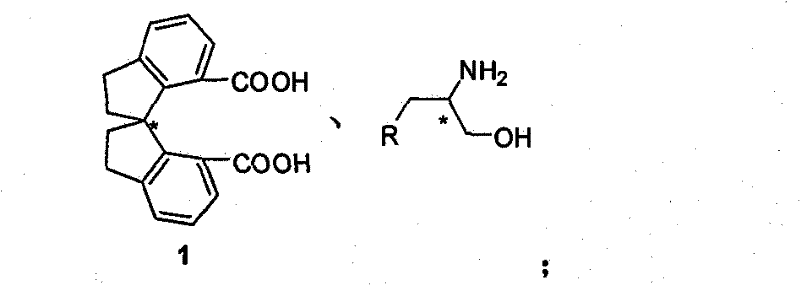
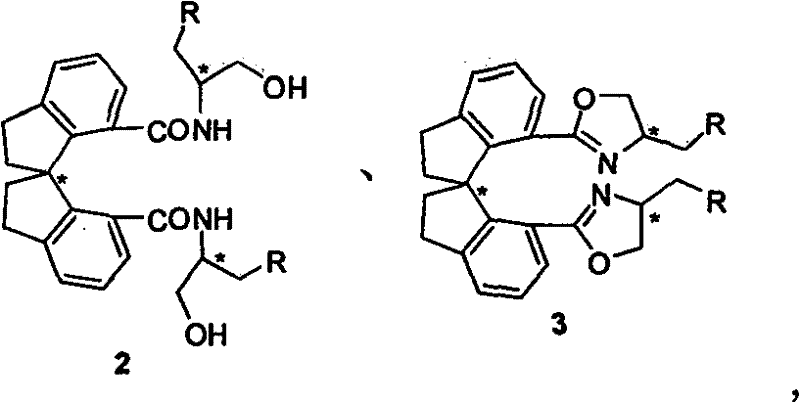
Beta-naphthyl methyl substituted spiral bisoxazoline ligand, synthetic method and application thereof
ActiveCN101671313AOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesRegioselectivityDrug biological activity
The invention relates to a beta-naphthyl methyl substituted spiral bisoxazoline ligand, a synthetic method and an application. The ligand has the advantages of simple synthetic method, mild conditionand applicability for industrialization production. The ligand has a spiral framework and a feature of a plurality of chiral centers, therefore the ligand has high catalytic activity and chiral induction effect and strong adjustment capacity in an asymmetric catalytic reaction of transition metals. Pyrrolidine derivants with various optical activities are prepared through high region selectivity and high enantioselectivity from allenoic and o-iodoaniline by the catalysis of the ligand. With the important biological activity, the pyrrolidine derivant has a better prospect of industry and medicine applications.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
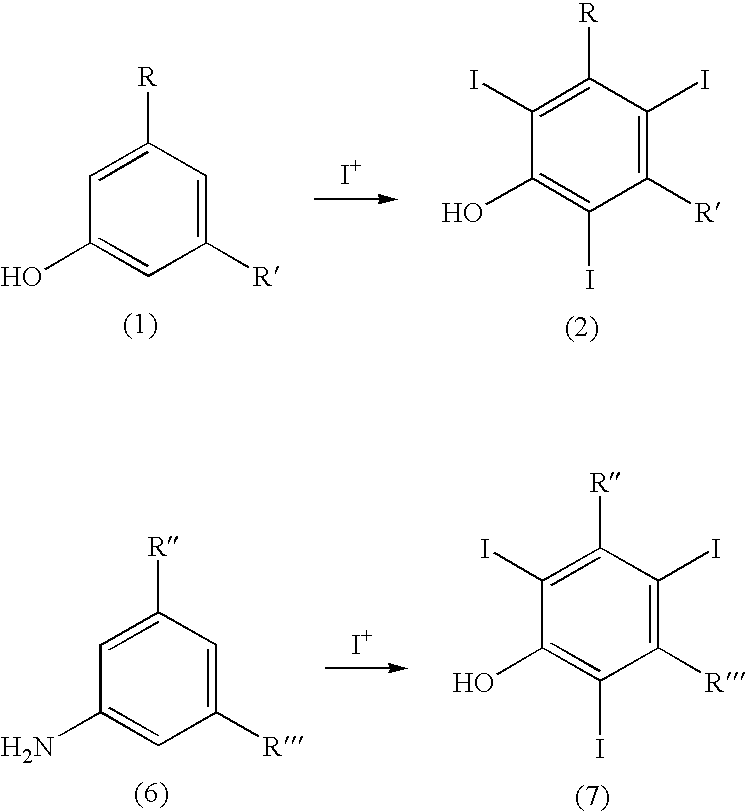
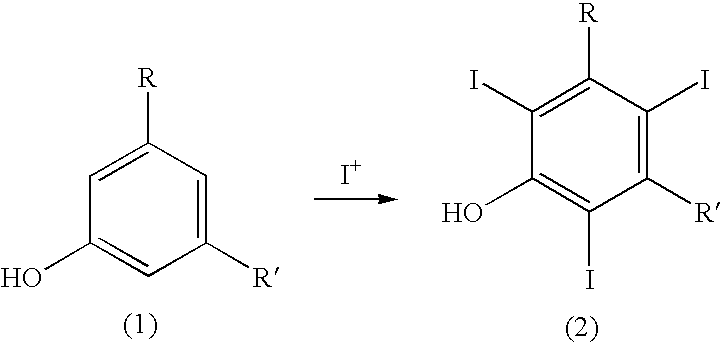
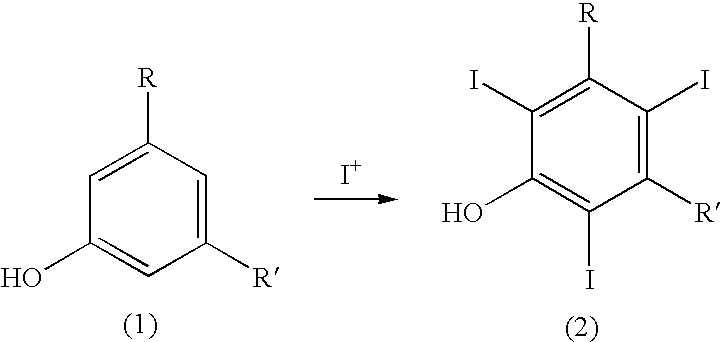
Process for the iodination of aromatic compounds
ActiveUS20100331567A1High purityHigh yieldElectrolysis componentsOrganic compound preparationAnilinePhenol
The present invention relates to a process for the preparation of iodinated phenols, —in particular, it relates to a process including the electrochemical iodination of 3,5-disubstituted phenols of formula (1) to the corresponding 3,5-disubstituted-2,4,6-triiodophenols of formula (2), which are useful intermediates for the synthesis of x-ray contrast media, and to the preparation of the contrast media themselves. Furthermore, the present invention includes the electrochemical iodination of 3,5-disubstituted anilines of formula (6) to the corresponding 3,5-disubstituted-2,4,6-triiodoanilins of formula (7).
Owner:BRACCO IMAGINIG SPA
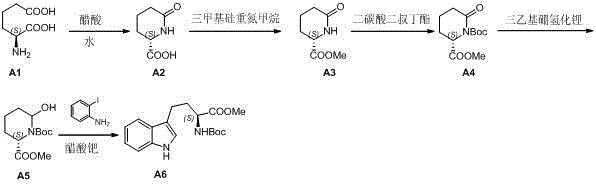
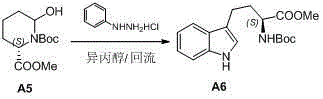
Preparation method of L-N-Boc-high tryptophan methyl ester
InactiveCN102911106AEasy to operateSimple post-processingOrganic chemistryPtru catalystPyrrolidinones
The invention discloses a preparation method of L-N-Boc-high tryptophan methyl ester, which can be mainly used for solving the problems that the reaction steps are more, the cost is low, the operation is difficult, and the single chirality of a final compound can not be ensured in an existing synthesizing method. The preparation method comprises the steps of conducting cyclization reaction to generate L-2-pyrrolidone-6-formic acid 1 by taking L-2-amino adipic acid as initial materials under the action of glacial acetic acid and water; 2. conducting an esterification reaction on the compound 1 and trimethyl silicon diazomethane to obtain L-2-pyrrolidone-6-methyl ester 2; 3. protecting N in the compound 2 by using Boc, then conducting the reduction reaction due to the action of a reducing agent, namely lithium triethylborohydride, and reducing carbonyl in the L-2-pyrrolidone-6-methyl ester protected by N-tert-butylcarbazate; and 4. finally synthesizing the high tryptophan methyl ester protected by the L-N-tert-butylcarbazate by two methods: synthesizing classical fisher benzazole in one method, and removing one molecular water by L-2-pyrrolidinol-6-methyl ester and iodoaniline, rearranging, and conducting Heck reaction under the action of a palladium catalyst to obtain the L-N-Boc-high tryptophan methyl ester in the other method.
Owner:SHANGHAI STA PHARMA R&D CO LTD
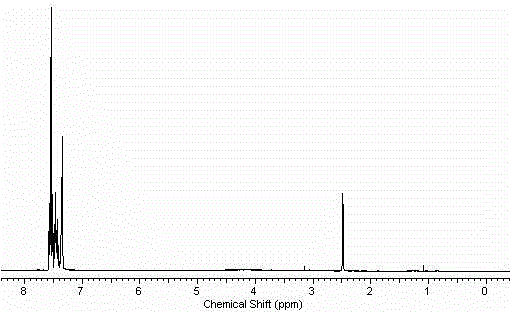
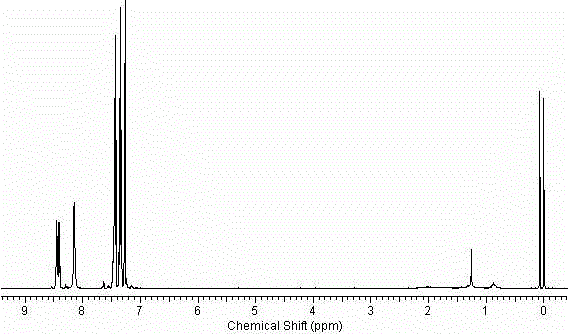
9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof
ActiveCN108727376AEasy post-processingHigh yieldOrganic chemistryX-ray constrast preparationsNonaneOrganic solvent
The invention discloses 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds. A preparation method of the compound comprises steps as follows: 9-nitrogen(6'-amino)hexylamine-9-9-aza-bicyclo[3.3.1] nonane-3 alpha-alkylamidophenyl formate and a chloroacetyl triiodoaniline derivative are mixed in an organic solvent in the mole ratio being 1:(1.0-1.5) and subjected to a reaction at the room temperature under the catalytic action of cesium hydroxide for 20-30 h, and a product, namely, the 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds, is obtained. The invention also discloses an application of the 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as a contrast medium for early diagnosis of breast cancer.
Owner:CHINA JILIANG UNIV
4-iodophenyl substituted carborane derivative and preparation method thereof
PendingCN112079856AIncrease profitImprove solubilityGroup 3/13 element organic compoundsBoronic acidCombinatorial chemistry
The invention discloses a 4-iodophenyl carborane derivative and a preparation method thereof. Commercialized organic reagents 4-iodoaniline and a diacetonitrile decaborate complex and several easily-synthesized substituted alkynes are adopted as the starting raw materials, carrying out diazotization reaction, Sonogashira coupling reaction, iodine exchange and alkyne addition reaction are carried out to form a series of 4-iodophenyl carborane with different substituent groups. The preparation method provided by the invention has the advantages of strong group applicability, easy purification and separation, high synthesis yield and high reference value.
Owner:YANCHENG INST OF TECH
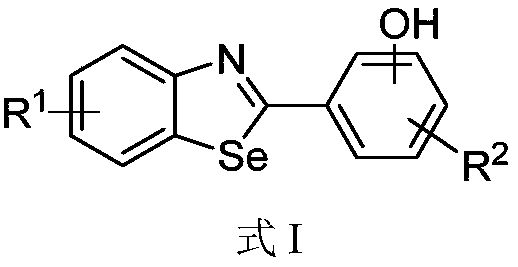
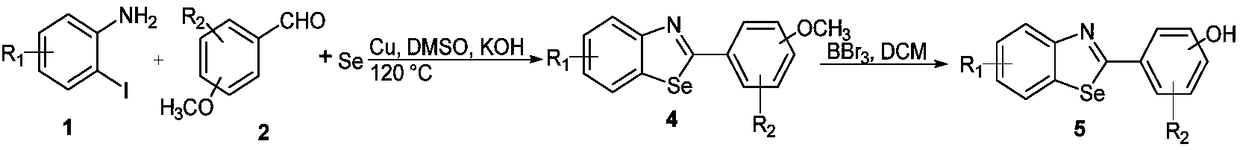
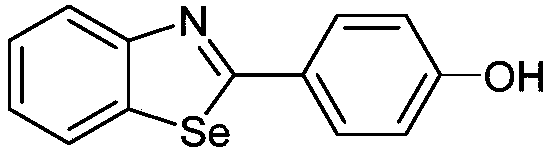
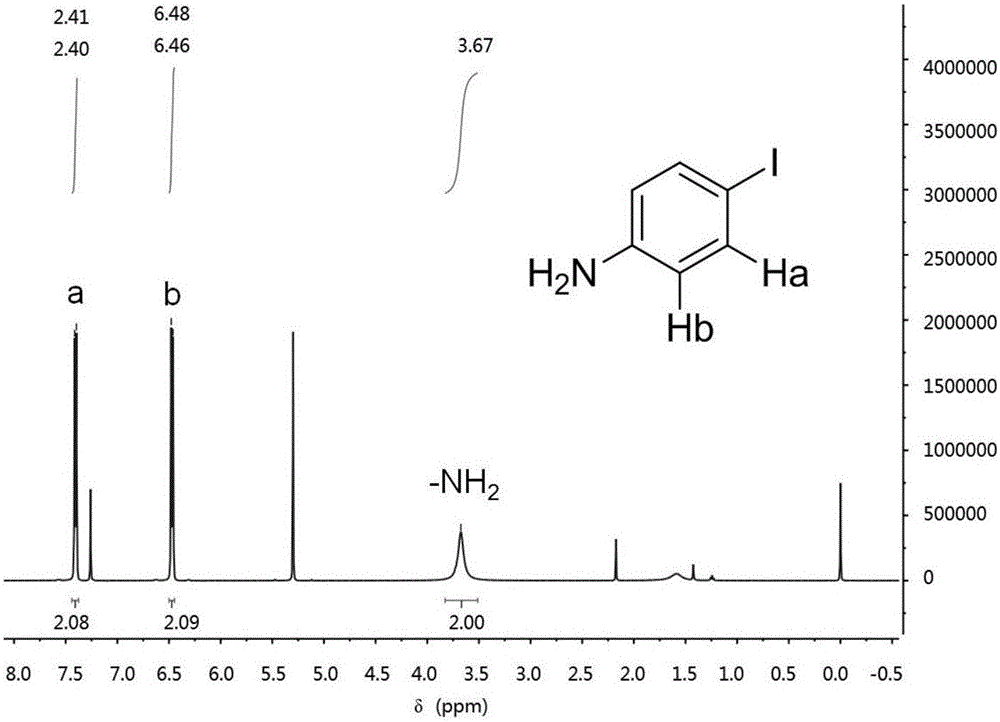
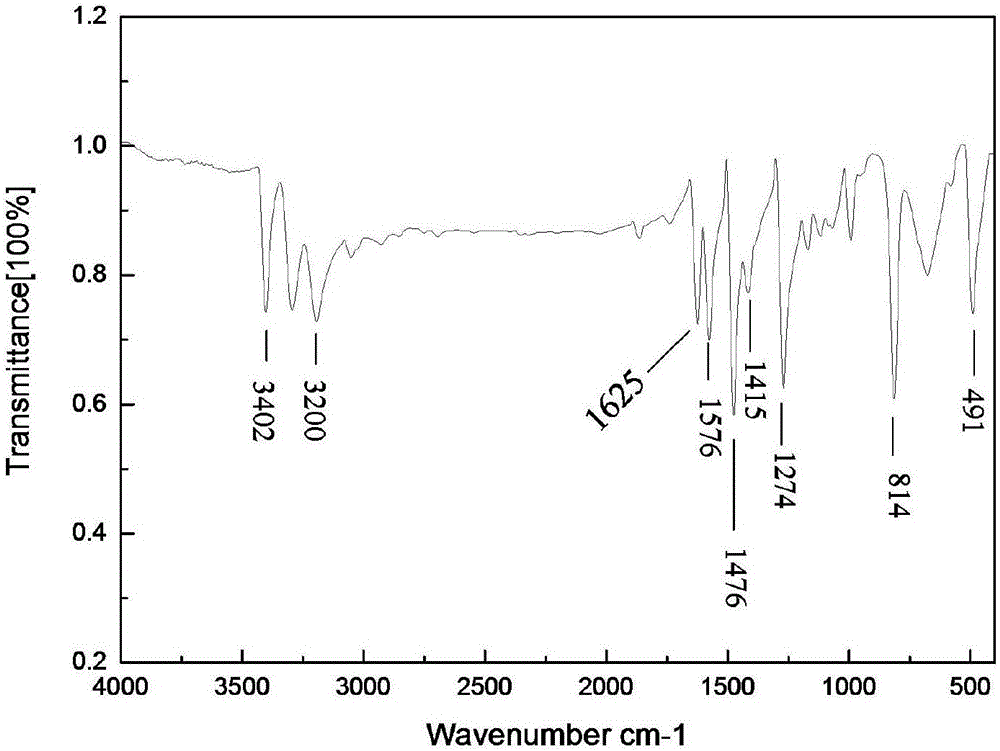
Benzoselenazole compound as well as preparation method and application thereof
InactiveCN108863985AERβ agonistic activity is goodMild conditionsOrganic chemistryAntineoplastic agentsDiseaseOestrogen receptor
The invention discloses a benzoselenazole compound as well as a preparation method and application thereof and belongs to the technical field of medicines. The preparation method of the benzoselenazole compound is characterized by preparing a series of methoxy-containing benzoselenazole compounds from o-iodoaniline derivatives, benzaldehyde derivatives and selenium under catalysis of copper through a one-pot process, and finally carrying out boron tribromide demethylation to obtain a hydroxy-containing benzoselenazole compound. In-vitro experiments show that the benzoselenazole compound as a ligand of an oestrogen receptor ER beta shows excellent agonist effect; the correlation research shows that the compound as an ERb ligand has treatment effect in animal model of rheumatic arthritis andintestinal diseases caused by inflammation, so that the benzoselenazole compound has potential treatment effect in treatment of rheumatic arthritis and intestinal diseases.
Owner:WUHAN UNIV
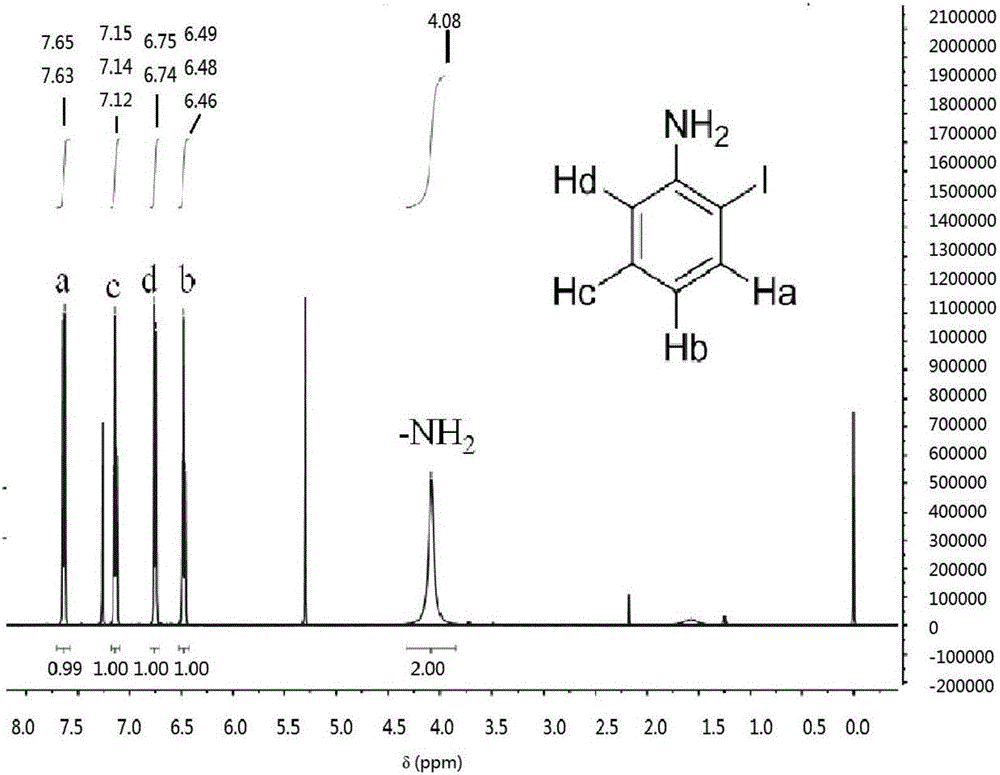
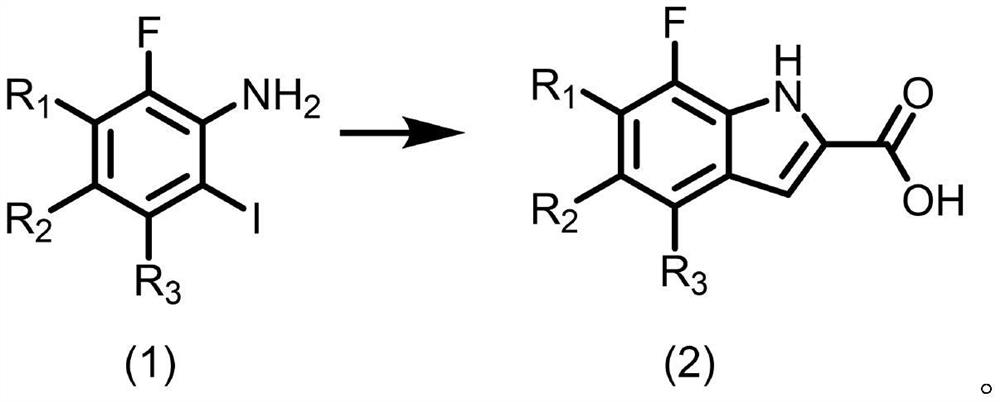
Preparation method of o-iodoaniline
ActiveCN106542958AWide variety of sourcesLow priceHalogenated hydrocarbon preparationGreen environmentSodium bicarbonate
The invention discloses a preparation method of o-iodoaniline. The synthesis route is represented by a formula in the invention. The preparation method possesses following advantages: raw material aniline is widely available and cheap, and is beneficial for industrialized utilization of the preparation method; in a certain solvent, sodium bicarbonate / I2 are used for preparing paraiodoaniline via direct iodination of aniline, and yield is high; paraiodoaniline is taken as a raw material, a certain catalyst is adopted, and rearrangement reaction is carried out so as to realize chemical conversion of paraiodoaniline into o-iodoaniline, processing steps are few, conditions are mild, and green environment-protection requirements are satisfied; and both paraiodoaniline and o-iodoaniline are preferable pharmaceutical intermediates.
Owner:GUIZHOU UNIV
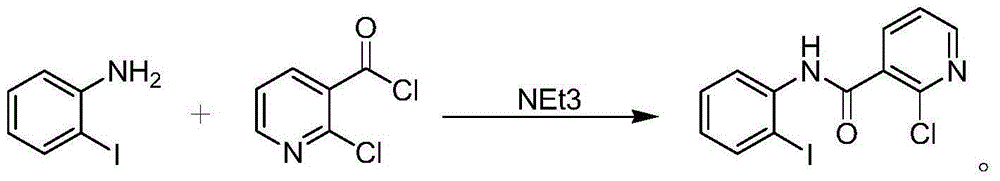
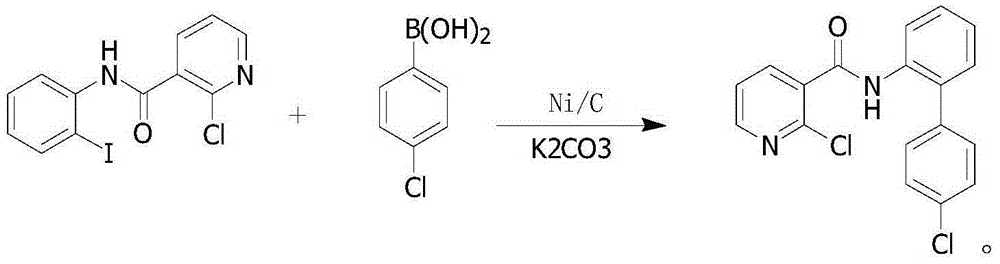
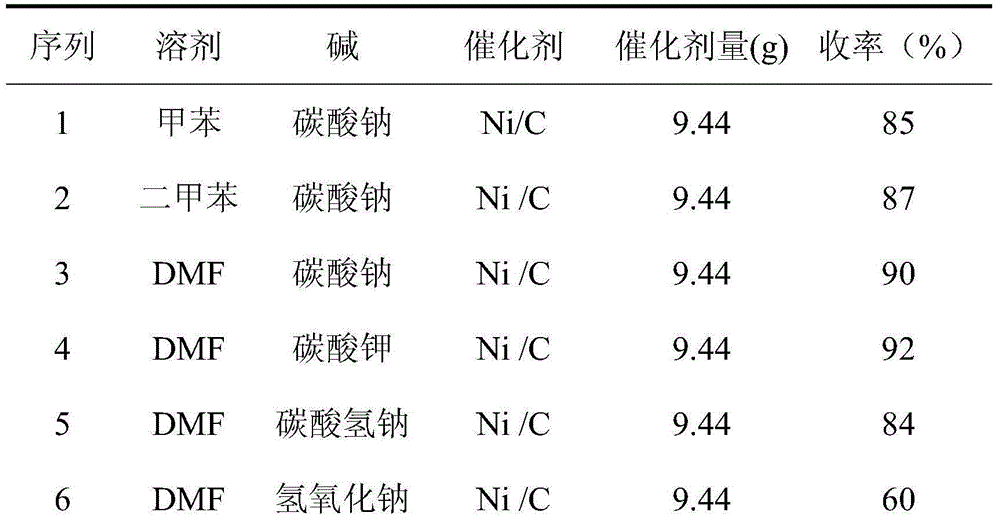
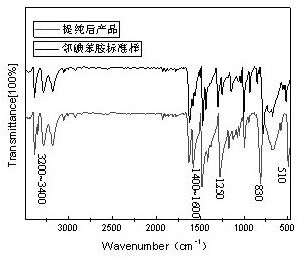
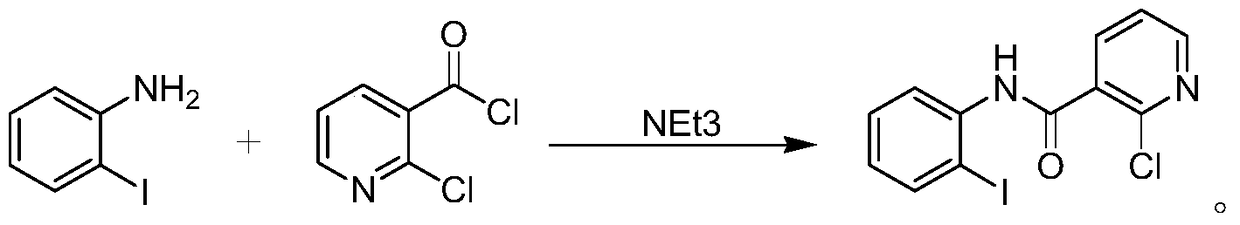
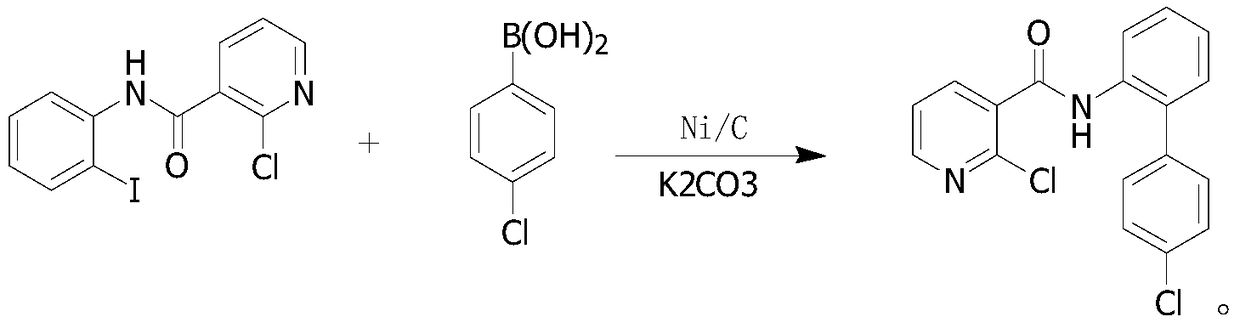
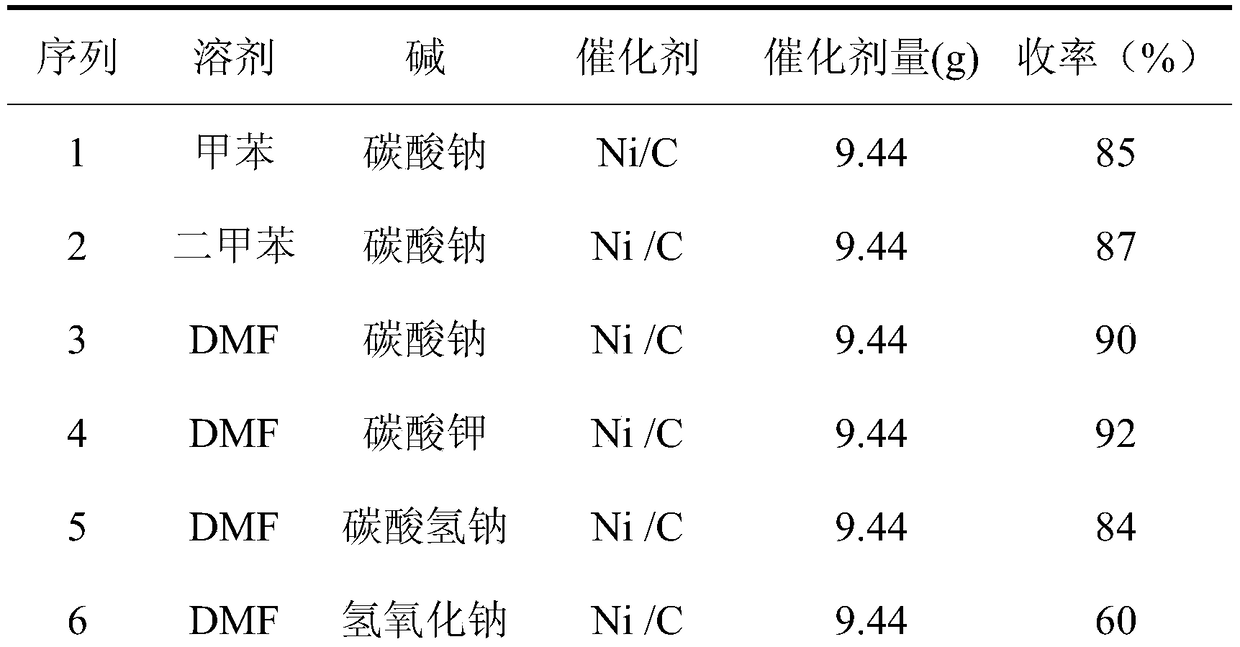
Preparation method of boscalid
ActiveCN105085389AReduce manufacturing costRaw materials are easy to getBiocideOrganic chemistryChloridePyridine
A preparation method of boscalid comprises following steps: o-iodoaniline and 2-chloronicotinyl chloride have a condensation reaction to produce an intermediate product 2-chloro-N-(2-iodophenyl)pyridine-3-formamide;2-chloro-N-(2-iodophenyl)pyridine-3-formamide which is not purified has a Suzuki coupling reaction with 4-chlorophenylboronic acid directly, and boscalid is produced. A Ni / C preparation method is provided, and Ni / C is taken as a catalyst for the Suzuki coupling reaction, so that the production cost is greatly reduced; further, production raw materials are available, the yield is high, and the preparation method of boscalid is applicable to large-scale industrial production.
Owner:利民化学有限责任公司
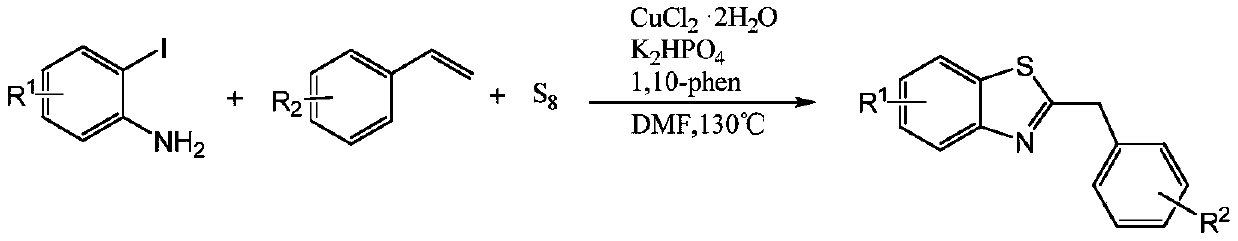
Method for preparing 2-benzothiazole substituted aniline compound under catalysis of copper compound
ActiveCN112239431AEasy to operateRaw materials are easy to getOrganic chemistryThiazoleOrganic synthesis
The invention belongs to the field of organic synthesis and metal catalysis, and discloses a method for preparing a 2-benzothiazole substituted aniline compound through catalysis of a copper compound.According to the invention, elemental sulfur which is cheap, easy to obtain, low in toxicity, easy to operate, stable and odorless is used as a sulfur source, commercially available isatin derivatives and 2-iodobenzidine are used as raw materials, and a series of 2-benzothiazole substituted aniline compounds are obtained at high yield through a cascade reaction under the catalytic action of a copper compound; 2-aminophenylmercaptan with unpleasant odor is prevented from being used as a raw material, and the invention has the advantages of simple reaction operation, mild conditions, easily available raw materials, short synthesis route and the like.
Owner:GUANGZHOU UNIVERSITY
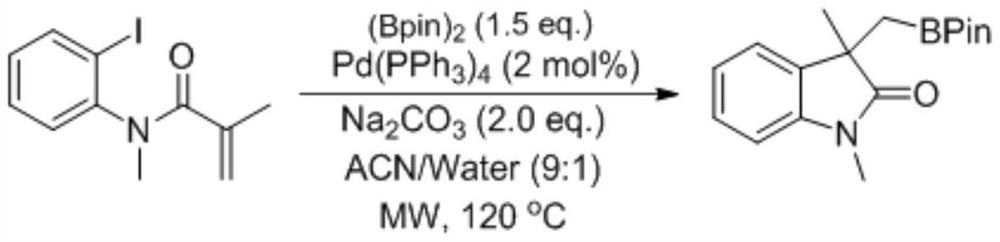
Preparation method of boron-containing indolinone derivative
ActiveCN111057080AImprove toleranceReaction raw materials are readily availableGroup 3/13 element organic compoundsBulk chemical productionPtru catalystOrganic solvent
The invention discloses a preparation method of a boron-containing indolinone derivative, particularly relates to a method for synthesizing the boron-containing indolinone derivative by catalyzing a cyclization reaction of an o-iodobenzidine derivative and bis(pinacolato)diboron through copper, and belongs to the field of organic synthesis. The method comprises the steps: taking the o-iodobenzidine derivative and bis(pinacolato)diboron as raw materials, carrying out a reaction in an organic solvent at the temperature of 40-140 DEG C under the catalysis of a copper catalyst and the combined action of a ligand and an alkaline compound, and after the reaction is finished, carrying out post-treatment to obtain the boron-containing indolinone derivative. The o-iodobenzidine derivative which iseasy to prepare and commercially available bis(pinacolato)diboron are used as raw materials, and rapid construction of the boron-containing indolinone derivative is realized through one-step construction of a ring and two chemical bonds. The method is mild in reaction condition and simple and convenient to operate, and has the advantages of easily available reaction raw materials, wide substrate applicability, easy separation of target products and the like.
Owner:ZHEJIANG UNIV OF TECH
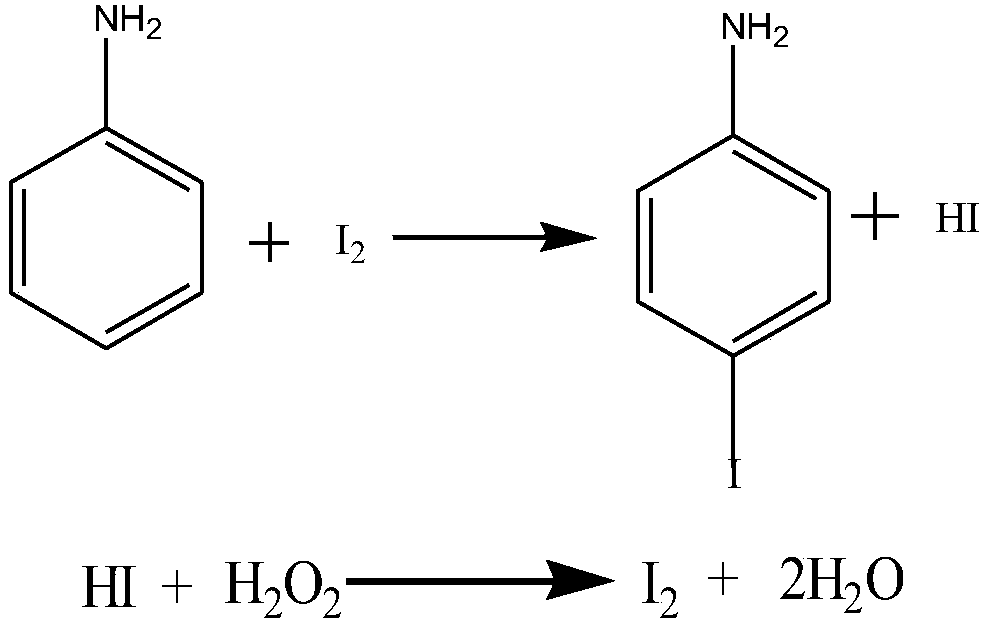
P-iodoaniline and preparation method thereof
PendingCN110724061AProcess pollution is smallAmino compound purification/separationOrganic compound preparationOrganic synthesisSulfite salt
The invention provides p-iodoaniline and a preparation method thereof, and belongs to the technical field of organic synthesis. A purpose of the invention is to solve the problems of excessive elemental iodine consumption and increased three wastes caused by a large amount of sulfate generated by reaction in the existing p-iodoaniline preparation method. The method comprises: 1, adding aniline andwater into a first reaction container according to a certain ratio, adding elemental iodine into the first reaction container in a dropwise manner, stirring to prepare a first solution, cooling the first solution to 0-20 DEG C, adding hydrogen peroxide with a mass concentration of 30% in a dropwise manner, and carrying out sampling analyzing until the mass percentage content of aniline is less than 0.5% to obtain a second solution; 2, adding sodium sulfite into the second solution, stirring for 0.4-0.5 h to obtain a third solution, and carrying out suction filtration on the third solution toobtain a brown crystal; and 3, pouring the brown crystal into a second reaction container, and carrying out extraction purification treatment to obtain the p-iodoaniline finished product. According tothe invention, with the method, the consumption of elemental iodine is low, the crude product content is high, and the generation of sulfate is avoided.
Owner:新岸诺亚(北京)催化科技有限公司 +1
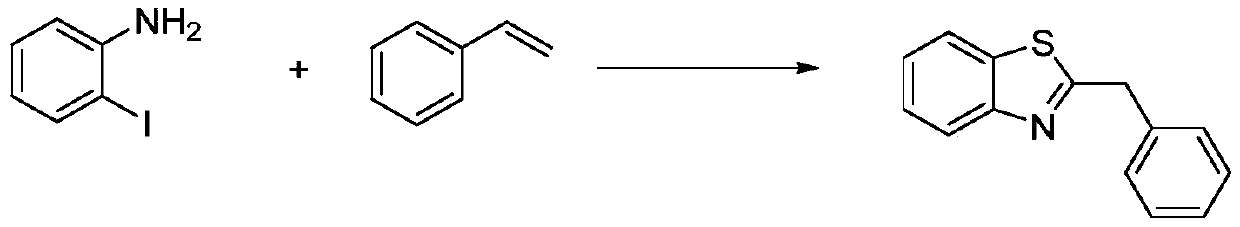
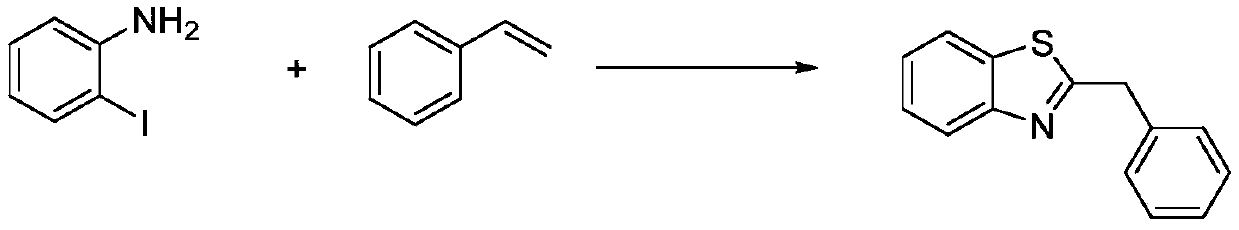
Method for synthesizing 2-substituted benzothiazole by one-pot method
The invention relates to a method for synthesizing 2-substituted benzothiazole by a one-pot method. The preparation method comprises the following specific steps: dissolving a 2-Iodoaniline compound,a styrene compound, elemental sulfur, an alkaline medium, a catalyst and a ligand in an organic solvent, and stirring for 10-24 hours at 100-140 DEG C to react to obtain 2-substituted benzothiazole; wherein the molar ratio of the 2-Iodoaniline compound to the styrene compound is 1: (1-3); and cooling, extracting, drying and column chromatography separation are carried out to obtain a pure product.The method has the advantages that (1) the reaction conditions are mild, the reaction activity is high, the reaction yield is high, the product selectivity is high, and the substrate expansion rangeis wide; (2) use of a large amount of solvents and noble metal compounds in a catalyst system is avoided, cost is low, the method is safe and convenient, and low environmental pollution is caused by the reaction system; and (3) no mixed solvent or oxygen is used in the reaction system, so that potential risks existing in oxygen use are avoided, and a reference value is provided for industrial application.
Owner:NANJING UNIV OF TECH
A kind of preparation method of boron-containing indolinone derivative
ActiveCN111057080BImprove toleranceReaction raw materials are readily availableGroup 3/13 element organic compoundsBulk chemical productionPtru catalystOrganic solvent
The invention discloses a preparation method of boron-containing indolinone derivatives, in particular to a method for synthesizing boron-containing indolinone derivatives through the cyclization reaction of copper-catalyzed o-iodoaniline derivatives and biboronic acid pinacol esters , belongs to the field of organic synthesis. The method uses o-iodoaniline derivatives and biboronic acid pinacol ester as raw materials, and under the catalysis of copper catalyst, through the joint action of ligands and basic compounds, in an organic solvent, the reaction is carried out at a temperature of 40-140° C. , and the boron-containing indolinone derivatives are obtained after post-treatment after the reaction. The invention uses easily prepared o-iodoaniline derivatives and commercially available pinacol esters as raw materials, and realizes the rapid construction of boron-containing indolinone derivatives by constructing one ring and two chemical bonds in one step. The reaction conditions are mild, the operation is simple, and the reaction raw materials are readily available, the substrates are widely applicable, and the target products are easily separated.
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of benzothiazolone compound
The invention discloses a novel economical, mild, simple and efficient method for preparing a benzothiazolone compound. According to the method, o-iodoaniline, elemental sulfur and DMF are adopted as raw materials, the benzothiazolone compound is efficiently prepared under an economical and cheap copper catalysis system, a target product yield as high as 90% can be obtained within 3-hour reaction time only, and the synthesis strategy is compatible with wide-range functional groups.
Owner:WENZHOU UNIVERSITY
A kind of polyquinazoline compound and its preparation method and application
The invention discloses a polyquinazoline compound and its preparation method and application. The preparation method comprises the following steps: under the action of a catalyst and an alkali Multi-component polymerization reaction to obtain polyquinazoline compounds. The method of the invention has mild conditions, and the polymerization monomer is simple and easy to obtain. The polyquinazoline compound obtained in the invention has better solubility, processability and higher thermal stability, and has potential applications in the fields of metal ion detection and self-assembly.
Owner:SOUTH CHINA UNIV OF TECH
A kind of preparation method of substituted 2,3-dihydroquinolone compound
ActiveCN112239456BStrong designabilityWide range of toleranceSilicon organic compoundsPtru catalystPalladium catalyst
Owner:ZHEJIANG SCI-TECH UNIV
A kind of preparation method of boscalid
ActiveCN104016915BLow costEasy to recycleOrganic compound preparationCarboxylic acid amides preparationPalladium catalystRaw material
The invention relates to the field of compound preparation, particularly a preparation method of a novel bactericide boscalid. The method comprises the following steps: in a polar aprotic solvent or high boiling solvent, carrying out Suzuki reaction on a palladium catalyst p-chlorophenylboric acid and o-acetaminobrombenzene in the presence of alkali and quaternary ammonium salt to obtain an intermediate product 4'-chloro-2-acetaminobiphenyl; and deprotecting the intermediate product in the presence of concentrated hydrochloric acid, and condensing with 2-chloronicotinoyl chloride. According to the method, the Pd(OH)2 / C is used as the catalyst to perform the Suzuki coupling reaction, and is recovered and reutilized many times; and thus, compared with the prior art, the method provided by the invention greatly saves the catalyst cost, is convenient for recovering the catalyst, and avoids using the high-cost raw material o-iodoaniline.
Owner:CHONGQING TECH & BUSINESS UNIV +1
Preparation method of substituted 2,3-dihydroquinolone compound
ActiveCN112239456ASimple stepsGood response applicabilitySilicon organic compoundsPtru catalystPalladium catalyst
The invention discloses a preparation method of a substituted 2,3-dihydroquinolone compound, wherein the preparation method comprises the following steps: adding a palladium catalyst, a ligand, a carbon monoxide substitute, an additive, N-pyridylsulfonyl-o-iodobenzidine and olefin into an organic solvent, reacting at the temperature of 100-120 DEG C for 24-48 hours, and after the reaction is completed, carrying out aftertreatment to obtain the substituted 2,3-dihydroquinolone compound. The preparation method is simple to operate, cheap and easily available in initial raw materials, high in reaction efficiency and good in substrate compatibility, the 2-aryl and 3-alkyl substituted 2,3-dihydroquinolone compound can be synthesized through substrate design, the operation is convenient, and thepracticability of the method is broadened.
Owner:ZHEJIANG SCI-TECH UNIV
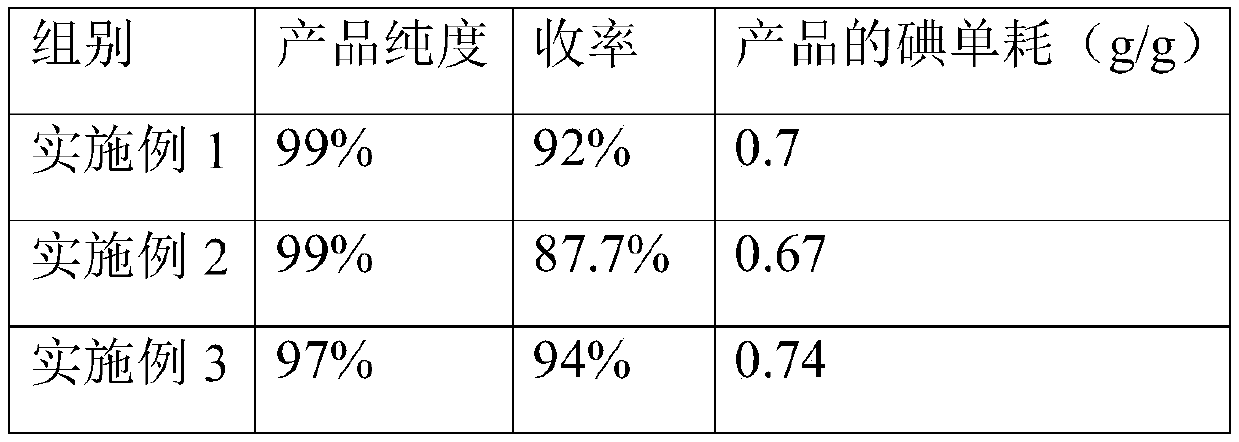
A method for melting and crystallizing separation and purification of o-iodoaniline
ActiveCN108047054BReduce lossesLow costAmino compound purification/separationOrganic solventPhysical chemistry
The invention discloses a method for melting, crystallizing, separating and purifying iodoaniline. The method comprises the following steps: 1) grinding an iodoaniline and paraiodoaniline mixture rawmaterial into powder to obtain a product A; 2) putting the product A into a crystallizer, starting the crystallizer, heating the product A and completely melting the product A to obtain a product B; 3) cooling the product B to perform crystallization, growing the crystal after the crystallization final temperature is arrived, and discharging mother liquid to obtain a product C; and 4) heating andsweating the product C step by step until the sweating final temperature and discharging the sweat, wherein the residual crystal is the purified iodoaniline. The method provided by the invention has the characteristics of no need of organic solvent, environmental friendliness, high separation and purification efficiency, small loss amount of iodoaniline, low cost and high purity of the iodoaniline, and is suitable for industrialized popularization.
Owner:GUIZHOU UNIV
The preparation method of boscalid
ActiveCN105085389BReduce manufacturing costRaw materials are easy to getBiocideOrganic chemistryChlorideFormamide
A preparation method of boscalid comprises following steps: o-iodoaniline and 2-chloronicotinyl chloride have a condensation reaction to produce an intermediate product 2-chloro-N-(2-iodophenyl)pyridine-3-formamide;2-chloro-N-(2-iodophenyl)pyridine-3-formamide which is not purified has a Suzuki coupling reaction with 4-chlorophenylboronic acid directly, and boscalid is produced. A Ni / C preparation method is provided, and Ni / C is taken as a catalyst for the Suzuki coupling reaction, so that the production cost is greatly reduced; further, production raw materials are available, the yield is high, and the preparation method of boscalid is applicable to large-scale industrial production.
Owner:利民化学有限责任公司
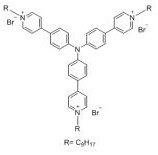
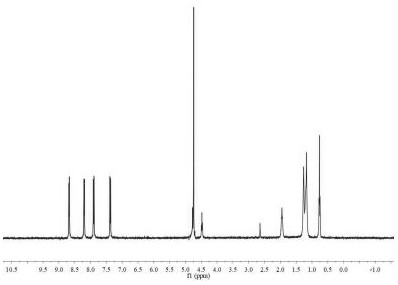
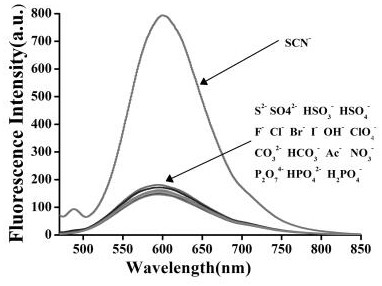
A fluorescent probe for recognizing thiocyanate and its preparation and recognition method
ActiveCN110423609BReduce manufacturing costNo pollutionOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
Owner:GUIZHOU UNIV
Beta-naphthyl methyl substituted spiral bisoxazoline ligand, synthetic method and application thereof
ActiveCN101671313BOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesMethyl palmoxirateDrug biological activity
The invention relates to a beta-naphthyl methyl substituted spiral bisoxazoline ligand, a synthetic method and an application. The ligand has the advantages of simple synthetic method, mild condition and applicability for industrialization production. The ligand has a spiral framework and a feature of a plurality of chiral centers, therefore the ligand has high catalytic activity and chiral induction effect and strong adjustment capacity in an asymmetric catalytic reaction of transition metals. Pyrrolidine derivants with various optical activities are prepared through high region selectivity and high enantioselectivity from allenoic and o-iodoaniline by the catalysis of the ligand. With the important biological activity, the pyrrolidine derivant has a better prospect of industry and medicine applications.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
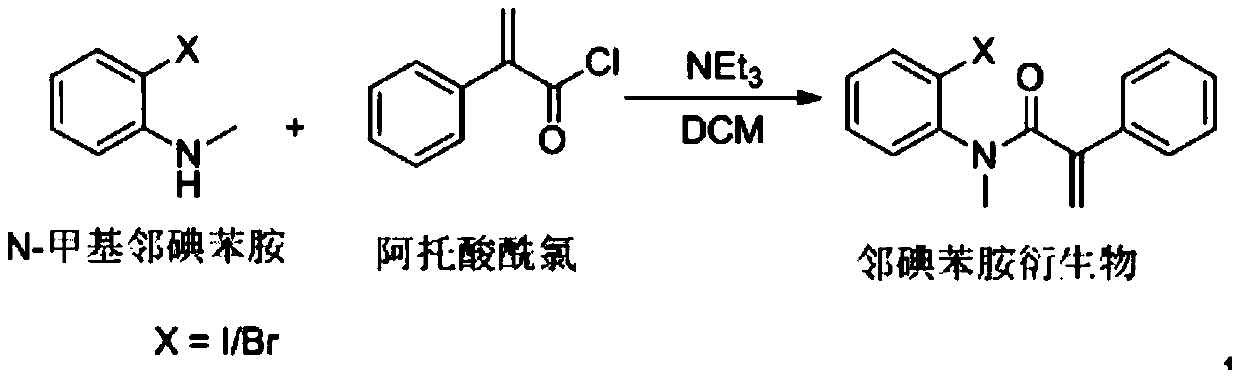
Preparation method of substituted iodobenzene with terminal double bonds
PendingCN114105775AReduce pollutionSimple preparation processOrganic compound preparationCarboxylic acid amides preparationChromatographic separationOrganosolv
The invention discloses a preparation method of substituted iodobenzene with a terminal double bond, and the substituted iodobenzene with the terminal double bond has the following structural formula. Comprising the following steps: taking substituted o-iodoaniline and a compound containing terminal double bonds as initial raw materials, and reacting in an organic solvent to obtain substituted iodobenzene with terminal double bonds. The method disclosed by the invention is simple in preparation process, low in cost, high in reaction speed, simple to operate and small in environmental pollution, and a crude product does not need to be subjected to column chromatography separation; the substituted iodobenzene with the terminal double bonds can be subjected to a series of subsequent derivatization to obtain various forms of derivatives, so that natural products, medicines and bioactive molecules are prepared through subsequent derivatization reaction, and the substituted iodobenzene has important application value.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
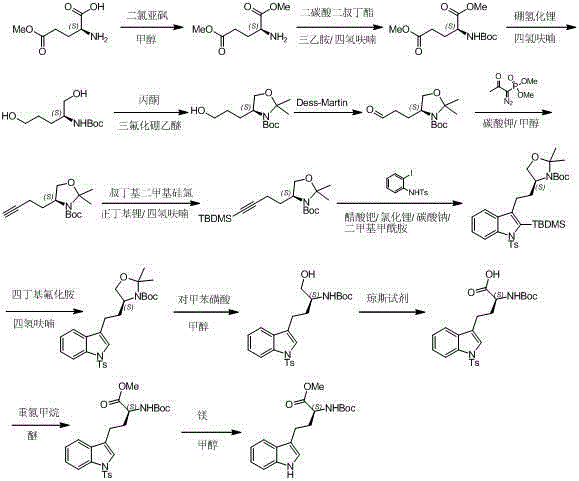
Synthetic method of Reziya base tetracyclic skeleton
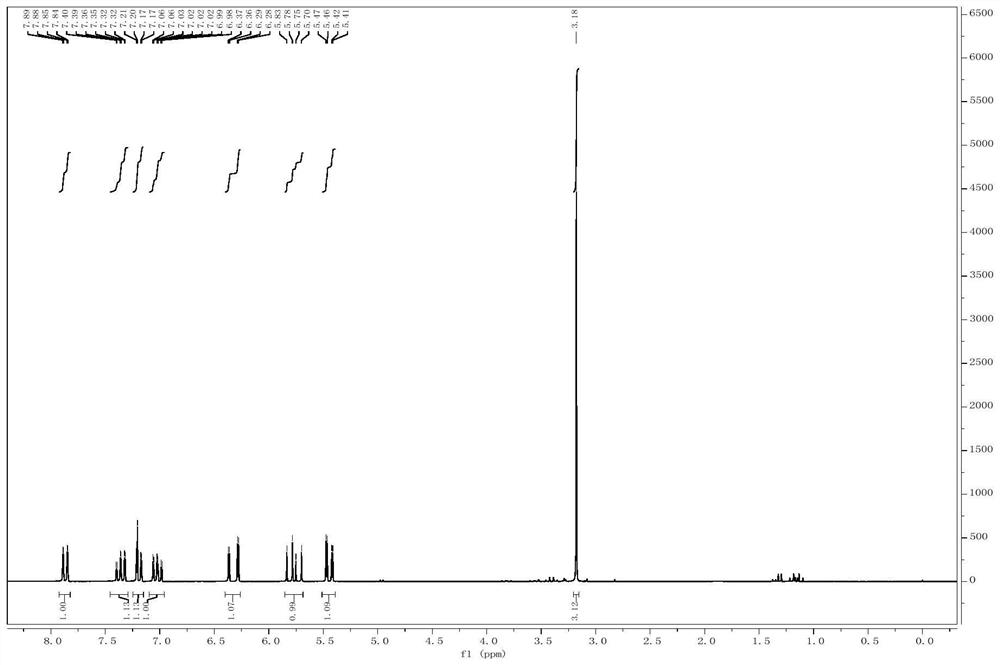
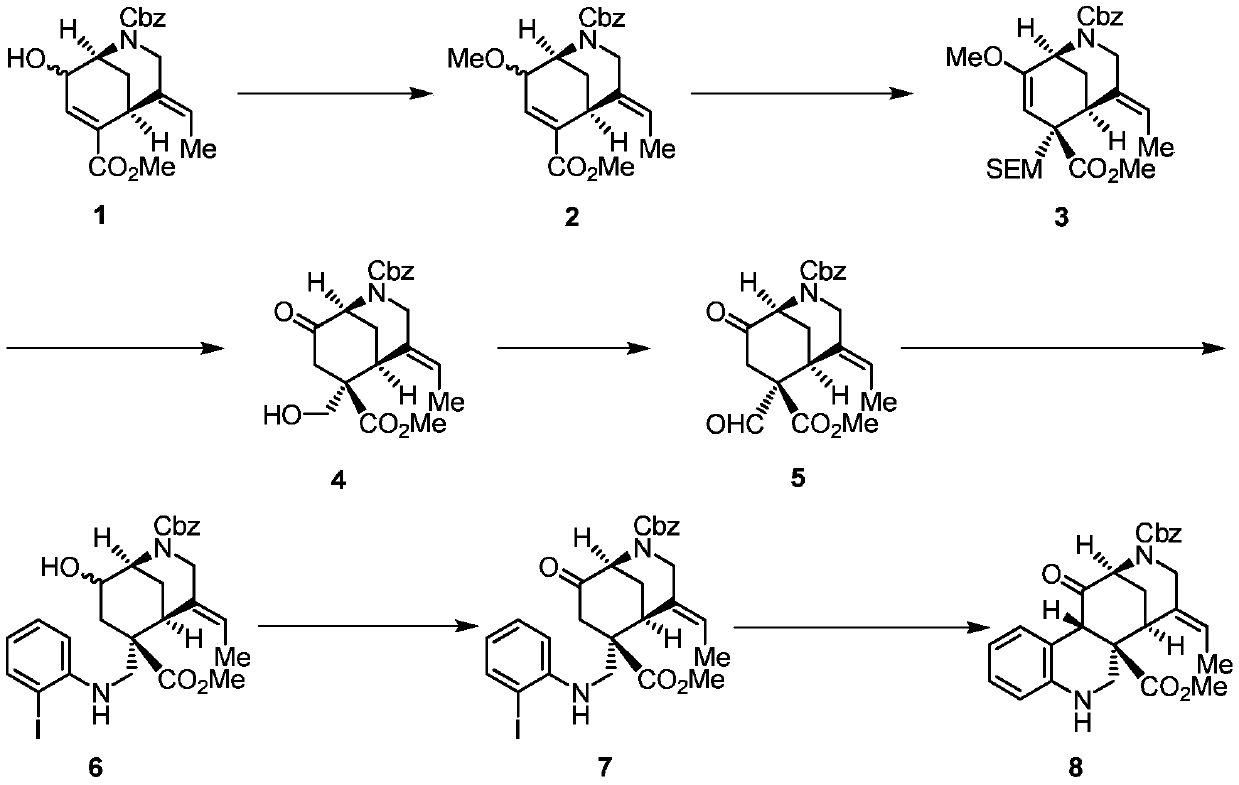
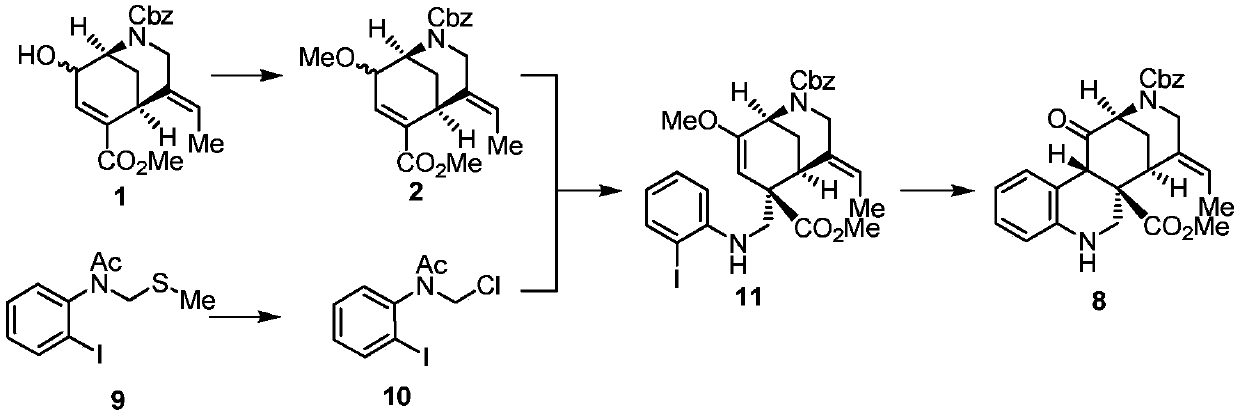
The invention discloses synthesis methods of a rhazimine tetracyclic skeleton. One of the synthesis methods adopts the following linear synthesis route: gamma-hydroxy-alpha,beta-unsaturated ester having a [3,3,1] ring system structure is adopted an initial raw material; a methanol group protects the hydroxyl group of allyl alcohol, and deconjugation alkylation is creatively used to achieve the migration of a double bond and construct a whole carbon quaternary carbon center; lithium tetrafluoroborate undergoes silicon deprotection and alkenyl ether structure hydrolysis; an aldehydic carbonyl group obtained by oxidizing a primary alcohol and o-iodoaniline undergo reducing amination, and a ketocarbonyl group is reduced; a secondary alcohol is oxidized; and the rhazimine tetracyclic skeleton is constructed through the alpha-position coupling of the carbonyl group. The other one of the synthesis methods adopts the following convergent synthesis route: a methyl-protected allyl alcohol structure and chloride connected with the o-iodoanide fragment undergoes deconjugation alkylation, and then intramolecular coupling is carried out to construct the rhazimine tetracyclic skeleton. The synthesis methods have the advantages of simple operating steps, low requirements of reaction conditions, and high-efficiency synthesis of the target compound.
Owner:EAST CHINA NORMAL UNIV
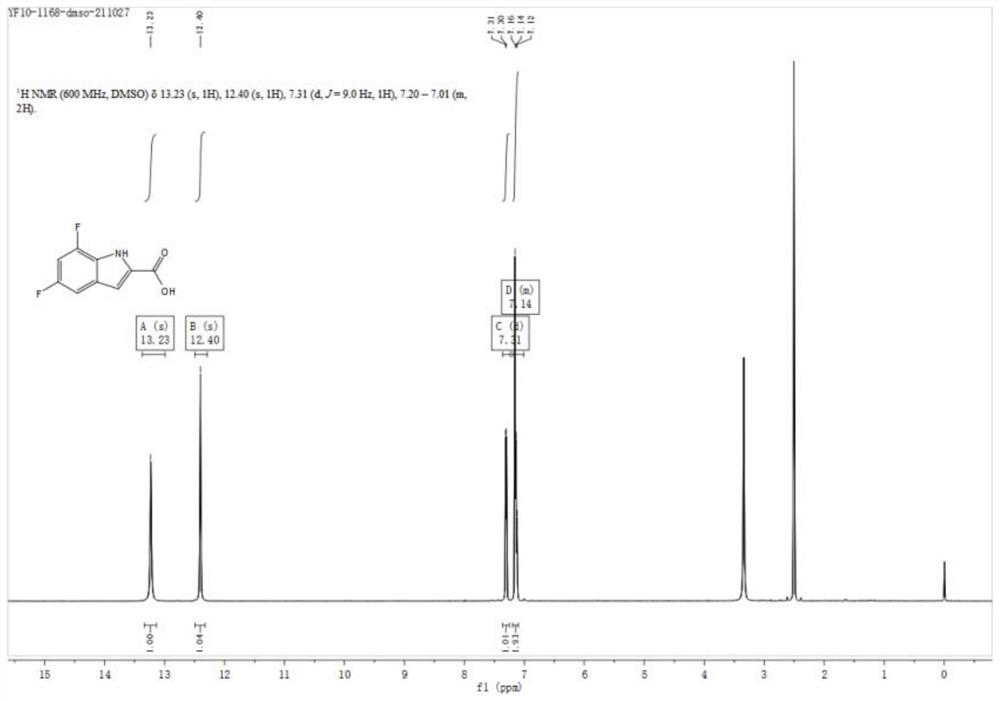
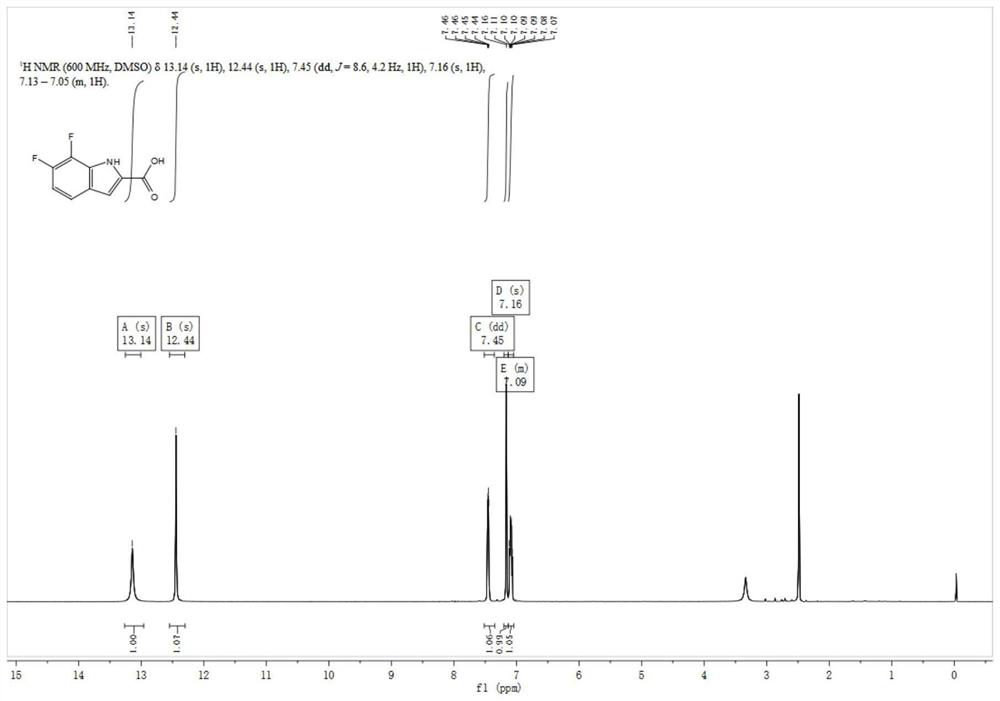
Preparation method of fluoro indole carboxylic acid compound
PendingCN114380732AReduce dosageSimplify the difficulty of purificationOrganic compound preparationAmino compound preparationPtru catalystOrganic synthesis
The invention discloses a preparation method of a fluoro indole carboxylic acid compound, and relates to the field of organic synthesis. Aiming at the current situations that in the prior art, the ring closing yield of a 7-position fluoro indole carboxylic acid compound is low and the use amount of a catalyst is large, a fluoro iodoaniline compound with a general formula (1) is used as a raw material and reacts with pyruvic acid in the presence of mixed alkali, anhydrous sulfate and a catalyst, and the fluoro indole carboxylic acid compound is prepared with high yield and high purity; the method is simple and convenient to operate and treat, mild in reaction condition, high in comprehensive yield and higher in safety, and is a process synthesis route which is high in cost performance and suitable for industrial large-scale production.
Owner:上海毕得医药科技股份有限公司
9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses
ActiveCN108727376BEasy post-processingHigh yieldOrganic chemistryX-ray constrast preparationsOrganic solventFormate
The invention discloses 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds. A preparation method of the compound comprises steps as follows: 9-nitrogen(6'-amino)hexylamine-9-9-aza-bicyclo[3.3.1] nonane-3 alpha-alkylamidophenyl formate and a chloroacetyl triiodoaniline derivative are mixed in an organic solvent in the mole ratio being 1:(1.0-1.5) and subjected to a reaction at the room temperature under the catalytic action of cesium hydroxide for 20-30 h, and a product, namely, the 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds, is obtained. The invention also discloses an application of the 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as a contrast medium for early diagnosis of breast cancer.
Owner:CHINA JILIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
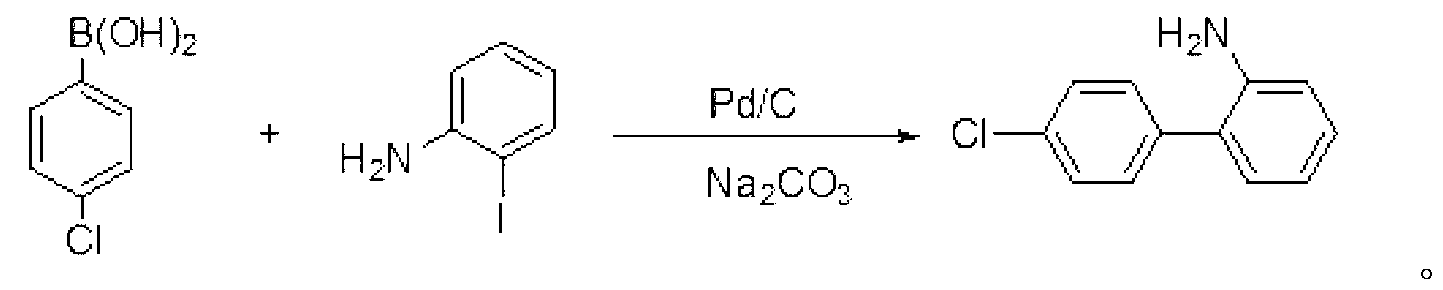
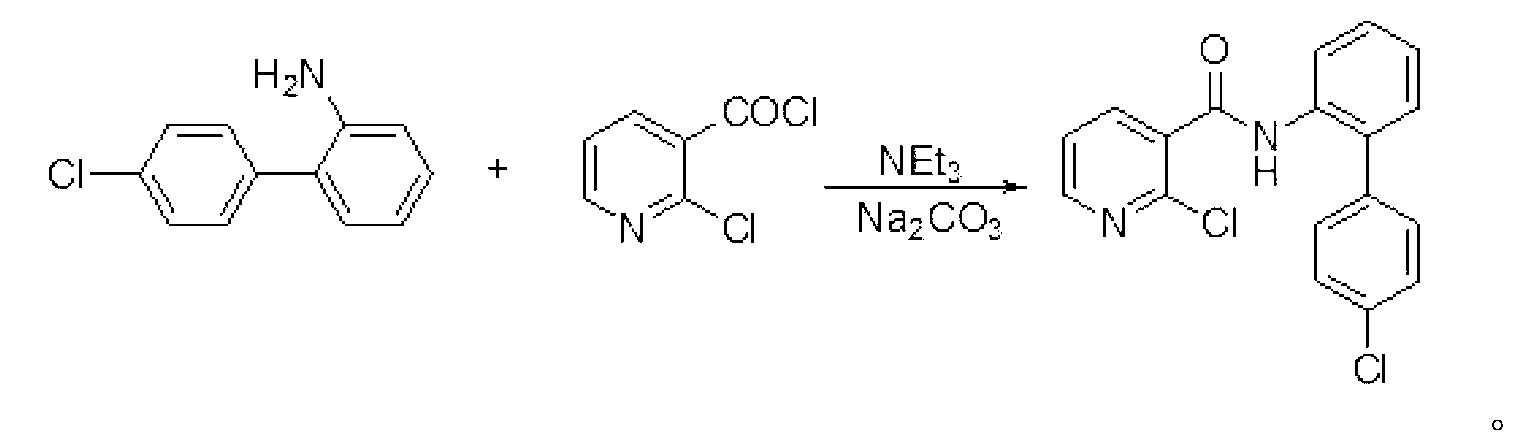



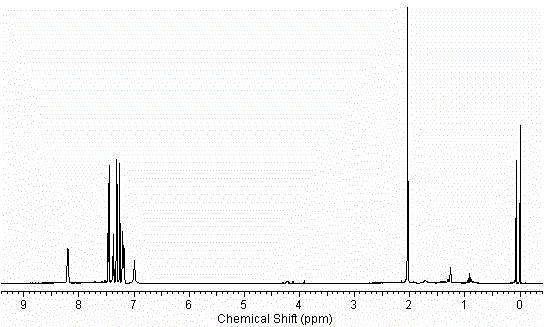
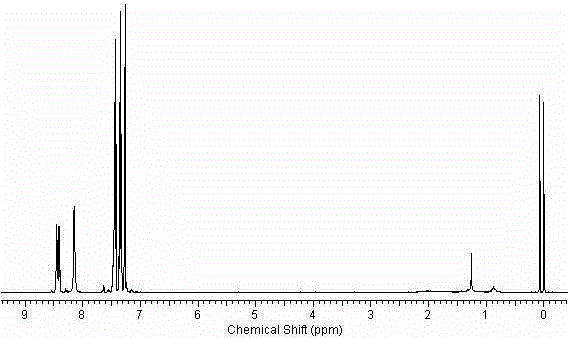
![9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/235b5907-fcab-4154-8cf7-38e6bd72e5d5/FDA0001667386470000011.png)
![9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/235b5907-fcab-4154-8cf7-38e6bd72e5d5/FDA0001667386470000013.png)
![9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof 9-aza-bicyclo[3.3.1] nonane coupled iodine-rich compounds as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/235b5907-fcab-4154-8cf7-38e6bd72e5d5/BDA0001667386480000021.png)







![9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses 9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses](https://images-eureka.patsnap.com/patent_img/9889df86-9fa8-4c75-a090-190fc39adc95/BDA0001667386480000021.png)
![9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses 9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses](https://images-eureka.patsnap.com/patent_img/9889df86-9fa8-4c75-a090-190fc39adc95/BDA0001667386480000023.png)
![9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses 9-Azabicyclo[3.3.1]nonane-coupled iodine-rich compounds and their preparation methods and uses](https://images-eureka.patsnap.com/patent_img/9889df86-9fa8-4c75-a090-190fc39adc95/BDA0001667386480000031.png)