Preparation method of o-iodoaniline
A technology of o-iodoaniline and p-iodoaniline, which is applied in the field of o-iodoaniline and its preparation, can solve the problems of poor selectivity, low yield of o-iodoaniline products, difficult separation of isomers, etc., and achieves mild conditions, low price, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of p-iodoaniline
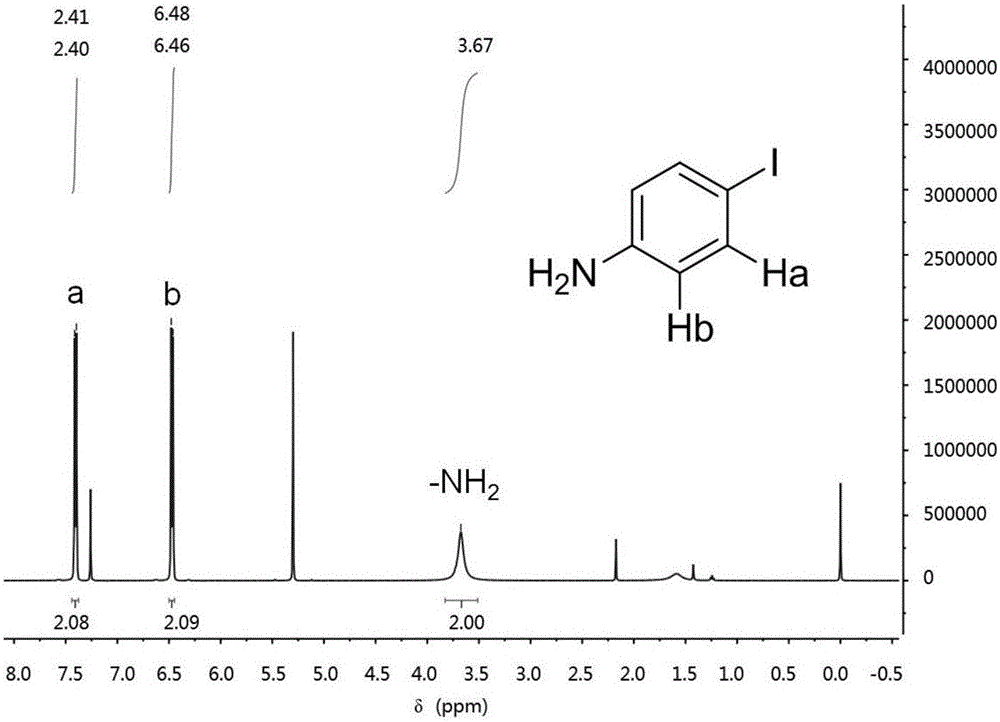
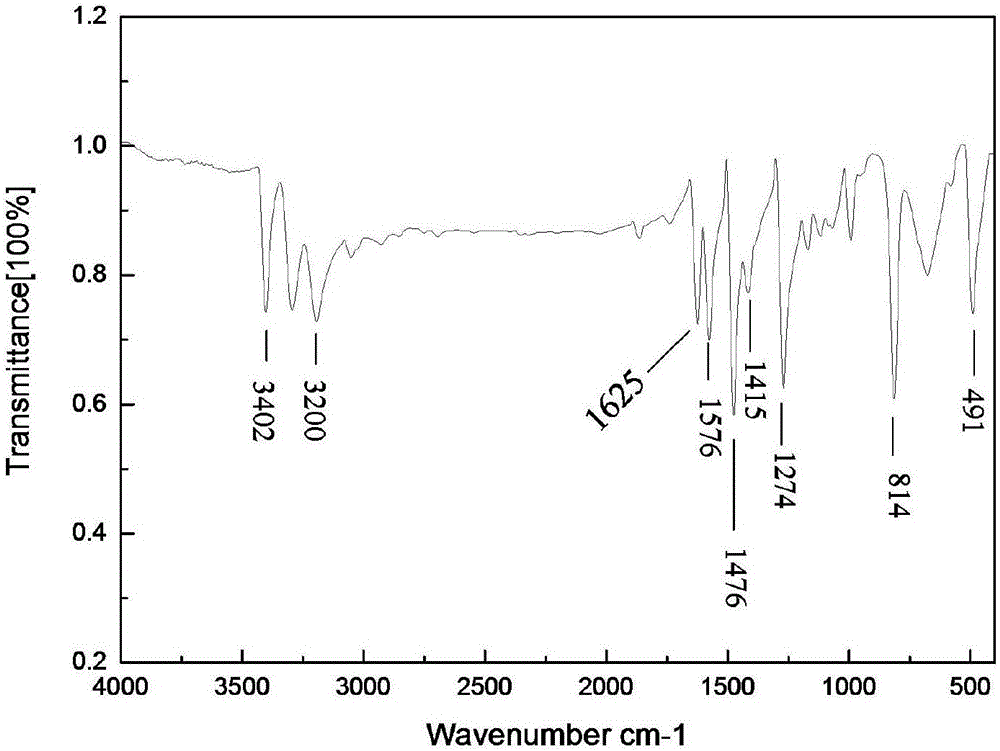
[0019] Add 4.1g of sodium bicarbonate, 3.1g of aniline and 50.0mL of ultrapure water into a conical flask equipped with magnetic stirring. After completely dissolving, the solution is cooled to 12.0-15.0°C under the action of a low-temperature thermostat, and stirred vigorously for 30 minutes. Add 0.024 mol (6.1 g) of iodine in batches, react for 80 min, and filter under reduced pressure to obtain a dark gray solid crude product. Wash with sodium bisulfite, ultrapure water and dry. Add 4.5 g of the dried crude product to a round bottom flask, add cyclohexane, and heat the solution to boil. After heating for about 5 minutes, wait for it to cool slightly, add activated carbon to decolorize, and quickly filter the hot solution in a hot state to remove insoluble impurities. The resulting solution crystallized on cooling. The crystals were obtained by suction filtration, washed with cyclohexane and ultrapure water, and dried to obtain th...
Embodiment 2
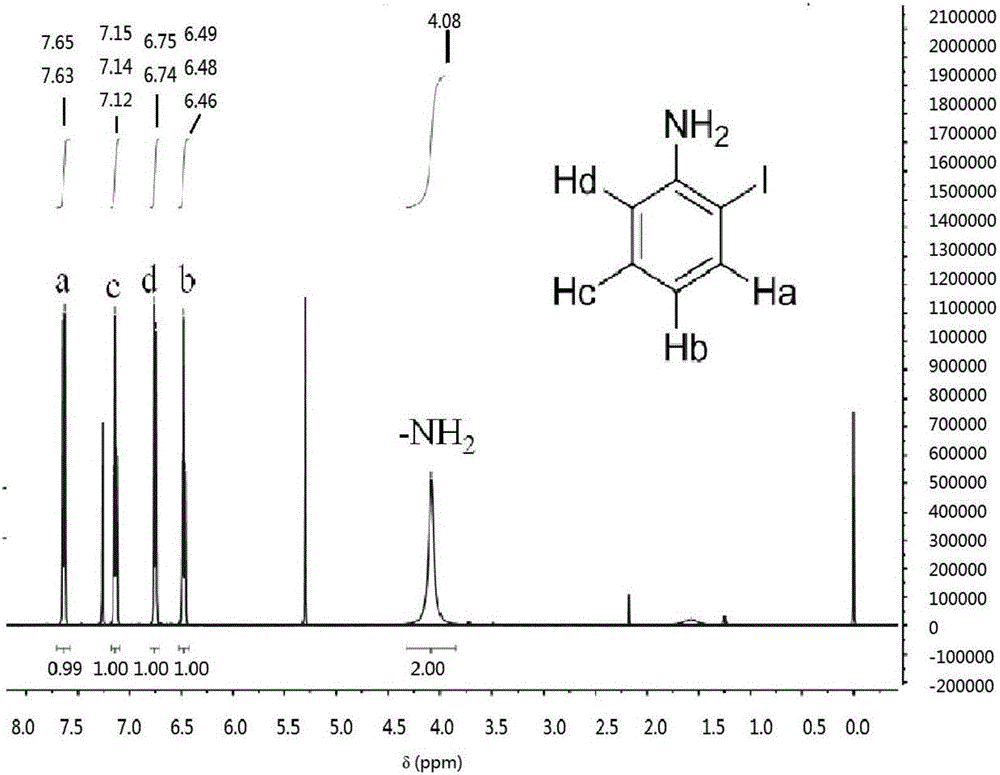
[0023] Add 10.0 g of p-iodoaniline (the synthesis method is the same as in Example (1)), 40.0 g of aniline, and 1.0 g of o-iodoaniline into a three-necked flask, and heat and react at 186.0-192.0° C. for 60 min. The resulting mixture solution was distilled off to remove most of the aniline and the residue was collected. The residue was heated with 100.0 g of n-hexane at 85.0-95.0°C for 30 min, and after cooling slightly, the upper layer of n-hexane solution was separated and frozen for crystallization. The crystals were washed with n-hexane and ultrapure water, and dried in air. Then use a mixed solvent of dichloromethane and ethyl acetate as the eluent, separate by column chromatography, evaporate the solvent under reduced pressure, and crystallize to obtain o-iodoaniline c in the form of white needles. Yield: 2.3g, 23%, white needle-like crystals; melting point (M.p.) 56.3-58.2°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com