4-iodophenyl substituted carborane derivative and preparation method thereof
A carborane and derivative technology, applied in the field of 4-iodophenyl substituted carborane derivatives and their preparation, can solve the problems of low yield, harsh reaction conditions and the like, and achieve high synthesis yield and preparation amount The effect of increasing, increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The embodiment of the present invention also provides a preparation method of the 4-iodophenyl substituted carborane derivative, comprising the following steps:
[0051] (1) Using the Sonogashira coupling reaction, and Under the palladium / copper catalyst effect, generate triazene-containing alkyne intermediates, wherein X is hydrogen, halo, triazene, alkyl, alkoxy, nitrogen heterocyclyl or ethynyl, when X When it is a hydrogen group, a halo group, an alkyl group, an alkoxy group or an azacyclic ring group, the alkyne intermediate containing a triazene is
[0052]
[0053] When X is an ethynyl group, the triazene-containing alkyne intermediate is
[0054]
[0055] in, The synthesis process refers to Journal of Organic Chemistry, 2014, 79(4), 1594-1610;
[0056] The consumption of described palladium / copper catalyst satisfies catalytic effect and gets final product, preferred following consumption: Relative to The molar consumption of, the consumption of de...
Embodiment 1
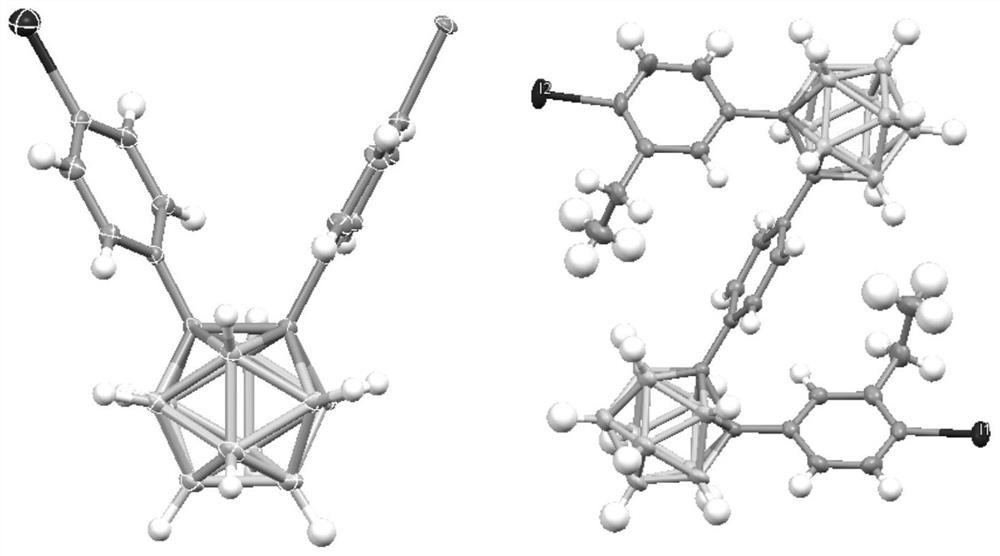
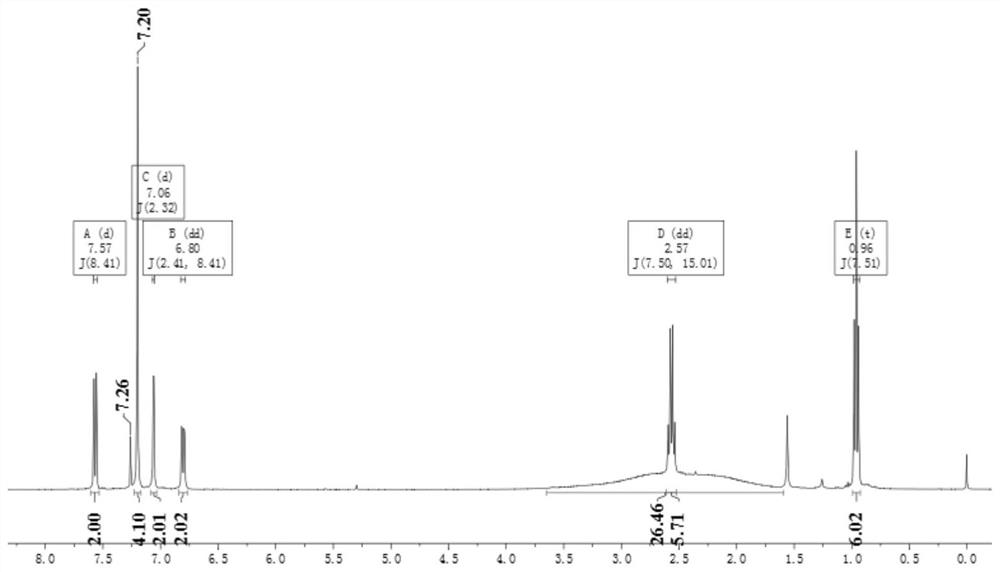
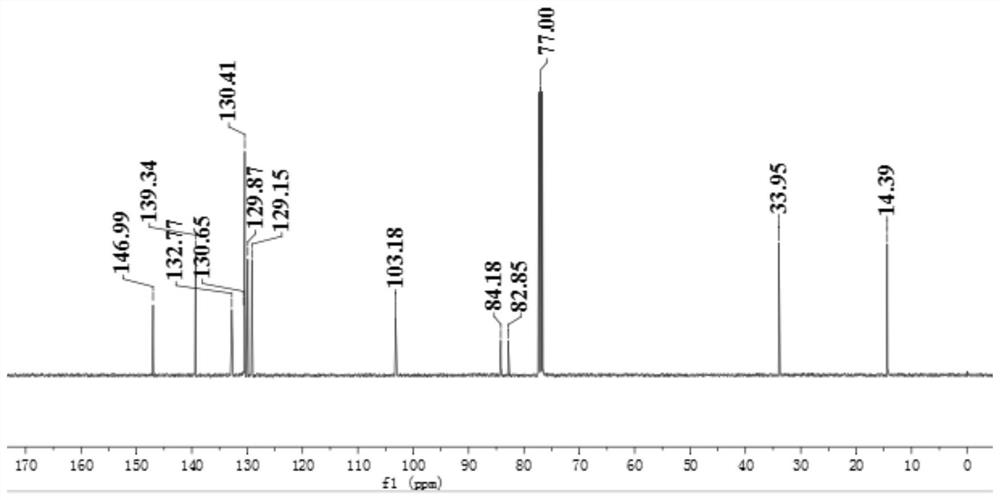
[0080] Example 1 The preparation of 1-(4-iodophenyl)-2-phenyl-1,2-carborane (R 1 =H, refer to DaltonTransactions, 2019, 48(33), 12549-12559)
[0081] (1) Synthesis of 3,3-diethyl-1-(4-iodophenyl)-1-triazene with reference to Journal of Organic Chemistry, 2014,79(4), 1594-1610: 25.0g (114.2mmol ) 4-iodoaniline was added to a mixed solvent consisting of 35mL of acetonitrile, 230mL of water and 35mL of concentrated hydrochloric acid (37% by mass fraction) (cooled to 0°C) to obtain a light gray suspension; 8.66g (125.6mmol) Sodium nitrite was dissolved in 23mL of water, cooled with ice and added to the above suspension to obtain a green solution, and stirred for 30min; 23.7g (171.2mmol) of potassium carbonate and 17.9mL of diethylamine (171.2mmol) were added to 175mL of water, cooled with ice, and then slowly added to the above green solution, and continued stirring for 1h. After the reaction was completed, it was extracted twice with ethyl acetate, the organic phase was separat...
Embodiment 2
[0085] Example 2 Preparation of 1-(4-iodophenyl)-2-(4-bromo)phenyl-1,2-carborane (Cab1, R 2 =Br)
[0086] (1) The preparation of 3,3-diethyl-1-(4-iodophenyl)-1-triazene is the same as in Example 1;
[0087] (2) In a 100 ml reaction tube, mix 6.0g (19.8mmol) 3,3-diethyl-1-(4-iodophenyl)-1-triazene and 3.9g (21.8mmol) 4- Bromophenylacetylene was added to a mixed solvent of 30 mL tetrahydrofuran and 15 mL triethylamine to obtain a reaction mixture; the reaction mixture was degassed twice through liquid nitrogen cooling-vacuumizing-thawing, and at the same time observe whether there was any After obvious bubbles escaped and no bubbles emerged, nitrogen was charged, and 417mg (0.6mmol) of Pd(PPh) was added under the protection of nitrogen. 3 ) 2 Cl 2 and 226mg (1.2mmol) of CuI, the reaction mixture was warmed up to 80°C, and the reaction was continued for 10h. After the reaction was completed, the solvent was removed by rotary evaporation, and the initial product was subjected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com