Synthetic method of Reziya base tetracyclic skeleton
A technology of Reziya base and synthesis method, which is applied in the field of synthesizing Reziya base tetracyclic skeleton, can solve problems such as large tension and complex structure, and achieve the effects of efficient route, simple operation steps and economical steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The present invention will be described in detail below.
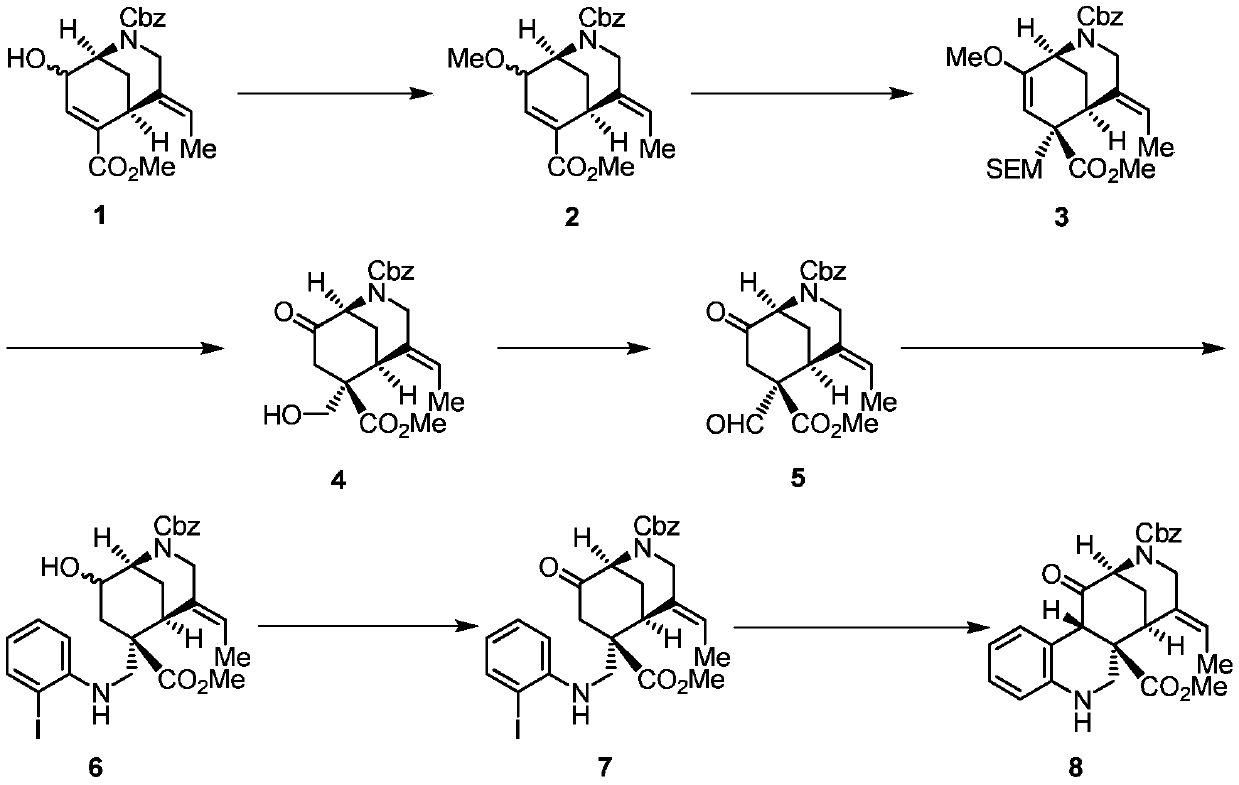
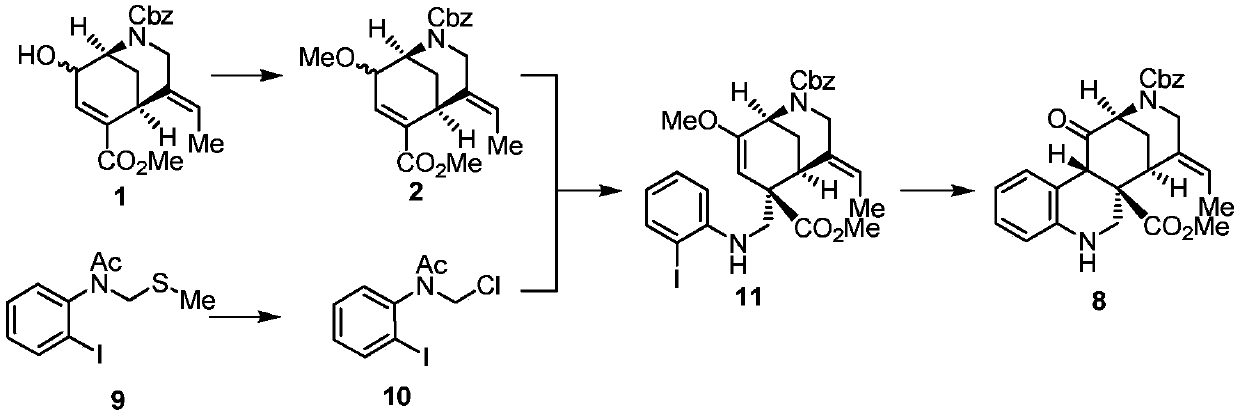
[0037] 1) Compound 1 (1.9g, 5.32mmol, 1.0equiv.), silver oxide (3.8g, 16mmol, 3.0equiv.) and tetrabutylammonium iodide (393mg, 1.06mmol, 0.2equiv.) were dissolved in heavy distilled dichloromethane. Then iodomethane (1.66ml, 26.6mmol, 5.0equiv.) was added and reacted overnight at room temperature. Excess methyl iodide was quenched with saturated sodium sulfite, water was added, extracted three times with ethyl acetate, washed with saturated brine, and dried over anhydrous sodium sulfate. The crude product was separated by column to obtain compound 2 (1.67g, 84%, Rf=0.39 (15% ethyl acetate / petroleum ether).
[0038] 1 H NMR (400MHz, CDCl 3 )δ7.41–7.29(m,6H),7.06(dd,J=14.4,3.7Hz,1H),5.53–5.36(m,1H),5.26–5.06(m,2H),4.53(d,J= 46.8Hz, 1H), 4.24–4.08(m, 1H), 3.89(d, J=22.3Hz, 1H), 3.74(s, 3H), 3.61–3.44(m, 4H), 3.31(s, 2H), 1.89(dd,J=27.6,12.6Hz,1H),1.77(dd,J=6.8,2.1Hz,3H).
[0039] 13 C NMR (101MHz, CDCl 3 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com