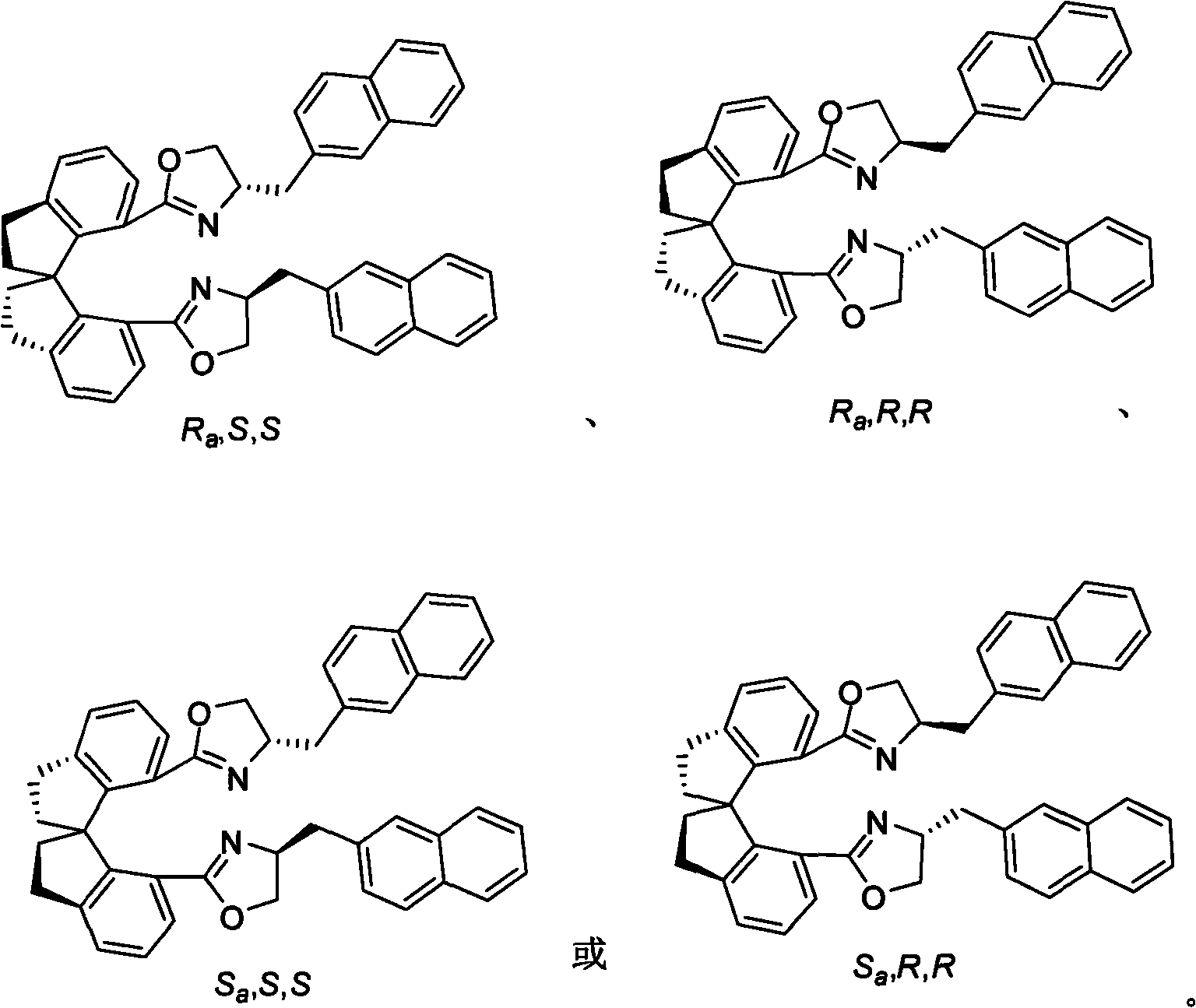
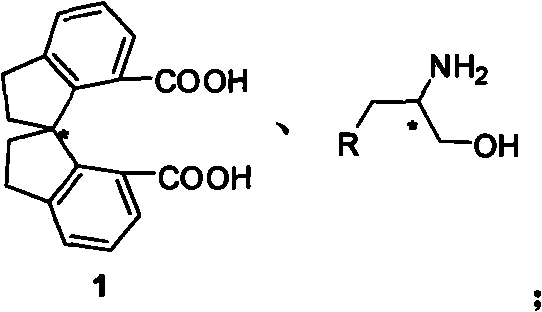
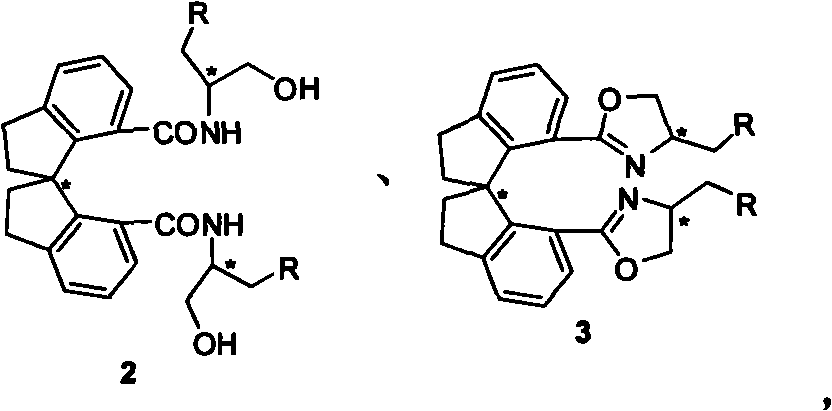
Beta-naphthyl methyl substituted spiral bisoxazoline ligand, synthetic method and application thereof
A bisoxazoline and spiro ring technology, which is applied in the field of spiro bisoxazoline ligands substituted by β-naphthylmethyl, can solve problems such as unsatisfactory effects, achieve good industrial and pharmaceutical application prospects, and the synthesis method is simple , the effect of emphasizing the adjustment ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Under inert gas protection, add 100mg (R a )-1, 160 mg aminoalcohol, 289 mg DCC and 94 mg nitrogen hydroxybenzotriazole. Add 10 mL of tetrahydrofuran under ice-cooling, keep at 0°C for 0.5-20 hours, then naturally rise to room temperature, react overnight, and TLC detects that the reaction is complete (petroleum ether: ethyl acetate = 1:1). The solvent was removed under reduced pressure, and the product was directly used in the next reaction by short column filtration.
[0038] Add 4 mg of DMAP to the reaction tube containing the product obtained in the previous step. After ventilation, add 5 mL of dichloromethane, add 0.3 mL of triethylamine under ice bath, add 143 mg of methanesulfonyl chloride after stirring for a while, and keep at 0 ° C for 0.5 After -5 hours, add 1.0 mL of triethylamine, let it rise to room temperature naturally, and react overnight, and TLC detects that the reaction is complete. Directly spin off the solvent, and column chromatograp...
Embodiment 2
[0041]
[0042] R 1 = H, R 2 = H, R 3 = n-heptyl
[0043] Pd(dba) 2 (6mg, 0.011mmol) and (R a , S, S)-3 (7mg, 0.011mmol) (ligand synthesized in Example 1) was complexed in 1mL tetrahydrofuran for 0.5-10 hours, then added Ag 3 PO 4 (34mg, 0.081mmol), 4a (75mg, 0.20mmol), 5a (112mg, 0.81mmol) and 1mL tetrahydrofuran. The reaction was reacted at 0-200°C, TLC followed the completion of the reaction, and directly spin-dried column chromatography to obtain a colorless viscous Thick liquid 44mg. (R)-6aa (57%, 96%ee): [α] 20 D =+9.4 (c=0.50, ethyl acetate); 1 H NMR (400MHz, d 6 -acetone) δ7.72(d, J=8.4Hz, 1H), 7.61(d, J=8.4Hz, 2H), 7.44(dd, J=7.6, 0.4Hz, 1H), 7.36-7.30(m, 1H ), 7.26(d, J=8.0Hz, 2H), 7.11-7.05(m, 1H), 5.48(d, J=2.0Hz, 1H), 4.99(d, J=2.0Hz, 1H), 4.80-4.75 (m, 1H), 2.31(s, 3H), 2.12-2.04(m, 1H), 1.86-1.76(m, 1H), 1.47-1.21(m, 10H), 0.86(t, J=6.8Hz, 3H ); 13 C NMR (100.5MHz, d 6 -acetone) δ146.1, 145.1, 144.9, 135.4, 131.3, 130.8, 130.4, 128.1, 125.3, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com