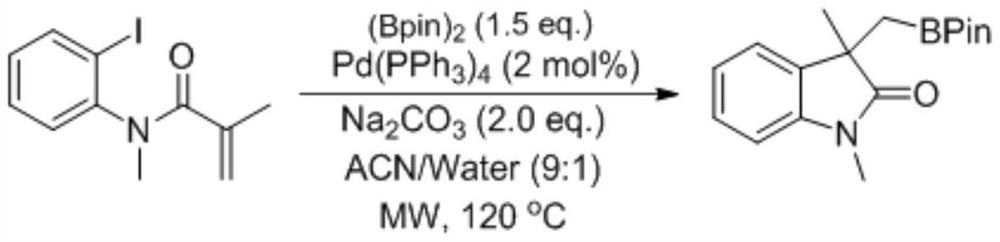
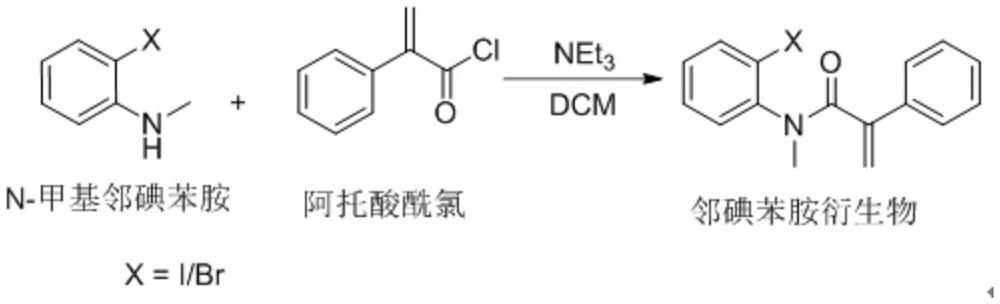
A kind of preparation method of boron-containing indolinone derivative
A technology for indolinone and derivatives, which is applied in the field of catalytic synthesis and achieves the effects of simple reaction raw materials, easy availability of reaction raw materials, and wide universality of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Into the reaction tube were sequentially added o-iodoaniline derivative 1a (72.6 mg, 0.2 mmol), cuprous iodide (3.8 mg, 10 mol%), triphenylphosphine (10.4 mg, 20 mol%), lithium tert-butoxide (16 mg) , 1.0eq.), pinacol diboronate (101.3mg, 2.0eq.), dichloroethane (2.0mL, 0.1M), the reaction mixture was reacted at 40°C for 5min, and directly passed through a rotary evaporator after the reaction After removing the solvent, the method of column chromatography separation (petroleum ether:ethyl acetate=15:1) was used to obtain the target product compound 1 in a yield of 98%.
[0025] 1H NMR (500MHz, CDCl3) δ 7.32 (t, J=1.7Hz, 1H), 7.30 (dd, J=11.8, 4.0Hz, 3H), 7.25 (dd, J=9.3, 5.7Hz, 2H), 7.19 (ddd, J=8.2, 4.2, 1.2Hz, 1H), 7.08–7.03 (m, 1H), 6.86 (d, J=7.6Hz, 1H), 3.20 (s, 3H), 1.92 (dd, J=58.5 ,15.2Hz,2H),0.96(s,6H),0.86(s,6H).
Embodiment 2
[0027] Into the reaction tube were sequentially added o-iodoaniline derivative 2a (88.2 mg, 0.2 mmol), cuprous bromide (5.6 mg, 20 mol%), tricyclohexylphosphine (22.4 mg, 40 mol%), potassium tert-butoxide (44.8 mg, 2eq.), pinacol diboronate (76.0mg, 1.5eq.), dichloromethane (2.0mL, 0.1M), the reaction mixture was reacted at 60°C for 15min, after the reaction was completed, it was directly removed by a rotary evaporator After solvent, the method of column chromatography separation (petroleum ether:ethyl acetate=10:1) was used to obtain the target product compound 2 in a yield of 66%.
[0028] 1H NMR(500MHz, CDCl3)δ7.32-7.27(m,4H),7.25-7.20(m,2H),7.18(d,J=7.9Hz,1H),7.03(d,J=1.6Hz,1H) ,3.19(s,3H),1.91(dd,J=44.7,15.4Hz,2H),1.01(s,6H),0.91(s,6H).
Embodiment 3
[0030] Into the reaction tube were sequentially added o-iodoaniline derivative 3a (86.2 mg, 0.2 mmol), cuprous chloride (1.9 mg, 10 mol%), 1,10-phenanthroline (3.6 mg, 10 mol%), tert-butanol Sodium (28.8mg, 1.5eq.), pinacol biboronate (50.6mg, 1.0eq.), toluene (2.0mL, 0.1M), the reaction mixture was reacted at 80°C for 5min, and directly after the reaction was completed by rotary evaporation After the solvent was removed by the instrument, a column chromatography method (petroleum ether:ethyl acetate=8:1) was used to separate the target product compound 3 with a yield of 80%.
[0031] 1H NMR(500MHz, CDCl3)δ7.62(d,J=7.8Hz,2H),7.31-7.27(m,4H),7.27-7.23(m,1H),6.95(d,J=8.2Hz,1H) ,3.24(s,3H),1.99–1.91(m,2H),0.99(s,6H),0.89(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com