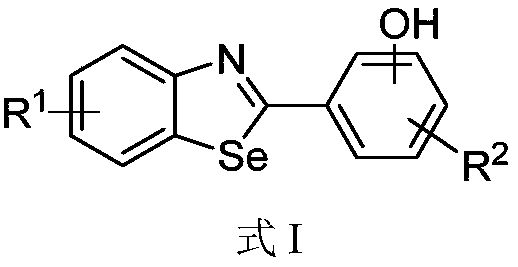
Benzoselenazole compound as well as preparation method and application thereof
A technology of benzoselenazoles and compounds is applied in the fields of benzoselenazoles and their preparation, and the application fields of ligands, and can solve the problem that the specific function of ERβ is not very clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
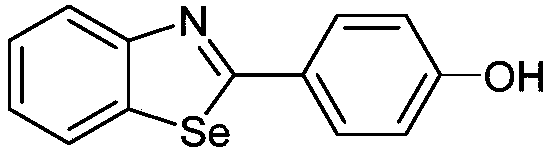
[0055] [Example 1] Preparation of 4-(benzo[d][1,3]selenazol-2-yl)phenol (compound 1)
[0056]
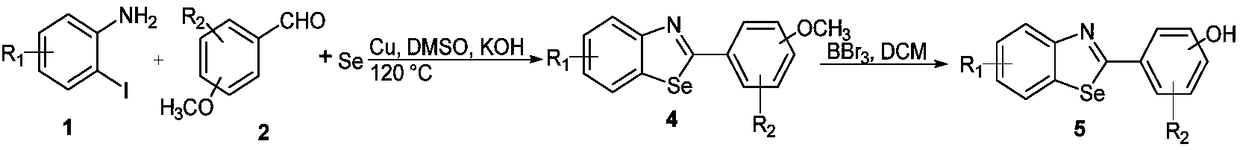
[0057]Under the condition of anhydrous and oxygen-free, add 0.1mmol o-iodoaniline, 0.12mmol 4-methoxybenzaldehyde, 0.3mmol selenium powder, 0.005mmol copper, 0.2mmol potassium hydroxide and 10ml DMSO solution, in a single port round bottom In the flask, reflux the reaction at 120° C., monitor the reaction by TLC, and separate by column chromatography to obtain pure methoxy-containing benzoselenazole compounds, and then use boron tribromide (3eq.) to demethylate to obtain the reaction product. The product was a white solid in 38% yield.
[0058] 1 H NMR (400MHz, Acetone-d 6 )δ9.14(s,1H),8.07(d,J=7.3Hz,1H),8.00(d,J=8.2Hz,1H),7.95(d,J=8.7Hz,2H),7.47(s, 1H), 7.35–7.28(m, 1H), 7.00(d, J=8.7Hz, 2H). 13 C NMR (100MHz, Acetone-d 6 )δ172.69, 161.37, 157.02, 138.71, 130.54, 128.88, 127.22, 125.99, 125.78, 124.96, 116.88.
Embodiment 2
[0059] [Example 2] Preparation of 4-(benzo[d][1,3]selenazol-2-yl)-2-fluorophenol (compound 2)
[0060]
[0061] Under anhydrous and oxygen-free conditions, add 0.1mmol o-iodoaniline, 0.12mmol 3-fluoro4-methoxybenzaldehyde, 0.3mmol selenium powder, 0.005mmol copper, 0.2mmol potassium hydroxide and 10ml DMSO solution, in a In a single-necked round bottom flask, reflux reaction at 120°C, monitor the reaction by TLC, separate by column chromatography to obtain pure benzoselenoazole compounds containing methoxy groups, and then use boron tribromide (3eq.) to demethylate to obtain the reaction Product, the product is a white solid, the yield is 35%. 1 H NMR (400MHz, Acetone-d 6 )δ9.50(s,1H),8.10(dd,J=7.9,1.2Hz,1H),8.02(dd,J=8.2,1.1Hz,1H),7.86(dd,J=11.8,2.2Hz,1H ),7.73(ddd,J=8.4,2.2,1.0Hz,1H),7.51(ddd,J=8.3,7.2,1.3Hz,1H),7.34(ddd,J=8.3,7.3,1.3Hz,1H), 7.16(t,J=8.6Hz,1H). 13 C NMR (100MHz, Acetone-d 6 )δ171.38, 156.78, 153.65, 151.24, 148.89, 148.76, 138.97, 129.43, 127.36, 126...
Embodiment 3
[0062] [Example 3] Preparation of 4-(5-chlorobenzo[d][1,3]selenazol-2-yl)phenol (compound 3)
[0063]
[0064] Under anhydrous and oxygen-free conditions, add 0.1mmol 2-iodo-3-chloroaniline, 0.12mmol 4-methoxybenzaldehyde, 0.3mmol selenium powder, 0.005mmol copper, 0.2mmol potassium hydroxide and 10ml DMSO solution, in a In a single-necked round bottom flask, reflux reaction at 120°C, monitor the reaction by TLC, separate by column chromatography to obtain pure benzoselenoazole compounds containing methoxy groups, and then use boron tribromide (3eq.) to demethylate to obtain the reaction Product, the product is a white solid, the yield is 34%. 1 H NMR (400MHz, Acetone-d 6 )δ8.10(d, J=8.5Hz, 1H), 7.99(d, J=2.1Hz, 1H), 7.95(d, J=8.7Hz, 2H), 7.34(dd, J=8.5, 2.1Hz, 1H),7.00(d,J=8.7Hz,2H). 13 C NMR (101MHz, Acetone-d 6 )δ175.31, 158.04, 137.09, 132.75, 132.67, 130.71, 128.48, 127.25, 125.72, 124.30, 116.94.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com