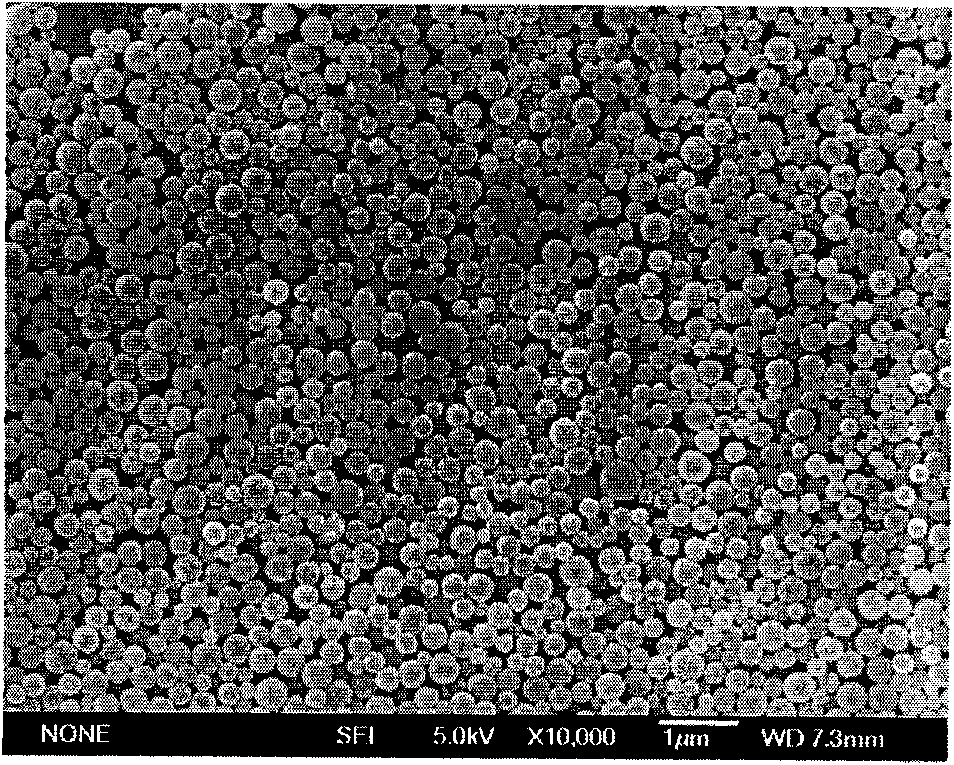
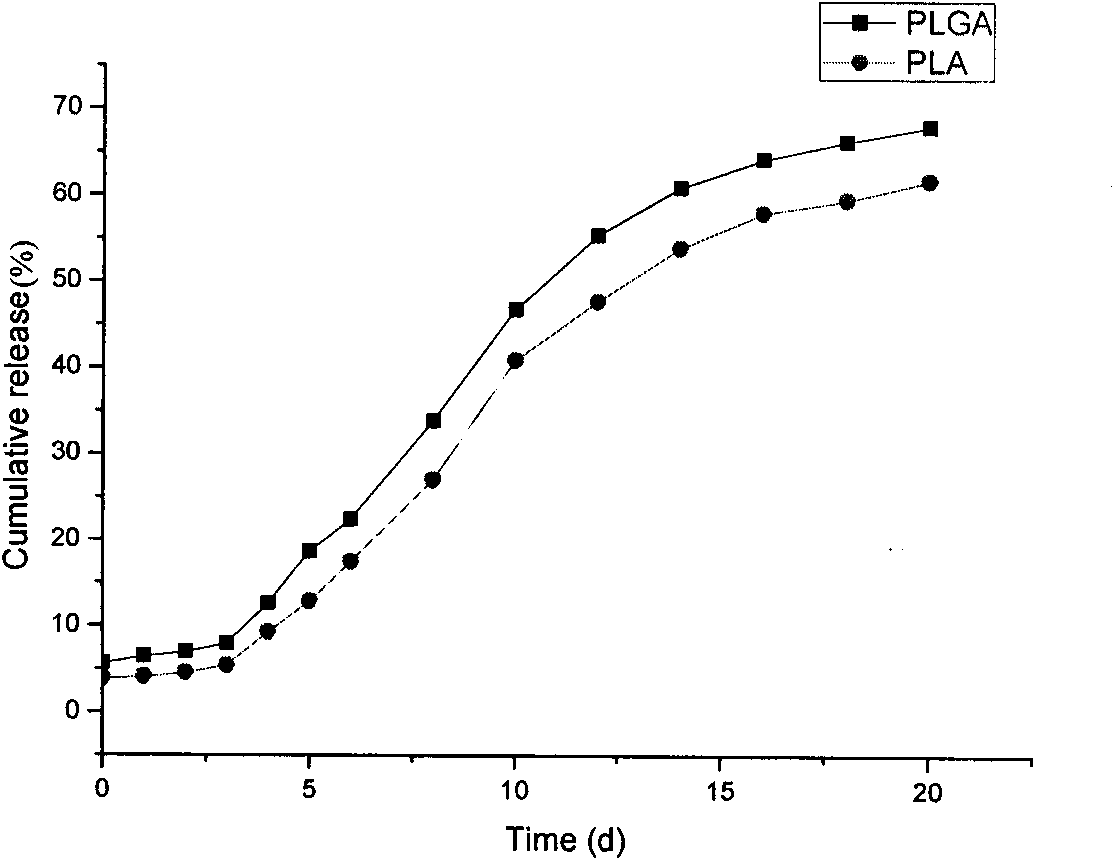
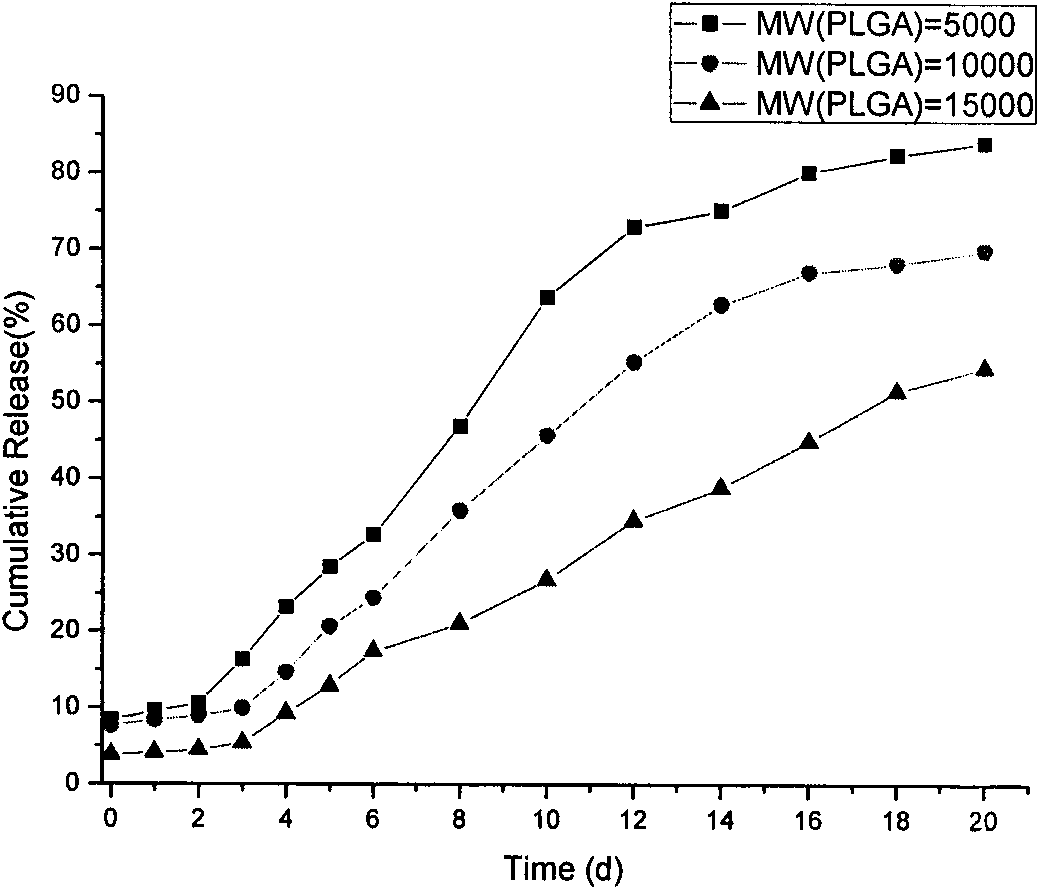
Polypeptide medicament sustained release microsphere or microcapsule preparation with uniform grain size and preparation method thereof
A technology for sustained-release preparations and drugs, which is applied in the field of sustained-release microspheres or microcapsule preparations of polypeptide drugs with uniform particle size, and can solve the problems of low drug embedding rate, difficulty in scale-up production, and poor preparation repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] The hydrophilic microporous membrane with a pore size of 5.2 μm is soaked in water to fully wet the membrane pores. Dissolve 10 mg of GLP-1 in 1 mL of deionized water as the inner aqueous phase (w 1 ). Dissolve 0.25 g of polylactic acid (PLA, molecular weight: 100,000 Daltons) in 5 mL of dichloromethane as the oil phase (o). Mix the inner water phase with the oil phase, and emulsify in an ice-water bath with a homogeneous emulsifier at 3000 rpm for 1 min to obtain w 1 / o Colostrum. Prepare 40mL of 1wt.% polyvinyl alcohol aqueous solution as the external water phase (w 2 ). Will prepare the obtained w 1 / o Colostrum is pressed into the outer water phase through the microporous membrane with a constant pressure of 50kPa to obtain w 1 / o / w 2 Complex emulsion. After the membrane emulsification was completed, the stirring was continued for 24 hours to remove methylene chloride, and the PLA microcapsules embedded with GLP-1 were obtained after centrifugation. The ob...
Embodiment 2
[0113] The hydrophilic microporous membrane with a pore size of 1.4 μm is soaked in water to fully wet the membrane pores. Dissolve 0.001mg Exendin-4 in 1mL deionized water as the inner aqueous phase (w 1 ). Dissolve 10 g of polylactic acid (PLA, molecular weight 3 kilodaltons) in 10 mL of ethyl acetate as the oil phase (o). Mix the inner water phase with the oil phase, and emulsify in an ice-water bath with a mechanical stirrer at 800 rpm for 1 min to obtain w 1 / o Colostrum. Prepare 10mL aqueous solution containing 0.1wt.% polyvinyl alcohol and 0.1wt.% NaCl as the external aqueous phase (w 2 ). Will prepare the obtained w 1 / o The colostrum is pressed into the outer water phase through the microporous membrane with a constant pressure of 100kPa to obtain w 1 / o / w 2 Complex emulsion. After the membrane was emulsified, it was evaporated under reduced pressure for 4 hours to remove ethyl acetate, and the PLA microcapsules embedded with Exendin-4 were obtained after cen...
Embodiment 3
[0115] The hydrophilic microporous membrane with a pore size of 2.8 μm is soaked in water to fully wet the membrane pores. Dissolve 45mg Exenatide in 1mL Tris buffer as the inner aqueous phase (w 1). Dissolve 0.15 g of polylactic acid-polyglycolic acid copolymer (PLGA, PLA:PGA=25:75, molecular weight 5 kilodaltons) in 1 mL of a mixed solution of dichloromethane and ethyl acetate (volume ratio 1:1 ), as the oil phase (o). Mix the inner water phase with the oil phase, and emulsify for 1 min in an ice-water bath using an ultrasonic breaker at 10w to obtain w 1 / o Colostrum. Prepare 50mL of aqueous solution containing 10wt.% polyvinyl alcohol and 0.1wt.% SDS as the external aqueous phase (w 2 ). Will prepare the obtained w 1 / o Colostrum is pressed into the outer water phase through the microporous membrane with a constant pressure of 75.0kPa, to obtain w 1 / o / w 2 Complex emulsion. After the membrane was emulsified, cross-flow diffusion dialysis was performed for 24 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com