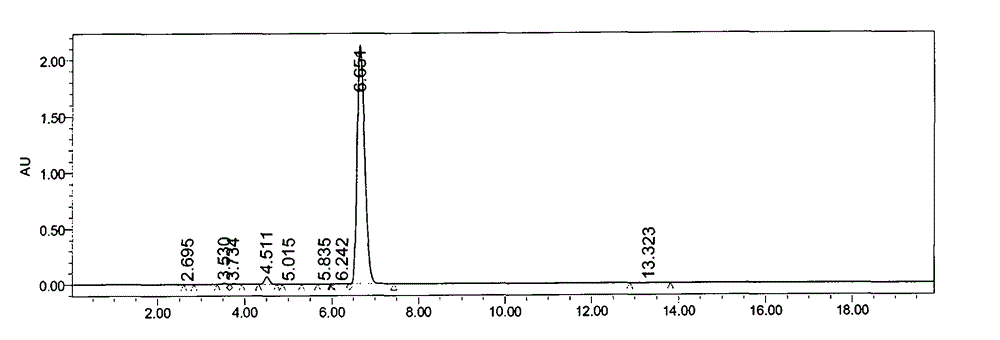
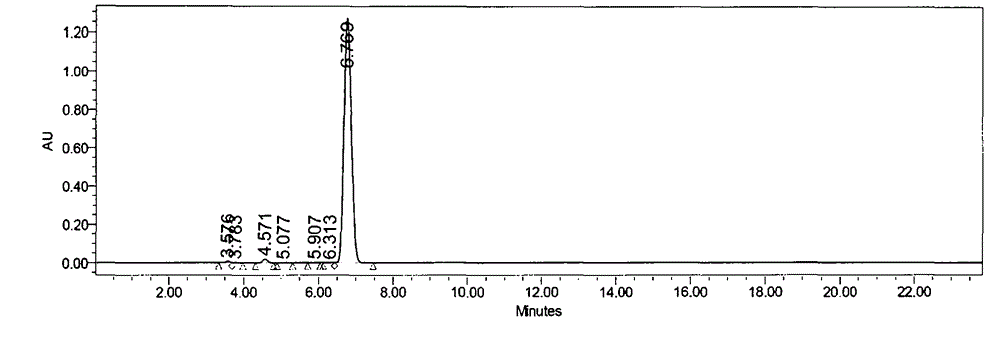
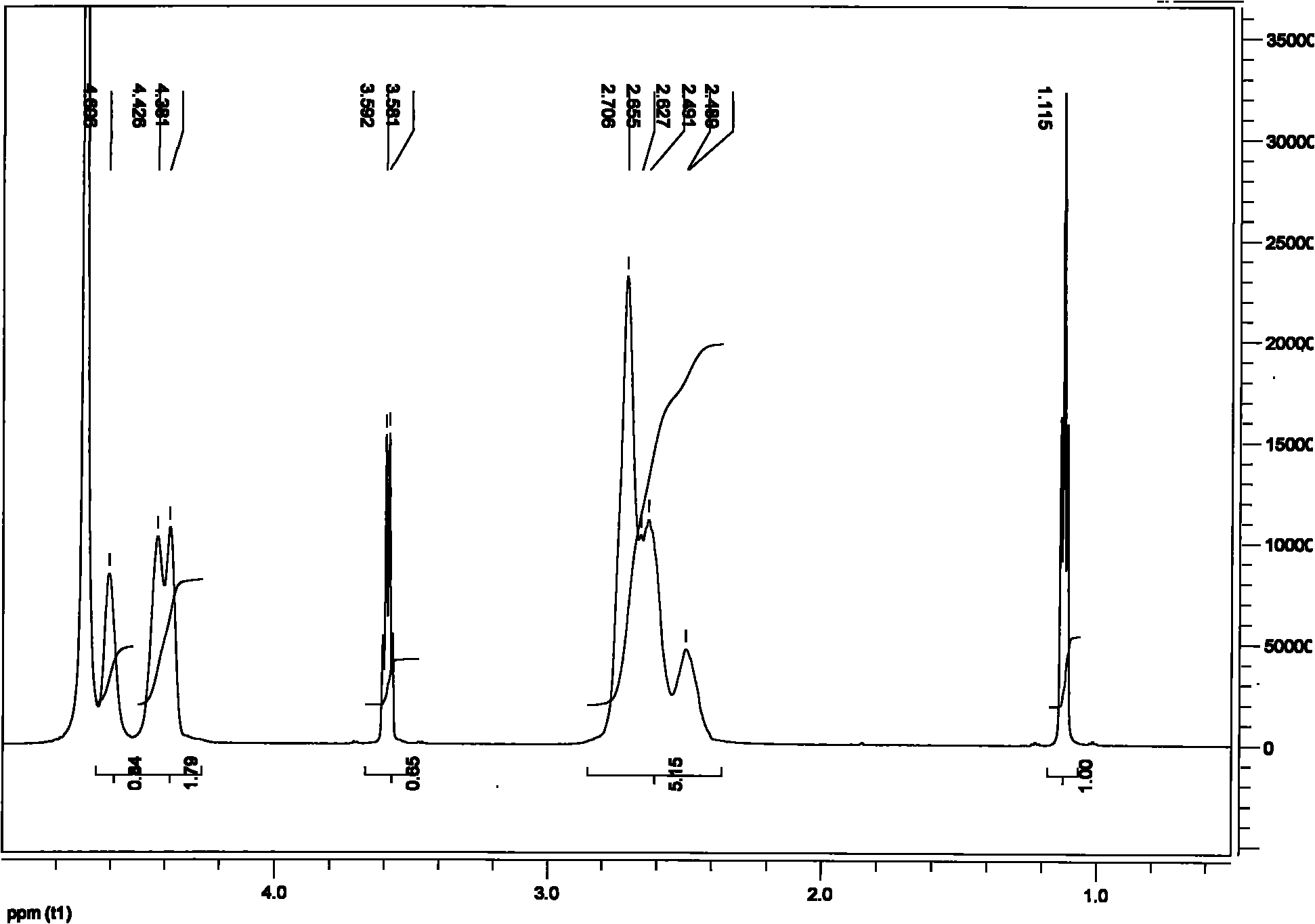
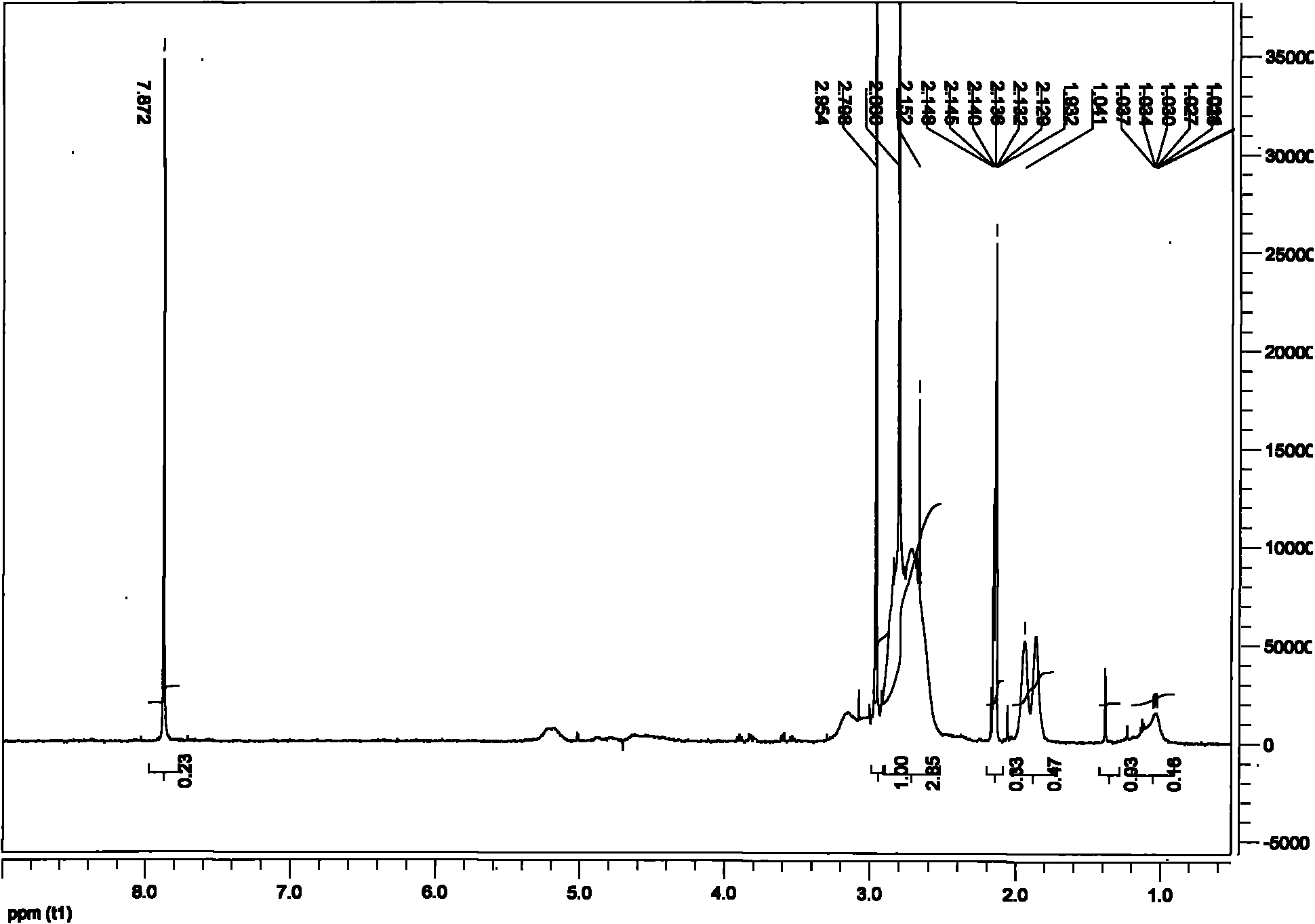
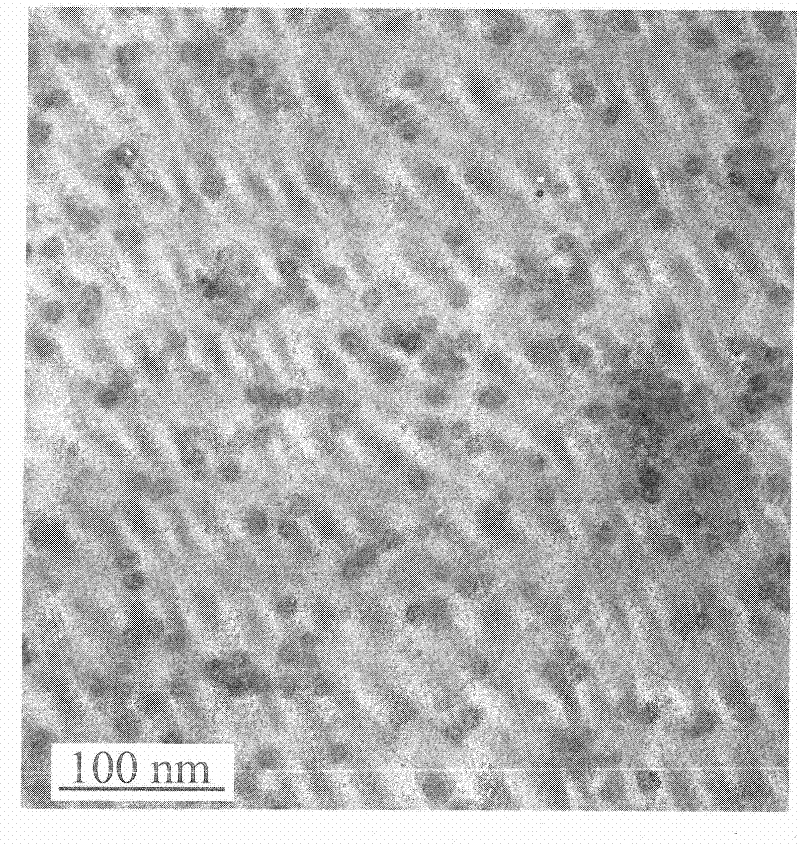
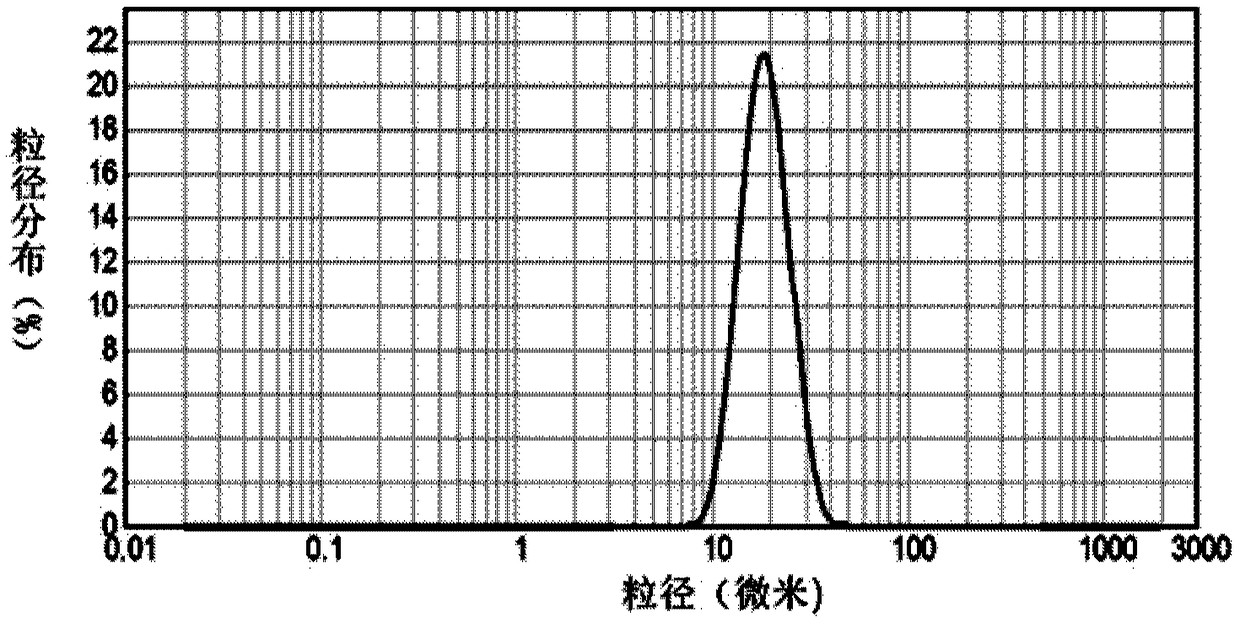
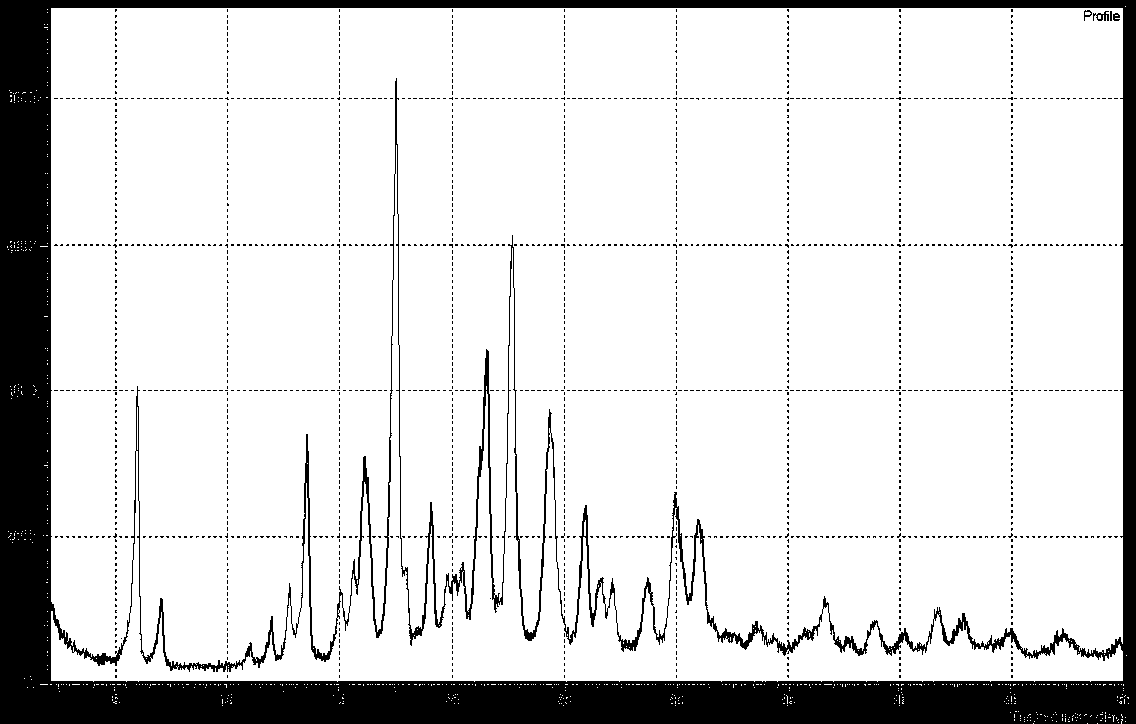
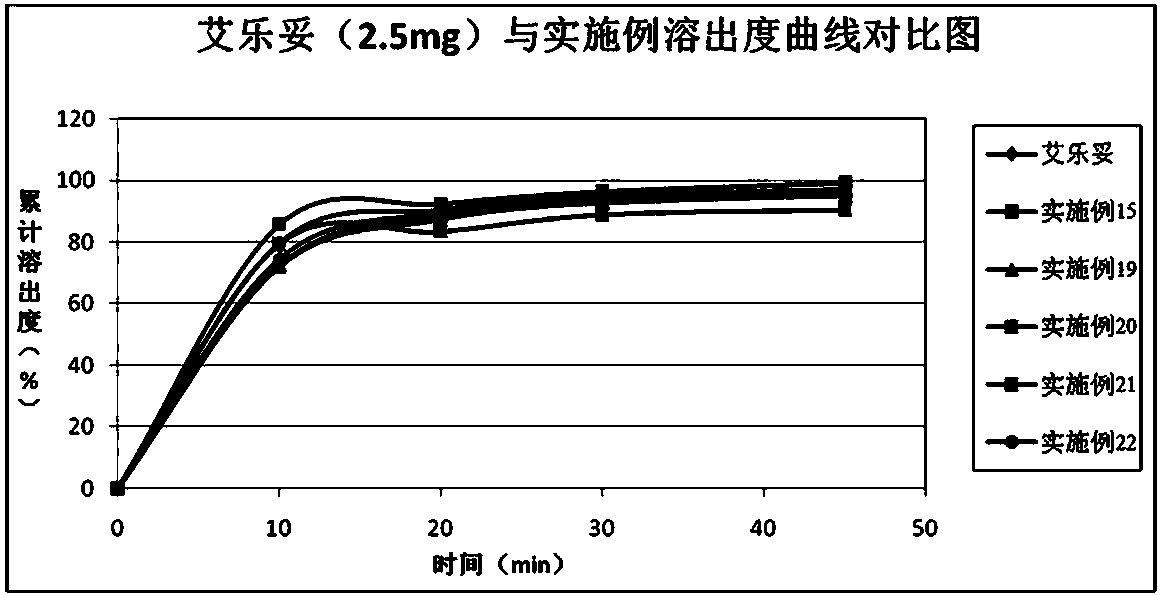
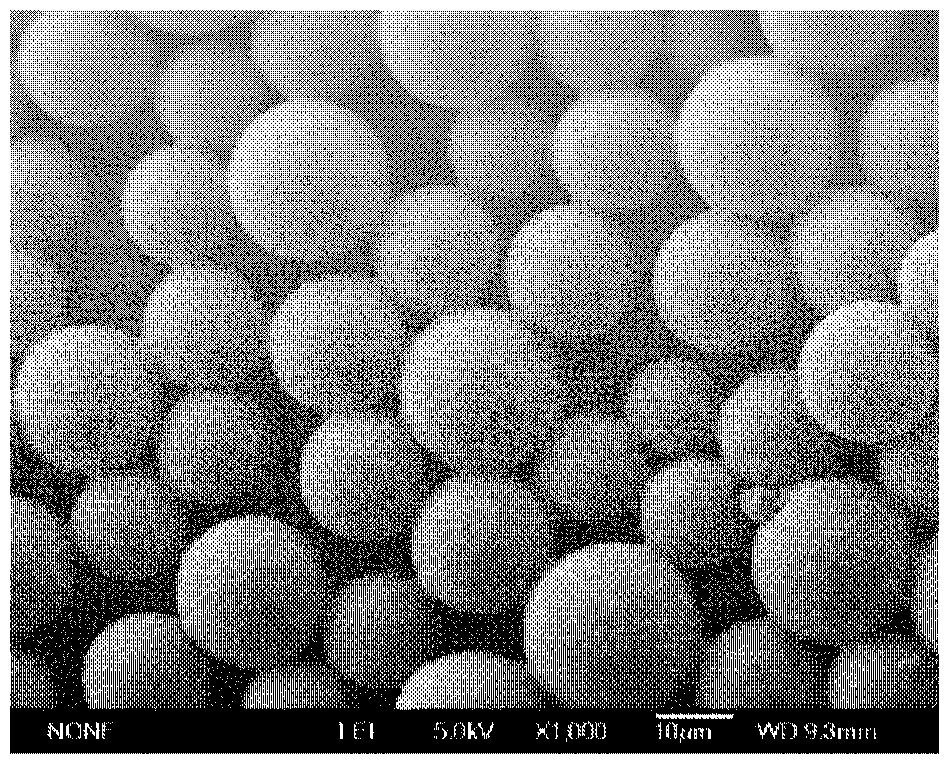Patents
Literature
218results about How to "Easy to scale up industrial production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing anode material of lithium ion battery in series of phosphate of olivine type
InactiveCN101049922AAchieve hybridEvenly distributedCell electrodesPhosphorus compoundsAluminium-ion batteryPhosphate
This invention relates to a method for preparing olivine-type phosphate-series lithium ion battery anode material. The method comprises: mixing one or more of ferrous salt solution, cobalt salt solution and manganese salt solution with oxalic acid or oxalate (precipitating agent) aqueous solution to obtain composite oxalate precursor, uniformly mixing with lithium source and phosphorus source by ball milling, and reacting in inert or weak-reductive atmosphere to obtain olivine-type phosphate-series lithium ion battery anode material. The method utilizes co-precipitation method for metal ion doping, and realizes molecular level uniform mixing among different ions. The obtained olivine-type phosphate-series lithium ion battery anode material has uniform chemical and physical compositions. The average particle size can be controlled within 0.3-10 mu.m. The first charge and discharge cycle specific capacity can reach 150 mAh / g at 0.1 C rate and room temperature. The livine-type phosphate-series lithium ion battery anode material has such advantages as high cycle performance and high charge / discharge performance.
Owner:CENT SOUTH UNIV
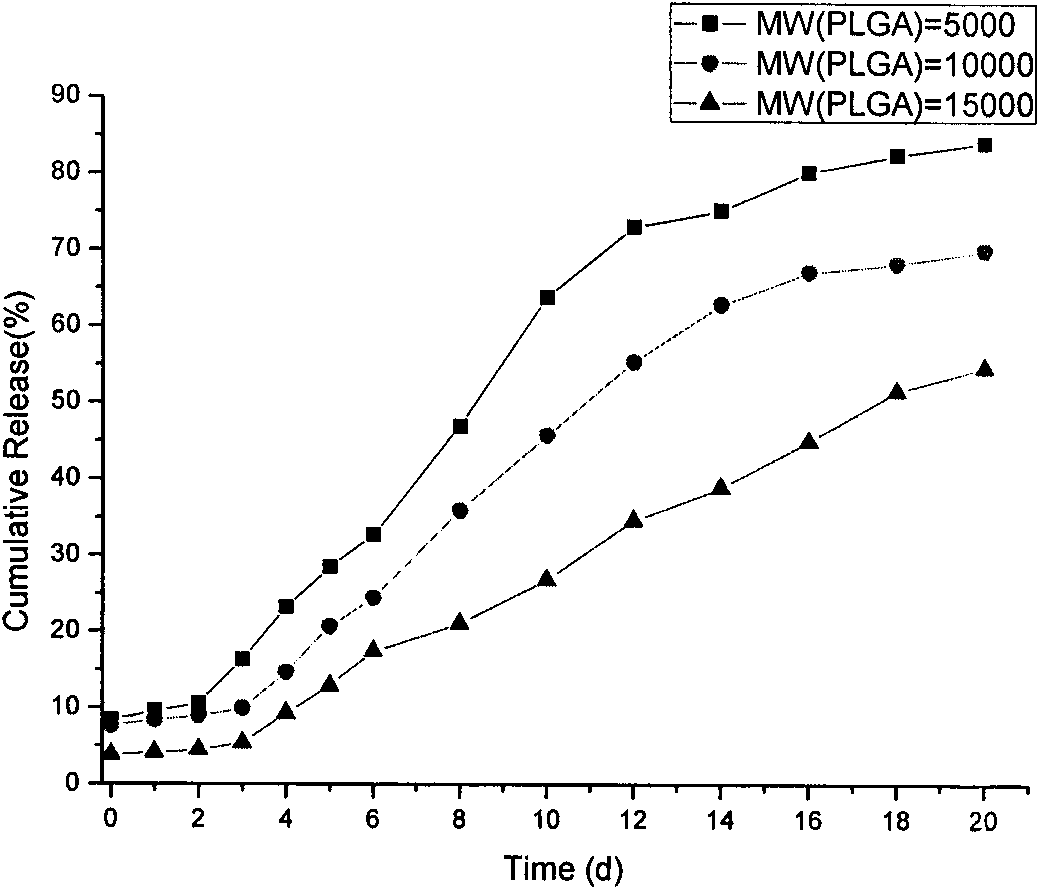
Polypeptide medicament sustained release microsphere or microcapsule preparation with uniform grain size and preparation method thereof
ActiveCN101559041ASmall particle sizeRegulated release ratePeptide/protein ingredientsMetabolism disorderMicrosphereMedicine
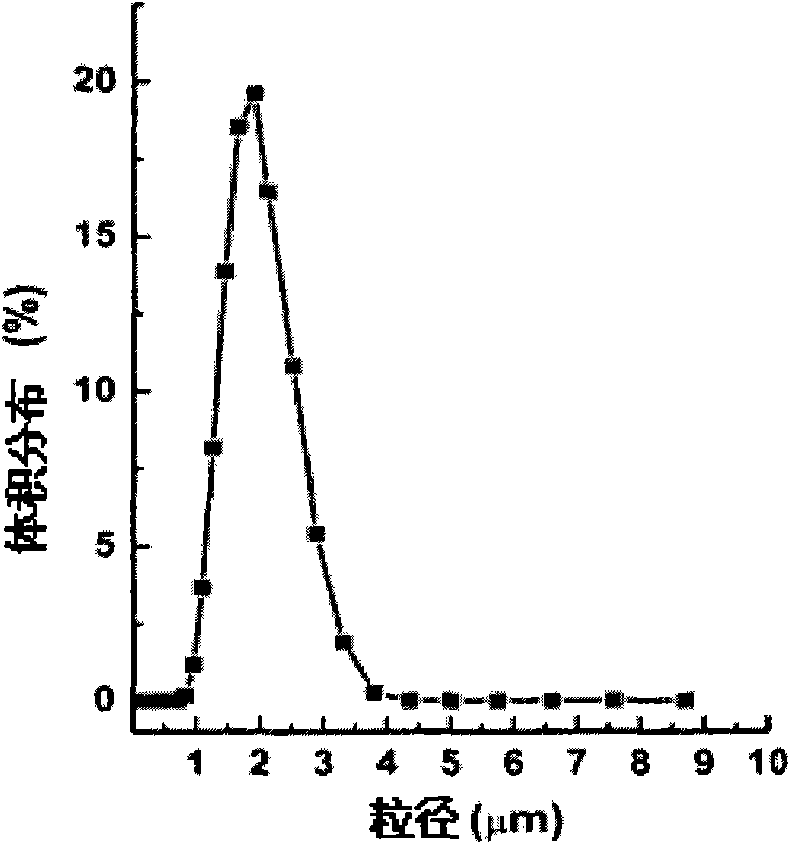
The invention discloses a polypeptide medicament sustained release microsphere or a microcapsule preparation with uniform grain size, a preparation method thereof and application. The average grain size of the microsphere or the microcapsule preparation is between 50 nanometers and 100 microns, and the grain size distribution coefficient CV value is less than 20 percent. The polypeptide medicament has a definite structure, has functions of therapy or adjuvant therapy of type-2 diabetes, and is preferably one or more of GLP-1, Exenatide, Exendin-4 and derivatives and analogs thereof. The microsphere or the microcapsule preparation uses a microsphere or a microcapsule with uniform grain size as a substrate to prepare the polypeptide medicament into a sustained release preparation through an embedding mode, and by changing the grain size of the microsphere or the microcapsule, the sustained release cycle is adjustable between one week and one month, and the microsphere or the microcapsule preparation can be applied to the therapy or the adjuvant therapy of the type-2 diabetes and body weight control. Besides, the microsphere or the microcapsule preparation has the advantages of simple preparation process and mild preparation course, and can protect the biological activity of the embedded polypeptide medicament.
Owner:辉粒药业(苏州)有限公司
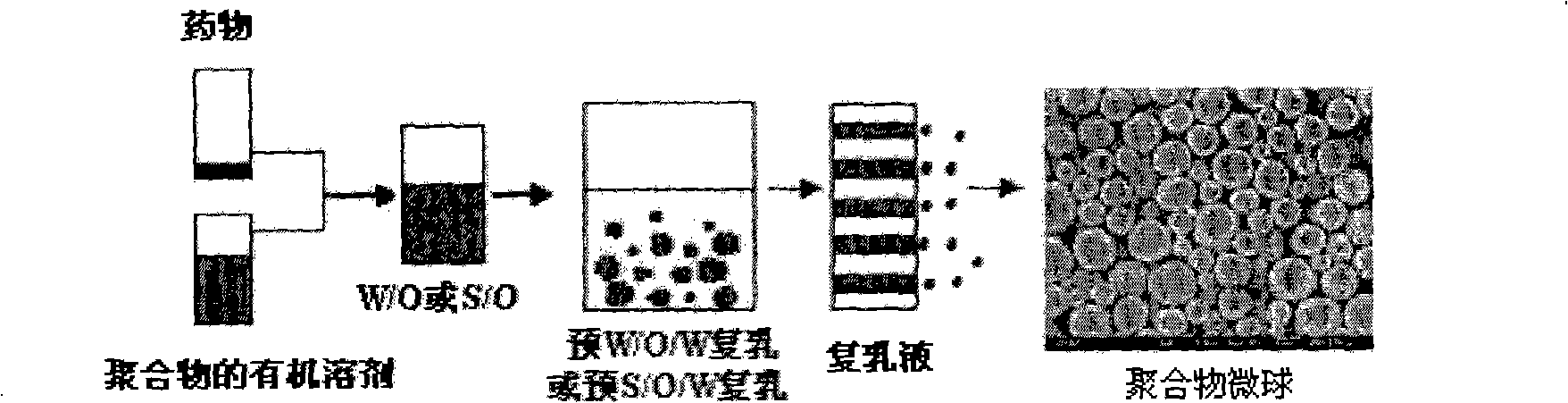
Method for preparing polymer microsphere
ActiveCN101269013AExpand the scope of applicationEasy to operateGenetic material ingredientsPharmaceutical non-active ingredientsDrugEmulsion
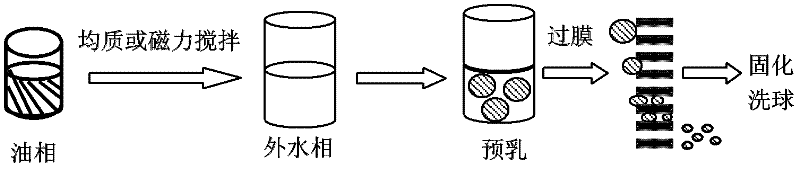
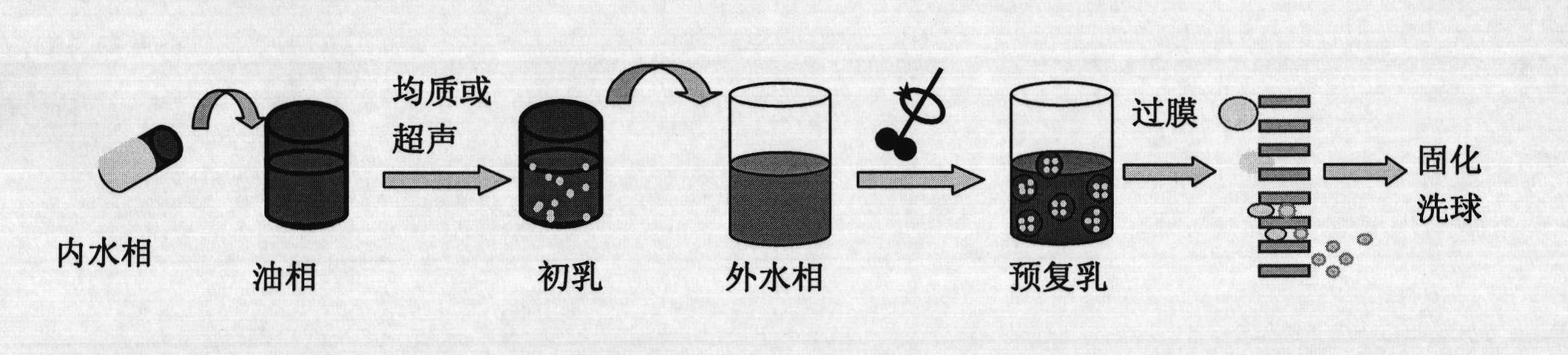
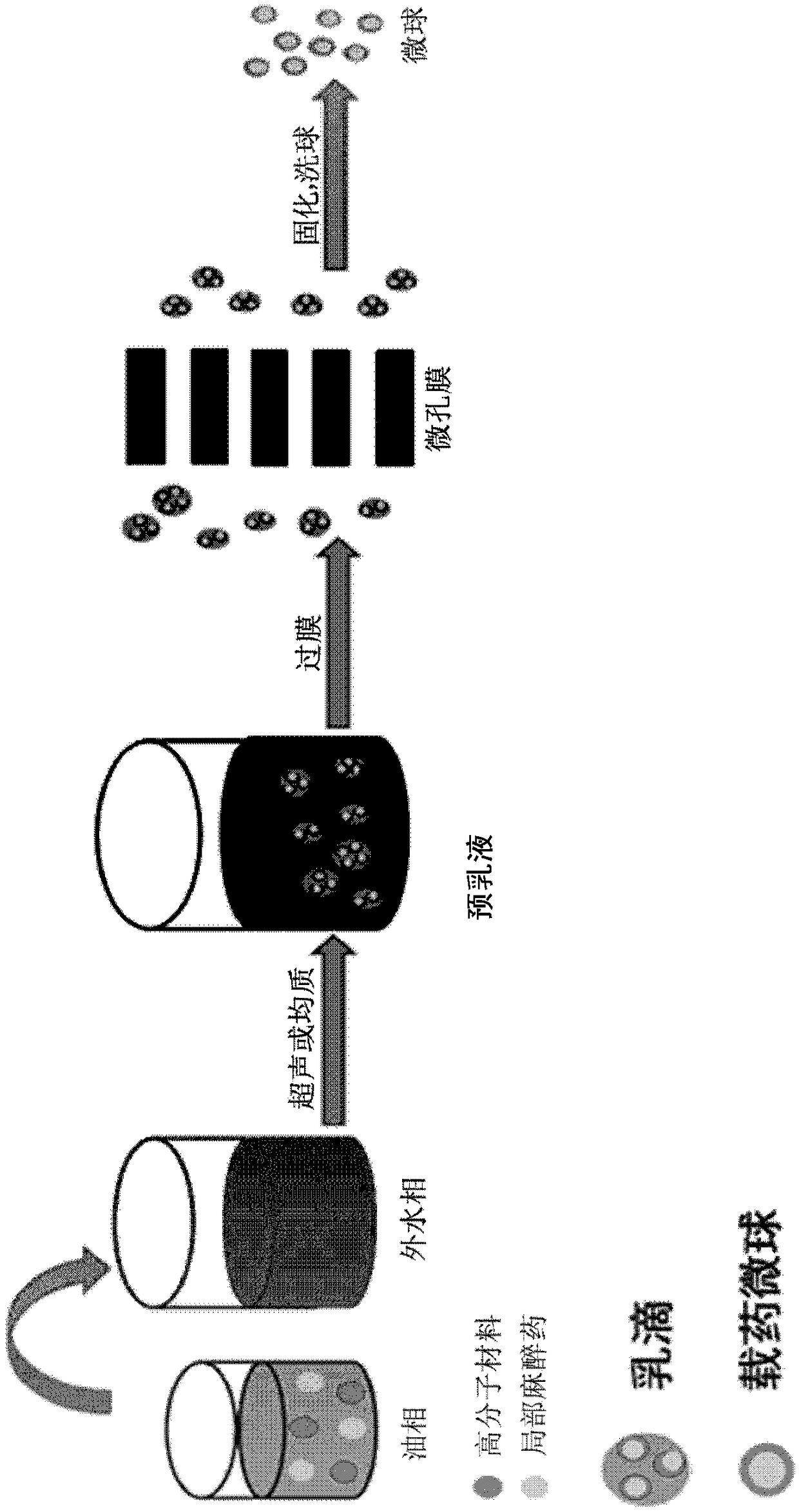
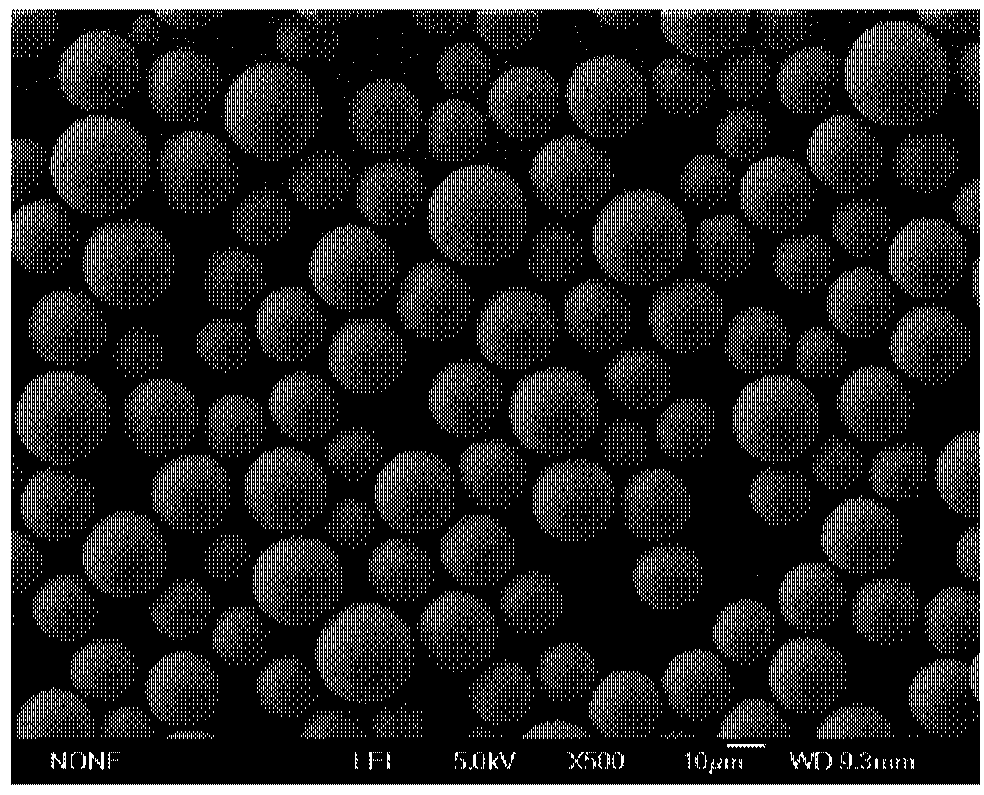
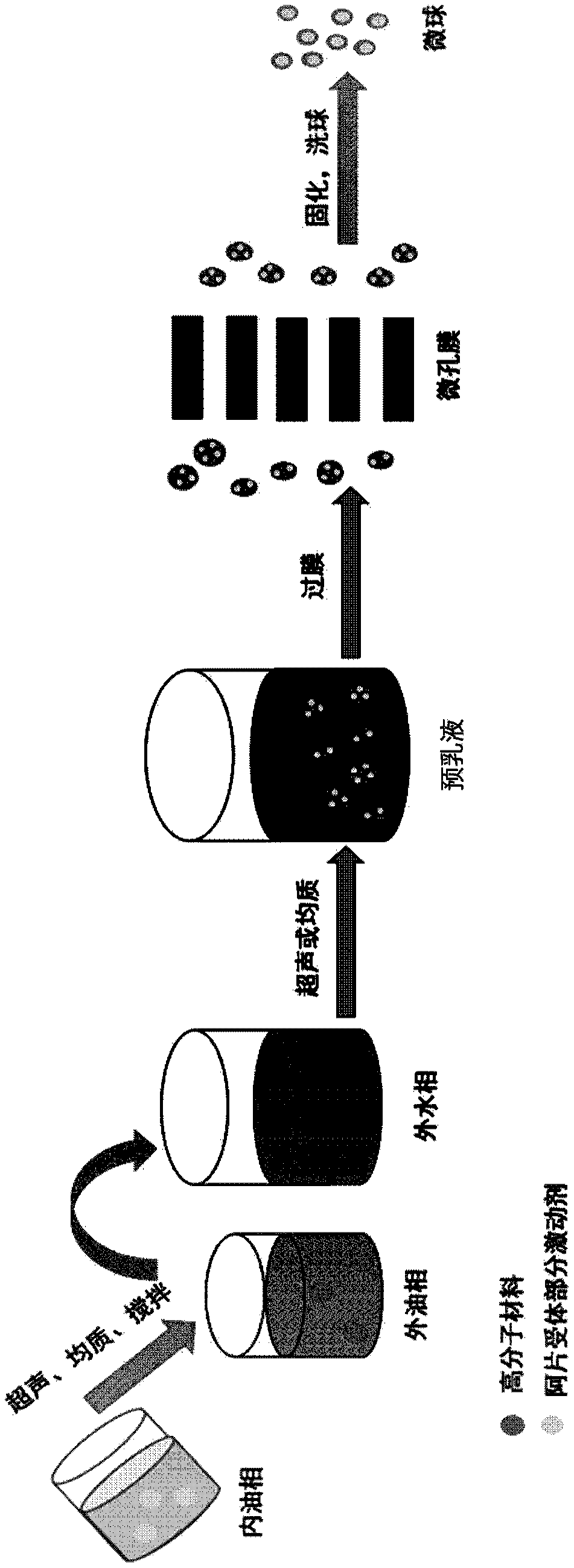
The invention discloses a preparation method of polymer microballoon sphere with uniform size, which can dissolve biodegradable polymer material in at least one organic solvent to form an O phase, and then adds the optional aqueous solution W1 containing medicine or medicine granule S into a fat phase O for emulsification to prepare colostrum; the obtained colostrum is added into an outer water phase W containing stabilizing agent to form pre-compound emulsion; then, the pre-compound emulsion is pressed through a microporous film by pressure to obtain compound latex; at last, after being solidified, the compound latex is centrifugated, washed, frozen and dried, so as to obtain the polymer microballoon sphere. The method of the invention is simple in technique, uniform in the grain diameter of the obtained product, good in repetitiveness of each batch product and easy in commercial process.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing ordered mesoporous aluminium oxide
InactiveCN101024503AGood dispersionUniform shapeAluminium oxide/hydroxide preparationAluminium hydroxide preparationSalt solutionMaterials science
The invention relates to a manufacturing method for ordered medium aperture alumina that includes the following steps: adding surface activator into inorganic aluminum salt as template agent, making precipitant solution, adding inorganic aluminum salt solution into hypergravity reactor, adding precipitant, when the reacting solution reaching a certain pH value, the precursor sol would be gained; taking aging, filtering, washing, and drying to the precursor sol to gain precursor powder. The powder could be made into ordered medium aperture alumina after taking sintering. The invention has the advantages of simple technology, safe to operate, low cost, etc. The specific surface of the product reaches 250-300m2 / g, and the shape has certain orderliness. It has crucial application value in adsorption and catalyzing process.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing ultracapacitor-used porous grapheme material
The invention discloses a method for preparing an ultracapacitor-used porous grapheme material, and belongs to the technical field of carbon material preparation. According to the method, coal pitch is used as the carbon source, nanometer calcium carbonate is used as a template, and potassa is used as an activating agent; the three components are ground in a dry method; the mixture is transferred to a corundum ceramic boat, is placed in a pipe furnace, and is subjected to heating activation under a normal pressure condition or a negative pressure condition to directly obtain the ultracapacitor-used porous grapheme material. The method adopts cheap coal pitch and nanometer calcium carbonate as the raw materials, and has the advantages being simple in technology, low in cost, suitable for industrialized production and the like; the material has a specific surface area within the range of 1330-1946 m<2> / g and an average aperture within the range of 2.32-3.08 nm, and can exhibit relatively high volume and energy intensiveness when being used as an electrode material of an ultracapacitor.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Butylphthalide synthesis method and purification technology
The invention relates to a butylphthalide synthesis method. The method comprises the steps that methyl 2-formyl benzoic acid is adopted as a starting material, THF is adopted as a solvent to react with an n-butyl magnesium chloride Grignard reagent, and acid regulation is performed to prepare a butylphthalide product. The invention further relates to a technology for preparing high-purity butylphthalide. The obtained crude butylphthalide product is subjected to hydrolysis treatment by an alkaline substance, acid regulation is performed to separate out solids, and filtering is performed to obtain a butylphthalide midbody; the acid regulation and alkali regulation processes are executed repeatedly, and finally ring closure and decompression desolvation are performed to obtain high-purity butylphthalide. According to the synthesis method, low-flash diethyl ether is prevented from being adopted as a solvent, the purification technology is easy to implement, the reagent can be purchased in bulk easily, column chromatography product purification and reduced pressure distillation under high temperature and high vacuum degree are not needed, and industrial enlarged production is easy.
Owner:福建省宝诺医药研发有限公司
New method for preparing polyaspartic acid hydrogels
The invention discloses a new method for preparing polyaspartic acid hydrogels, which comprises the following steps: preparing crosslinking and hydrolysis method-prepared crosslinked polyaspartic acid hydrogels by using polysuccinimide as a raw material and by using a heterogeneous suspension crosslinking method; and drying the crosslinked polyaspartic acid hydrogels to obtain solid polyaspartic acid hydrogel powder. In the step of preparing the crosslinking and hydrolysis method-prepared crosslinked polyaspartic acid hydrogels by using the heterogeneous suspension crosslinking method, a crosslinker and an alkaline mixture for hydrolysis are added into suspension of the polysuccinimide under a condition of the temperature of 10 below zero DEG C to 50 DEG C to perform crosslinking and hydrolysis at the time, and the reaction products are dried to obtain the polyaspartic acid hydrogels. The method has the advantages of simple preparation process, organic solvents are not used in the reaction processes, environment is protected, and the prepared polyaspartic acid hydrogels have high water absorption capacity and high anti-salt capacity. The invention belongs to the field of hydrogel preparation.
Owner:BEIJING UNIV OF CHEM TECH
Itraconazole composite powder and preparation method thereof
ActiveCN101780046ASimple processEasy to operateOrganic active ingredientsPowder deliverySolubilityAdditive ingredient
The invention relates to itraconazole composite powder and a preparation method thereof. The method comprises the following steps: dissolving itraconazole medicine into an organic solvent; adding the mixture of the itraconazole medicine and the organic solvent into a water solution with hydrophilic accessory ingredients for in-situ precipitation to separate out medicine; obtaining the nanometer amorphous itraconazole medicine grain turbid liquor; carrying out spraying drying or freeze drying on the obtained itraconazole medicine turbid liquor to obtain micron level itraconazole high molecular accessory ingredient composite powder; and then, dispersing the powder into water to obtain the uniform nanometer amorphous itraconazole medicine grain turbid liquor. The itraconazole high molecular accessory ingredient composite powder has good water-solubility and high dissolution speed, in addition, the operation is simple, the amplification is easy, the production cost is low, and the invention lays the foundation for the industrial production of the itraconazole medicine and the development and the utilization of novel preparations of the itraconazole medicine.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing porous flaky nano aluminum oxide
ActiveCN106830033AGood dispersionLarge specific surface areaNanotechnologyAluminium hydroxide preparationDispersityRoom temperature
The invention discloses a method for preparing porous flaky nano aluminum oxide. The method is characterized by comprising the following steps: (1) dissolving soluble salt and an expanding agent into deionized water, and stirring till the soluble salt and the expanding agent are completely dissolved; (2) adding an aluminum precursor into the solution obtained in the step (1), and stirring till the aluminum precursor is completely dissolved; (3) performing hydrothermal crystallization on the solution obtained in the step (2) under a dynamic condition; (4) after hydrothermal treatment, cooling the solution to the room temperature, filtering, washing, drying, and roasting, thereby obtaining the porous flaky nano aluminum oxide. The porous flaky nano aluminum oxide prepared by using the method has the advantages of being large in specific surface area, adjustable in pore, controllable in thickness, good in dispersity, high in thermal stability, rich in pentacoordinate aluminum, and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
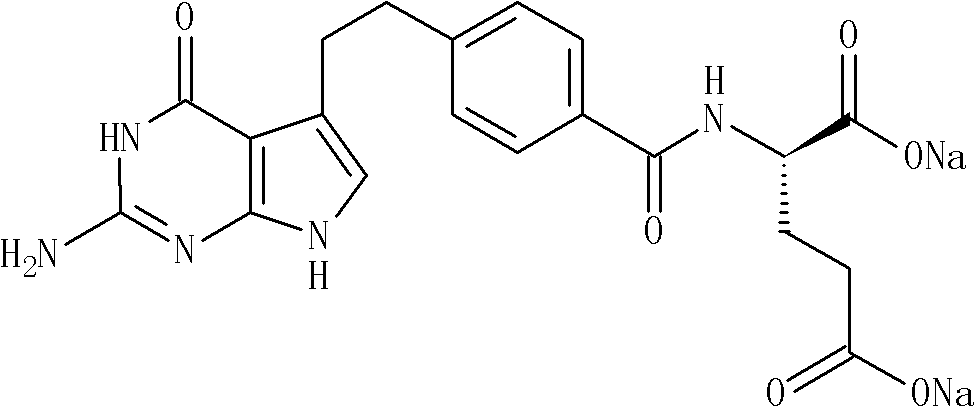
Industrialized production method of high-purity pemetrexed disodium
ActiveCN102086204AReduce Occupational InjuriesEasy to operateOrganic chemistryAcetonitrileSilica gel
The invention provides an industrialized production method of high-purity pemetrexed disodium, comprising the following steps of: (1) adding crude pemetrexed disodium into a reactor, adding water and stirring to dissolve at a temperature of 10-30 DEG C; (2) adding tetrahydrofuran or acetonitrile serving as a dissolvent into the reaction solution of the step (1), dissolving out a part of solids, adding kieselguhr or silica gel and stirring for 5-30 minutes; and (3) filtering the reaction solution of the step (2), adding dissolvent same as the dissolvent added in the step (2) into filtrate, crystallizing for 0.5-10 hours at a temperature of 10-30 DEG C, isolating solids, and drying for 0.5-10 hours at a temperature of 20-40 DEG C to obtain the high-purity pemetrexed disodium. By means of the production method, the shortcomings that in the prior art column chromatography, purification and heating are needed, the product purity is low, the operation is cumbersome and the industrialized production is difficult to realize are overcome; the production method is simple and convenient for operation, is easy to realize the industrialized production and has the advantages of few consumption of dissolvent, energy saving, environmental protection and low labor intensity; and the products have the advantages of white color, high purity, less than 0.05% of impurities in a single product and good stability.
Owner:NANJING HAIRUN PHARM CO LTD
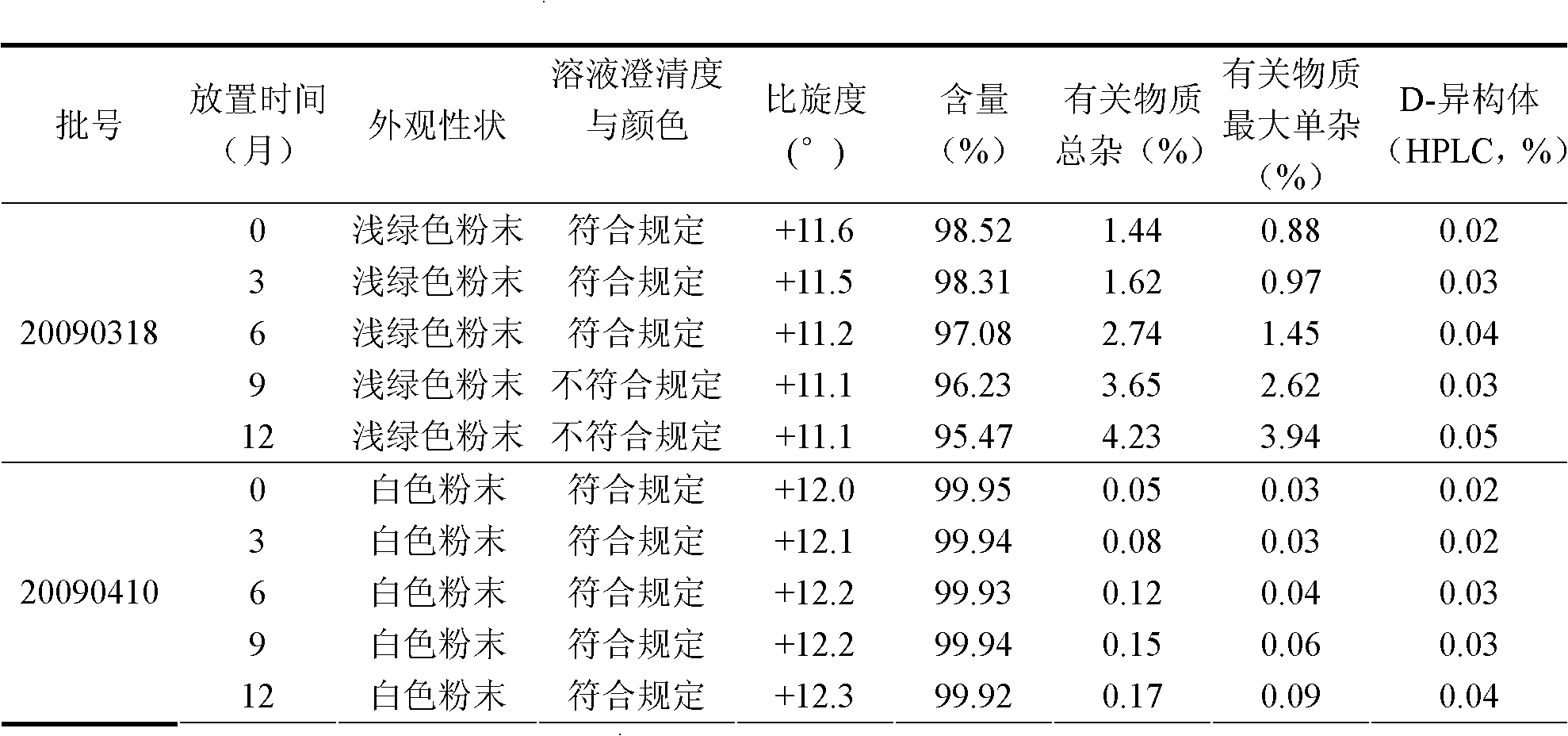
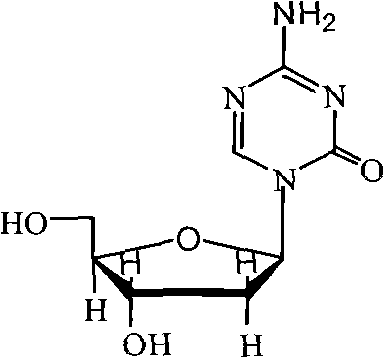
Industrialized production method for high-purity decitabine
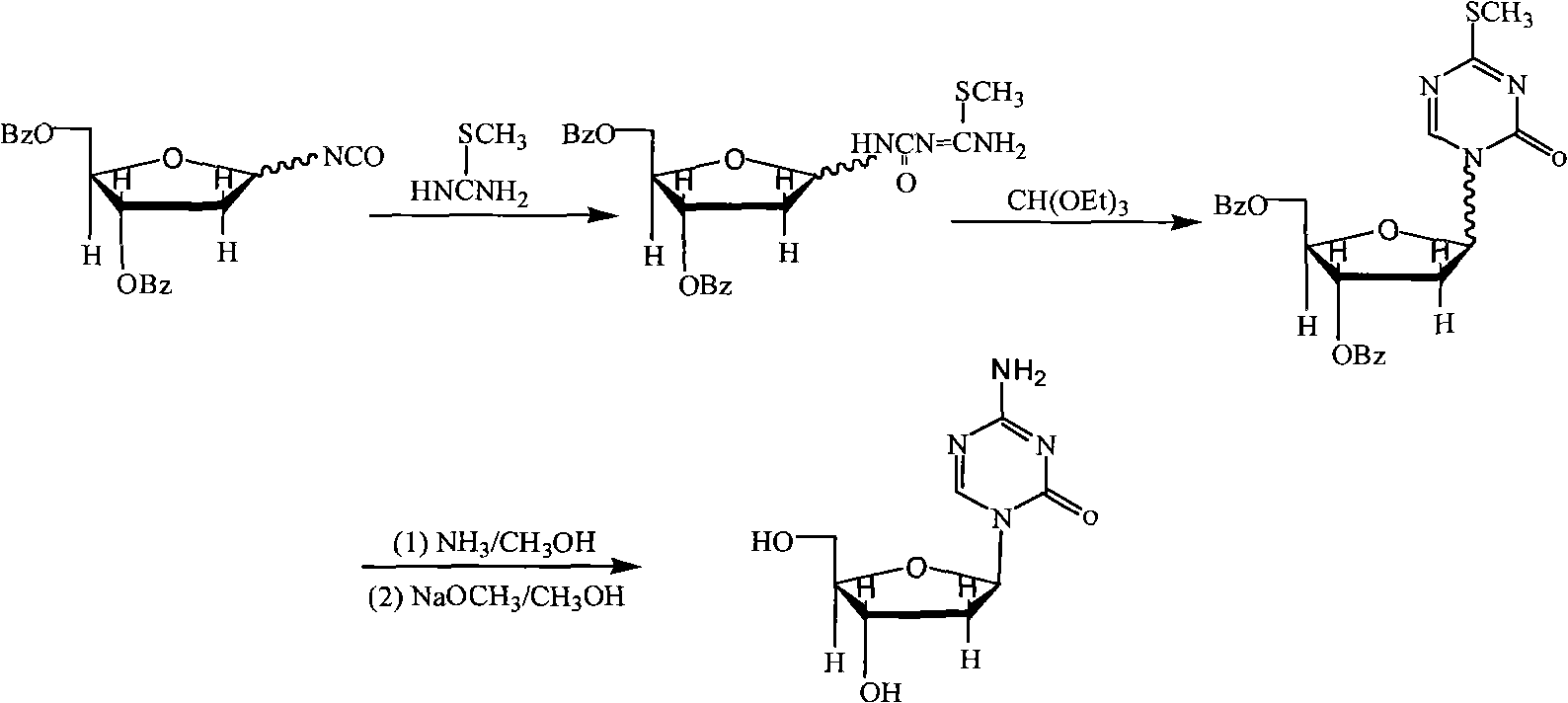
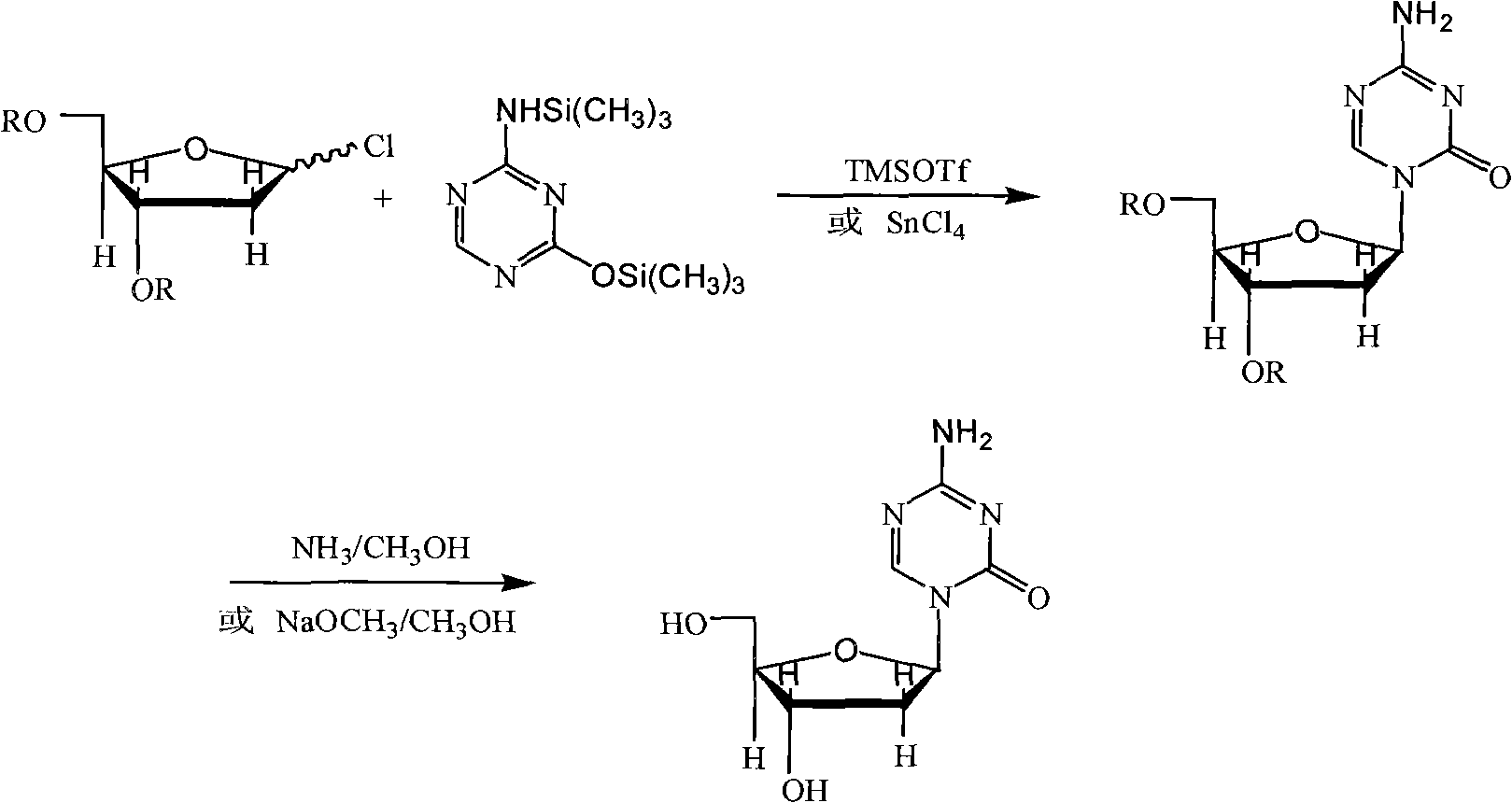
InactiveCN101948493AReduce the impactMeet quality requirementsSugar derivativesSugar derivatives preparationSodium methoxideSolvent
The invention provides an industrialized production method for high-purity decitabine. The method comprises the following steps of: 1, performing silanization reaction of 5-azacytosine, bis(trimethylsilyl)amine and trimethyl chlorosilane, which serve as raw materials, to prepare 2,4-bis(trimethylsilyl)-5-azacytosine; 2, performing reaction of the product obtained by the step 1 and 1-chloro-3,5-bis-(4-chlorobenzoyl)-2-deoxy-D-ribofuranose, which serve as raw materials, to prepare a crude product of 1-(3,5-bis-(4-chlorobenzoyl)-2-deoxy-beta-D-ribofuranose)-5-azacytosine; 3, dissolving the product obtained by the step 2 in a C5 to C7 hydrocarbon, stirring, filtering and drying to obtain a refined product; and 4, producing the high-purity decitabine by using the product obtained by the step 3, methyl alcohol and sodium methoxide as raw materials. The method overcomes the disadvantages of need of column purification, low purity, difficult industrial production in the prior art, and has the advantages of simple and convenient operation, small solvent consumption, small influence on the environment, low labor intensity, short period, high product purity, single impurity and less than 0.1 percent total impurity content.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Nickel-based catalyst prepared through solid-phase thermal dispersion and preparation method thereof
InactiveCN102091625ASimple processHigh economic utilizationMolecular sieve catalystsOrganic compound preparationSolid phasesMolecular sieve
The invention relates to a supported nickel-based catalyst and a preparation method thereof. The active ingredient of the catalyst is nickel, and the nickel accounts for 5 to 30 percent of the total mass of the catalyst; and silica, active carbon, silica gel, a molecular sieve or alumina is taken as a carrier. In the preparation method, the active ingredient is supported on the surface of the carrier by a solid-phase dispersion method, the dispersibility of the active ingredient is improved and the interaction force between the active ingredient and the carrier is enhanced through the heat treatment process, and a catalyst precursor is reduced through the liquid phase reduction process. Compared with the traditional immersion method, the method has the advantages that: a filtering and drying step is saved, the operating process is more simple, the catalyst preparation cost is low, the energy consumption is low, the method is environment-friendly, the activity of the prepared catalyst is equal to that of the catalyst prepared by the immersion method, and the method is suitable for industrial amplification production of the supported nickel-based catalyst.
Owner:NANJING UNIV OF TECH
Porous microspheres for medicine carriers, preparation method and medicine loading method
ActiveCN102258786AWide choiceIncrease load ratePharmaceutical non-active ingredientsSolubilityFreeze-drying
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
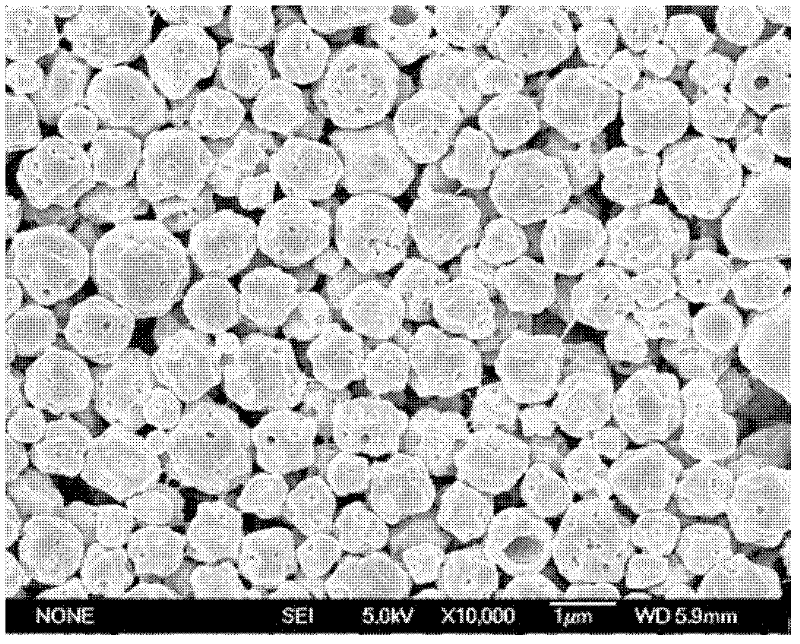
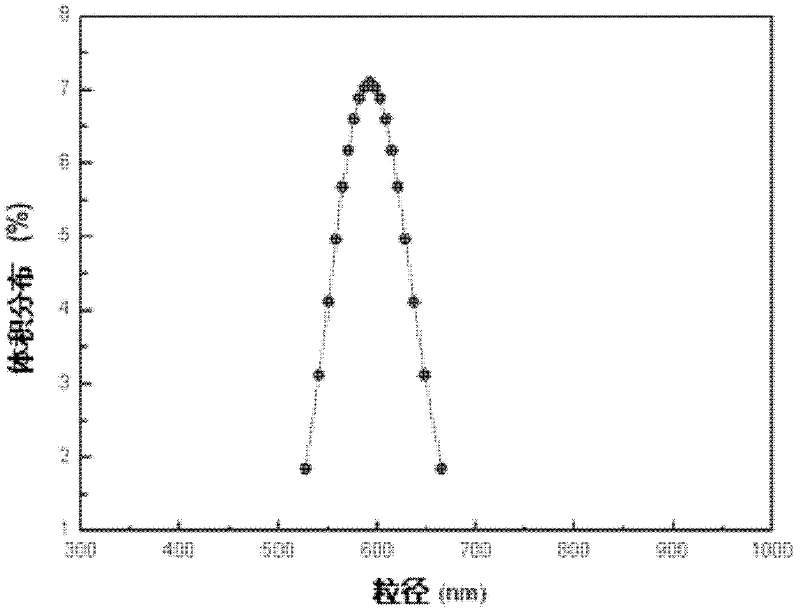
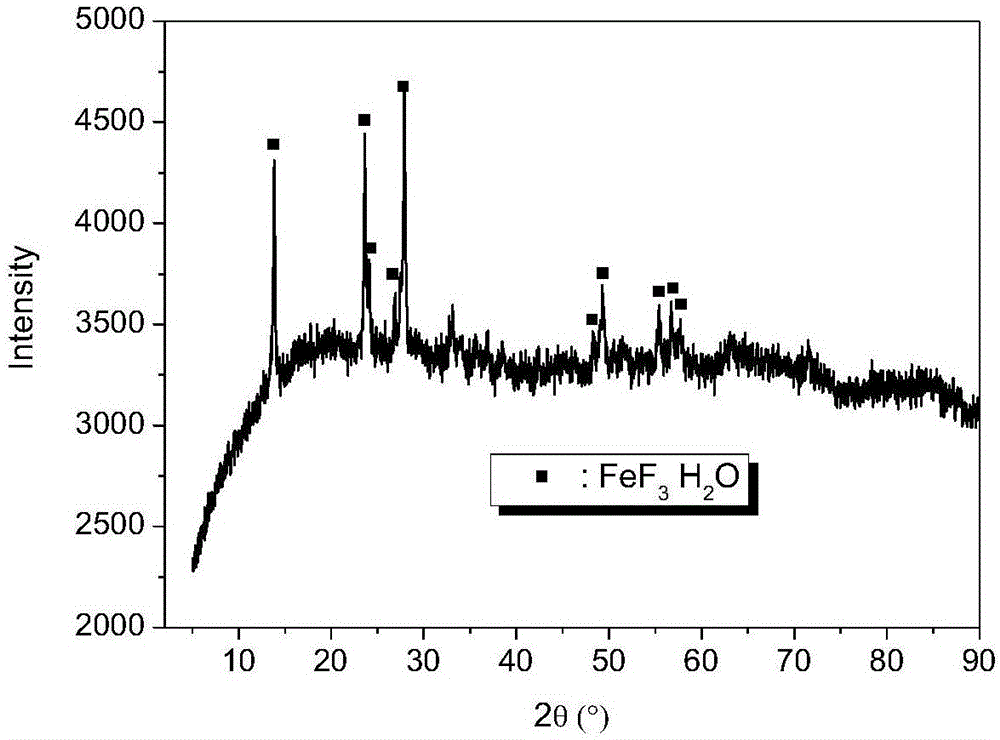
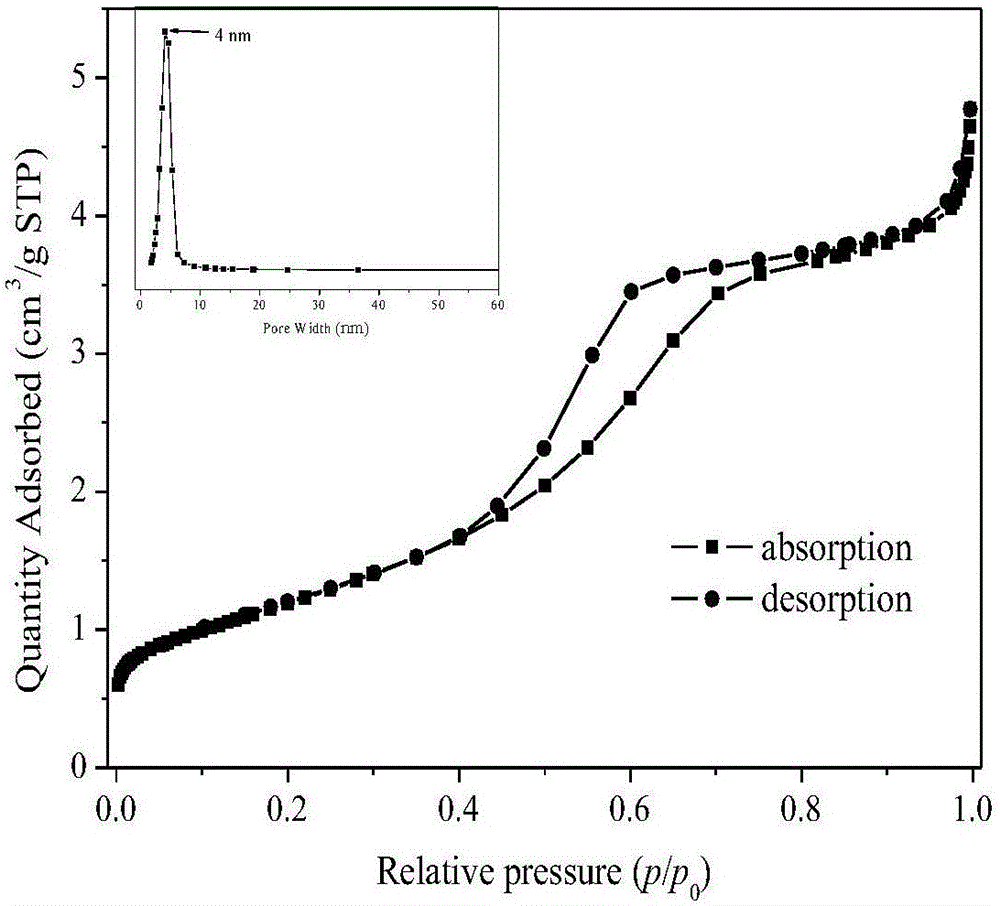
Nano iron fluoride-based composite material, and preparation method thereof
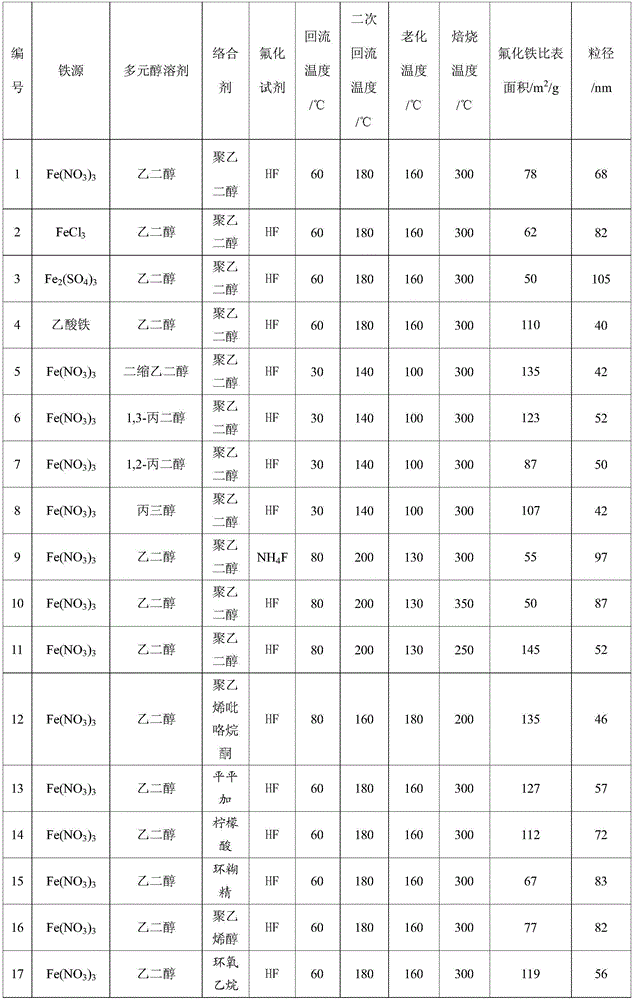
The invention discloses a preparation method of a nano iron fluoride-based composite material, which aims to solve the problems of high raw material cost, complex synthetic process, difficulty in uniform mixing of iron fluoride and carbon and oxygen elements, and inability for effective grain size control of the conventional preparation method. The preparation method of nanocrystalline iron fluoride comprises the following steps: (1) preparing a solution consisting of an iron source precursor, a polyalcohol solvent, and a complexing agent, and carrying out refluxing at a temperature of 30-80 DEG C; (2) adding a fluorinating reagent to a solution obtained through (1) for fluorination, and carrying out refluxing and stirring at a temperature of 140-200 DEG C to obtain a suspension; and (3) carrying out aging of the suspension at a temperature of 100-160 DEGC, carrying out washing and filtering of the aged suspension to obtain a solid, and finally calcinating the solid at a temperature of 200-350 DEG C to obtain the nano iron fluoride.
Owner:XIAN MODERN CHEM RES INST
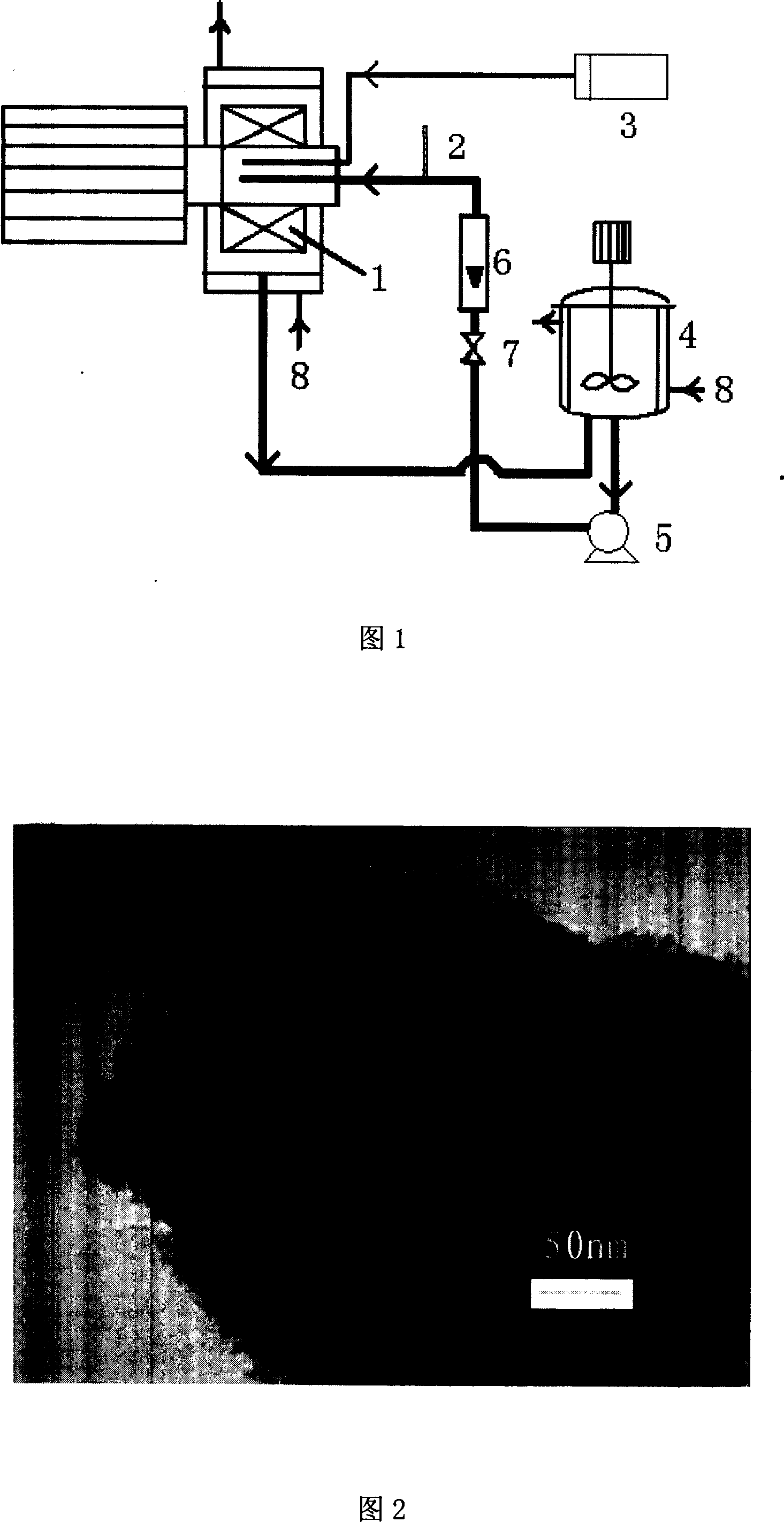
Recombinant human growth hormone (rhGH) long-acting sustained-release microcapsule and preparation method thereof
ActiveCN102370630ALow burst rateSmall particle sizePeptide/protein ingredientsMetabolism disorderOrganic solventFreeze-drying
The invention relates to the field of medicine, and specifically, relates to a recombinant human growth hormone (rhGH) long-acting sustained-release microcapsule and a preparation method thereof. The preparation method of the rhGH long-acting sustained-release microcapsule comprises the following steps of 1, dissolving a diblock amphiphilic polymeric material in an organic solvent to obtain an oil phase O, 2, adding an rhGH-containing aqueous solution W1 or rhGH-containing particles S into the oil phase O obtained by the step 1, and carrying out emulsification preparation to obtain W1 / O or S / O primary emulsion, wherein the rhGH-containing aqueous solution W1 is utilized as an inner water phase, 3, adding the W1 / O or S / O primary emulsion into a stabilizer-containing outer water phase W2 to obtain W1 / O / W2 or S / O / W2 composite pre-emulsion, 4, carrying out a filter pressing process on the W1 / O / W2 or S / O / W2 composite pre-emulsion through a millipore membrane to obtain W1 / O / W2 or S / O / W2 composite emulsion, and 5, removing the organic solvent in the W1 / O / W2 or S / O / W2 composite pre-emulsion, carrying out solidification, centrifugal washing and freeze drying of the organic solvent-free W1 / O / W2 or S / O / W2 composite emulsion. The rhGH long-acting sustained-release microcapsule obtained by the preparation method has the advantages of even size, high encapsulation efficiency, high activity, low burst release quantity, good repeatability, simpleness of operation, and benefit to drug effect activity keeping and industrialized mass production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Narcotic analgesic-loaded sustained-release microsphere and preparation method thereof and application thereof
ActiveCN109010307AGuaranteed repeatabilityGood for stabilityAntipyreticAnalgesicsDrugAnalgesic agents
The invention discloses a narcotic analgesic-loaded sustained-release microsphere and a preparation method thereof and application thereof. The narcotic analgesic-loaded sustained-release microspherecan be continuously released for 1 to 7 days, the burst release rate is less than 20% within 0.5 hour, and the drug embedding rate is higher than 80%, so that high drug embedding rate and low burst release rate and sustained release can be realized. The method has simple process, the obtained product has uniform particle size, batches of products have good repeatability, and the preparation methodis easy for industrial production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +2
Construction and application of recombinant strain converting L-threonine to L-2-aminobutyric acid
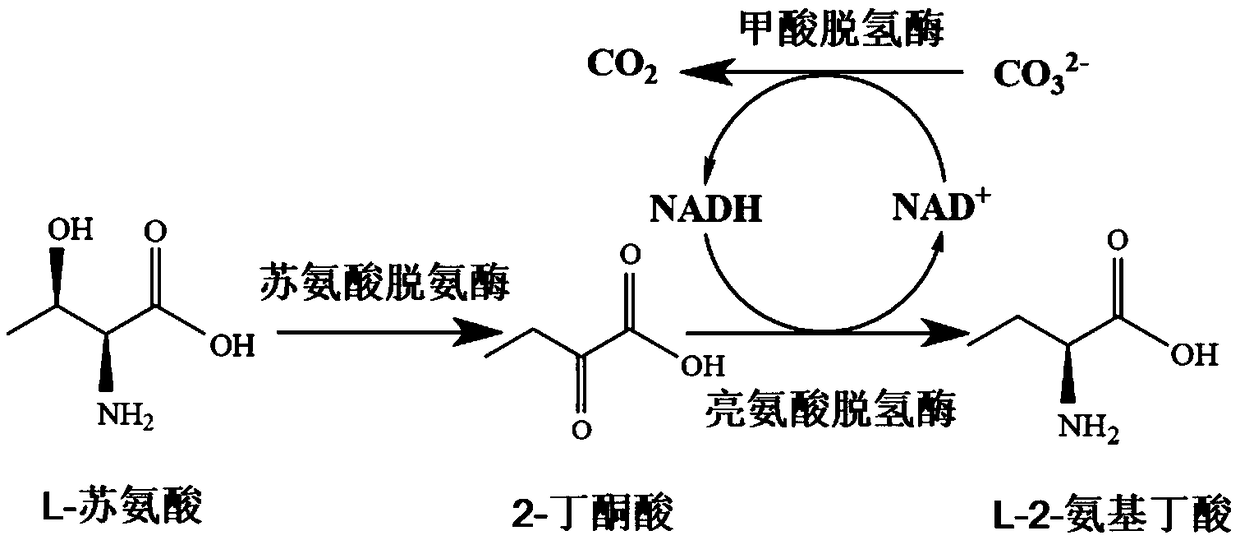
ActiveCN109266595ALow costEasy to scale up industrial productionCarbon-nitrogen lyasesBacteriaL-threonineSal ammoniac
The invention discloses a construction and application of a recombinant strain converting L-threonine to L-2-aminobutyric acid, belonging to the field of bioengineering technology. The production method of the invention utilizes a recombinant bacterium expressing two plasmids to simultaneously realize the high-efficient expression of three enzymes, and comprises the steps of: conversion of L-threonine to L-2-aminobutyric acid, coupled with a coenzyme regeneration system, converts NAD+ into NADH, so that the concentration of NADH in the system is relatively stable, and the conversion can be carried out efficiently. Furthermore, the CO<2> converted from ammonium formate can be dissolved in ammonia water, which has little environmental pollution and industrial application value. The method has the advantages of mild conversion condition, strong specificity, low cost and short conversion time. The L-2-aminobutyric acid is prepared adopting the method, 40g / L L-threonine is added, The concentration of the obtained product L-2-aminobutyric acid is 43.3 g / L, and the conversion ratio was over 99.9%.
Owner:JIANGNAN UNIV
Borophene nanosheet and preparation method thereof
PendingCN112758950AMitigate edge oxidation problemsThe method is simpleBoronNanotechnologyFluid phaseChemistry
The invention discloses a borophene nanosheet and a preparation method thereof, and mainly solves the problem of preparing a two-dimensional borophene nanosheet from boron powder at present. The borophene nanosheet has a typical two-dimensional layered structure, the thickness of the borophene nanosheet ranges from 0.3 nm to 10 [mu]m, the transverse size of the borophene nanosheet ranges from 100 nm to 100 [mu]m, and the mass content of boron is larger than 90%. The preparation method comprises the following steps: providing boron powder, adding the boron powder into a solvent, carrying out ultrasonic treatment in a water bath, then adding the obtained product into concentrated acid, carrying out ultrasonic treatment, and carrying out centrifugal drying to obtain an intercalation product; performing high-temperature expansion on the intercalation product to obtain expanded boron powder; and finally, performing liquid-phase stripping on the expanded boron powder to obtain the borophene nanosheet. The invention provides a simple, green, efficient and low-cost method for preparing the borophene nanosheet, and large-scale production can also be realized.
Owner:JIANGSU XFNANO MATERIALS TECH CO LTD
Method for preparing food-grade lutein crystal from marigold extract
The invention provides a method for preparing a food-grade lutein crystal from a marigold extract. The method comprises the following steps of: performing a saponification reaction on the marigold extract in a food-grade lower alcohol solvent by adding a solid alkali; diluting; then cooling and crystallizing; regulating the pH value; filtering; washing by adding an alcohol-water solution; and drying by adding a small amount of antioxidant, thereby obtaining lutein crystal powder. According to the method, the process is simple; the adopted solvent is recycled after being distilled, so that the treatment cost is low. Thus, the method is liable to industrial production. As the method is used, the defects of the existing lutein preparation method that the cost is high, the process is complicated, the time consumption is long and the lutein ester is unstable by performing reactions via harmful organic solvents under an acidic condition and at a high temperature are overcome. For the prepared lutein crystal, the actual yield is greater than 85%; and the purity reaches 80% to 90%. The prepared lutein crystal can be further prepared into an oil suspension or micro capsule powder which is applied to the fields of food additives, food supplements, functional foods and the like.
Owner:INNOBIO CORP LTD
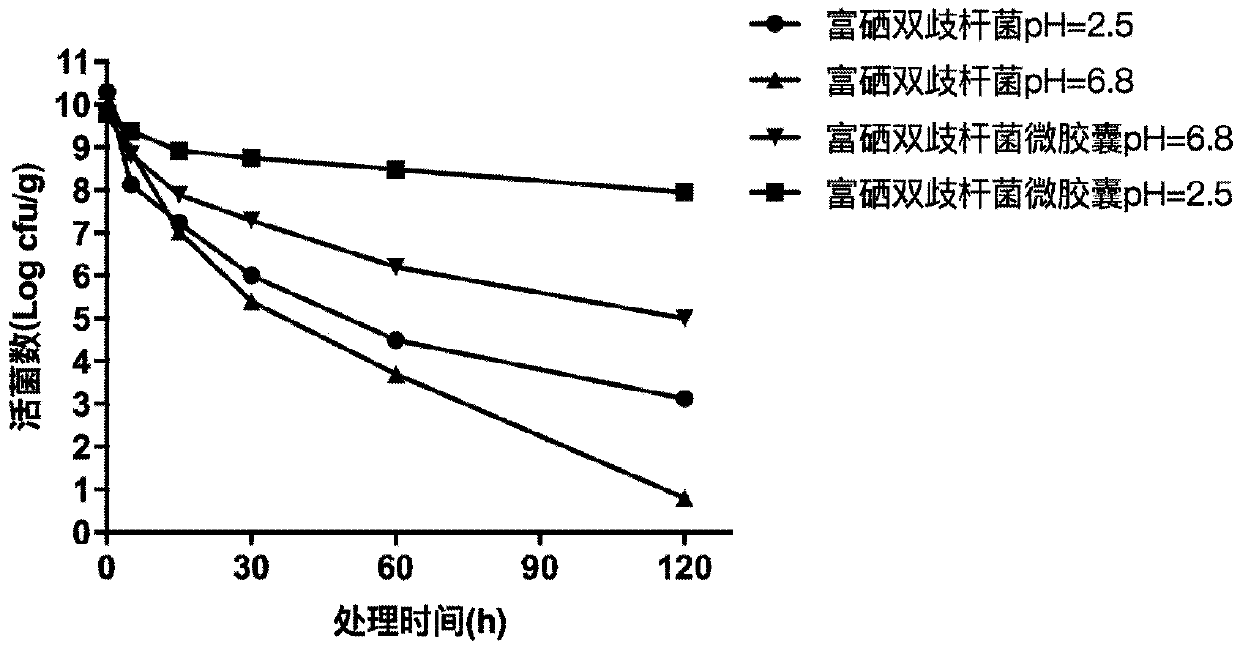
Selenium-enriched bacillus bifidus micro-capsules
InactiveCN109601811AHigh embedding rateImprove acid and bile salt resistance propertiesMilk preparationFood scienceVegetable oilFreeze-drying
The invention discloses selenium-enriched bacillus bifidus micro-capsules. The selenium-enriched bacillus bifidus micro-capsules are prepared by adopting a method comprising the following steps: uniformly mixing a sodium alginate solution with selenium-enriched bacillus bifidus thallus and calcium carbonate powder so as to obtain a mixture; dropwise adding the mixture into vegetable oil containingan emulsifier, and carrying out stirring so as to obtain an emulsion; sequentially adding vegetable oil containing glacial acetic acid and an emulsifier solution into the emulsion, carrying out stirring, and allowing standing; and then, separating oil phase so as to obtain aqueous phase, and centrifuging the aqueous phase so as to obtain the selenium-enriched bacillus bifidus micro-capsules. By screening reagents, materials and dosage ratio adopted in micro-capsule preparing, coating and freeze-drying processes, embedding rate of the selenium-enriched bacillus bifidus micro-capsules disclosedby the invention are improved. The embedding rate of the selenium-enriched bacillus bifidus micro-capsules is up to 98% while the particle size is smaller than 200 microns and the freeze-drying survival rate is up to 85%. According to normal-temperature stability results, viable number of the selenium-enriched bacillus bifidus micro-capsules is still about 10<8> cfu / g within 180 days.
Owner:JIANGSU DAYSEBIOTECH LTD +1
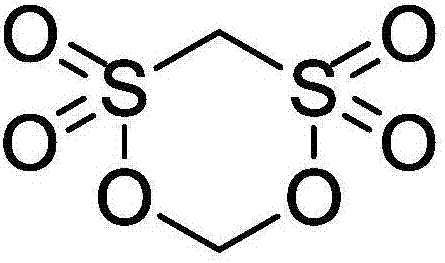
Methylene methanedisulfonate synthesis method
The invention provides a methylene methanedisulfonate synthesis method. The methylene methanedisulfonate synthesis method comprises the following preparation steps: adding methane-disulfonic acid and paraformaldehyde to a reaction vessel, stirring and mixing, then adding anhydrous magnesium sulfate, heating to 70-100 DEG C, carrying out isothermal reaction for 5-8 hours, cooling to room temperature, extracting solids by using dichloromethane, filtering to remove insoluble substances, and carrying out spin dry on a filtrate to obtain white methylene methanedisulfonate. The methylene methanedisulfonate synthesis method has the beneficial effects that a novel dehydrating agent is used, thereby causing reaction to be pure and environment-friendly; an organic solvent does not need to be added; the stirring of the system is easy; the reaction is complete, thereby improving the yield of the generated methylene methanedisulfonate; the reaction time is short; the aftertreatment is convenient; and the mass production can be realized simply and convenient.
Owner:泰兴华盛精细化工有限公司
Process for preparing micro Azithromycin powder
InactiveCN1973844AGranularity controllableShape is easy to controlAntibacterial agentsOrganic active ingredientsSolubilityAzithromycin
The process of preparing superfine Azithromycin powder belongs to the field of superfine medicine powder preparing technology. Specifically, the process includes marketable Azithromycin medicine material in organic solvent to obtain Azithromycin solution, adding the Azithromycin solution in counter solvent in certain volume through magnetic stirring at certain temperature to deposit Azithromycin crystal and obtain crystal slurry, ageing at certain temperature, filtering, washing and drying to obtain superfine Azithromycin powder product. The process is simple, safe, low in cost and suitable for industrial production, and the obtained superfine Azithromycin powder has high leaching rate and high solubility.
Owner:BEIJING UNIV OF CHEM TECH
High-activity iron-based catalysts for coal direct liquefaction and preparation methods for high-activity iron-based catalysts
InactiveCN102527432AHigh catalytic activityGood dispersionOrganic-compounds/hydrides/coordination-complexes catalystsLiquid hydrocarbon mixture productionHydrogenSolvent
The invention provides three high-activity iron-based catalysts for coal direct liquefaction and preparation methods for the three high-activity iron-based catalysts. The three catalysts are iron oleate, iron naphthenate and Fe3O4 hollow nanospheres respectively. The series of catalysts are oil-soluble, can contact with coal samples well and can react completely. A solvent tetrahydronaphthalene used in the process of preparing the Fe3O4 hollow nanospheres is the solvent used in coal direct liquefaction reaction; by adoption of the 'in-situ' synthesis technology, the catalysts can be directly used in the coal direct liquefaction reaction without aftertreatment; and the catalysts have high contact surface area and high activity in a reaction system. The catalysts are low in preparation cost and simple in preparation and are not required to be recycled. A small quantity of the catalyst is used in the coal direct liquefaction process, but the catalysts have high activity, so temperature and pressure required during the coal direct liquefaction are reduced, conversion ratio of the coal and the yield of oil are obviously increased, and industrial amplified application is realized.
Owner:XINJIANG UNIVERSITY
Method for preparing ordered mesoporous aluminum oxide in batch
InactiveCN101376517ALarge specific surface areaNarrow pore size distributionAluminium oxides/hydroxidesMaterials preparationNano structuring
The invention relates to a method for batch-preparing organized mesoporous alumina, which belongs to the nano structure material preparation field. In the method, a surface active agent is added to be used as a template agent after inorganic aluminum salting liquid is manufactured; precipitator liquid is confected at the same time; the confected precipitator is added at certain speed to obtain an inorganic precursor sol. The obtained inorganic precursor sol is processed through aging, then filtration, washing and drying to obtain inorganic precursor powders; the obtain inorganic precursor powders are calcined according to the set calcination process under nitrogen atmosphere to obtain the organized mesoporous alumina. The method has simple technology, safe operation and low cost, and is easy for industrialized enlarged production. The prepared mesoporous alumina has the advantages of large specific surface area (250 to 300m<2> / g), narrow pore-size distribution, vermiform pore canal, sequential size and shape, and important application value during the absorption and catalysis process.
Owner:BEIJING UNIV OF TECH
Preparation method of Cu-Im-Ga-Se quaternary semiconductor alloy
InactiveCN103572089AMild reaction conditionsReduce manufacturing costFinal product manufactureSemiconductor devicesIndiumInduction furnace
The invention aims to provide a preparation method of a Cu-Im-Ga-Se quaternary semiconductor alloy. The preparation method comprises the concrete steps: placing a quartz crucible with Cu-Im-Ga raw materials in a vacuum induction furnace, vacuumizing, introducing argon, controlling the vacuum degree of the induction furnace at 20-30mmHg, gradually heating to 500-700 DEG C, then, heating to 900-1100 DEG C, melting for 10-30min, and cooling in the furnace to obtain a Cu-Im-Ga ternary alloy; respectively filling the prepared Cu-Im-Ga ternary alloy and Se powder at two ends of a quartz tube, vacuumizing, and sealing the quartz tube; heating the Cu-Im-Ga-Se filling end of the quartz tube to 850-950 DEG C by using a tube furnace; heating the Se powder filling end of the quartz tube to 300-330 DEG C by using the tube furnace, and preserving the temperature for 0-20min; preserving the temperature of 300-500 DEG C for 1-20h; heating from 500 DEG C to 750 DEG C; cooling the Cu-Im-Ga-Se filling end to 750 DEG C when the temperature at the Se powder filling end is up to 750 DEG C, and reacting Im with Se at the temperature of 750 DEG C for 5-60h; and shutting off the tube furnace to stop heating, and cooling to the room temperature to obtain a solid Cu-Im-Ga-Se sample.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
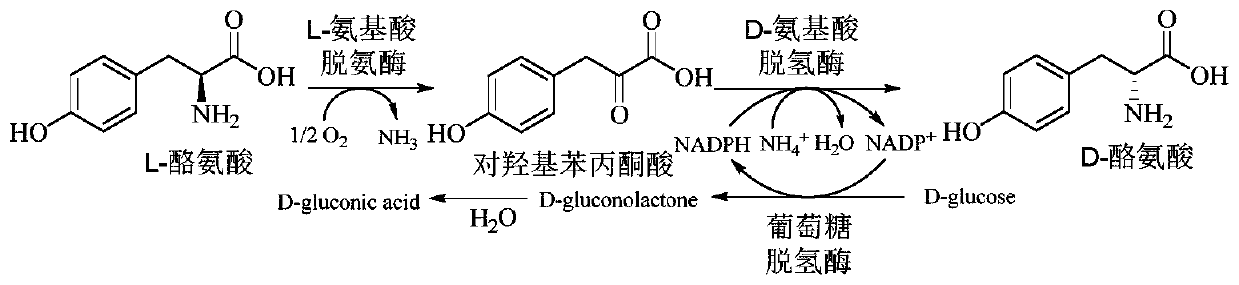
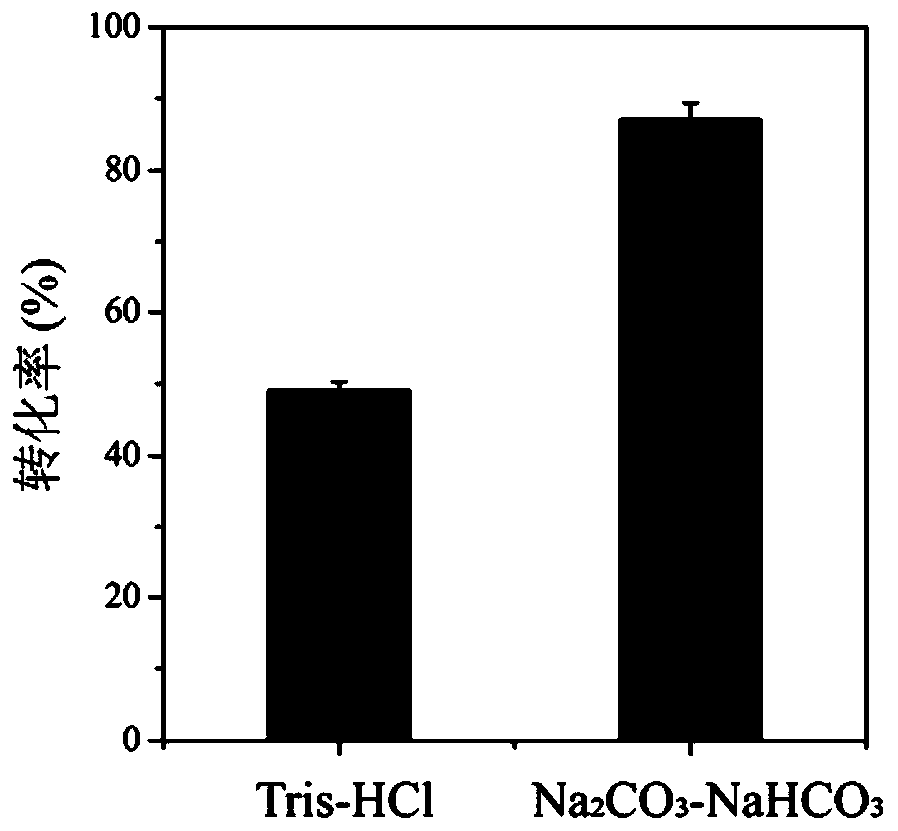
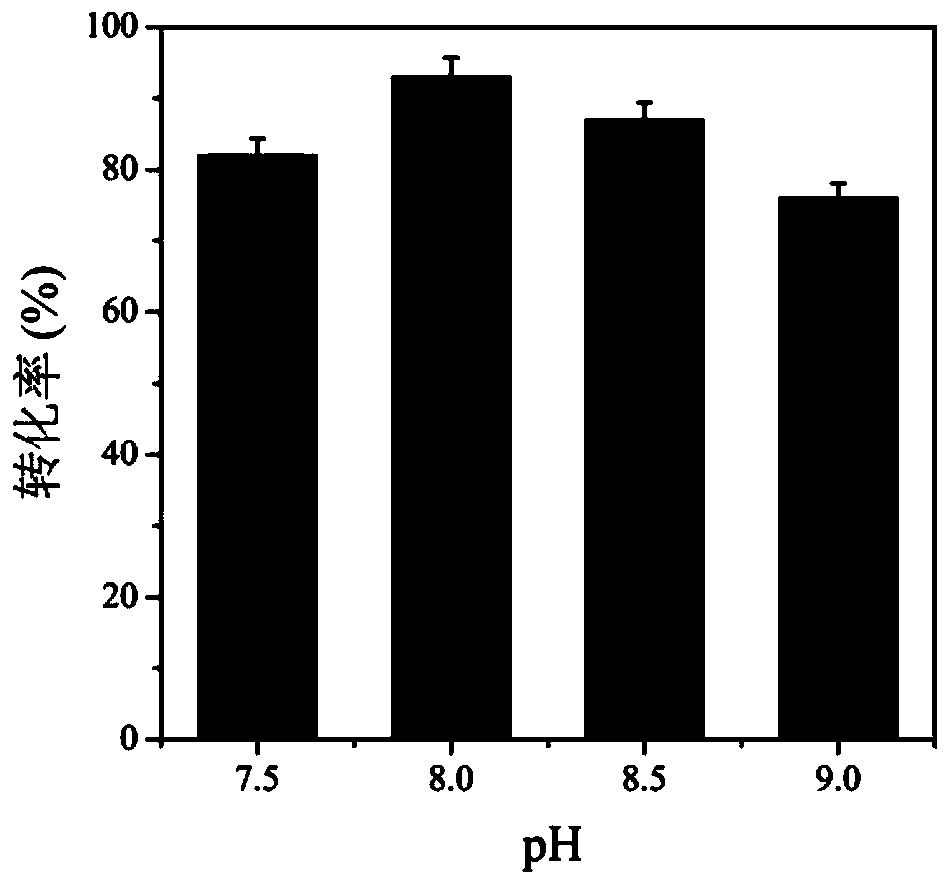
Method for improving production efficiency of D-tyrosine
InactiveCN110938580ALow costEasy to scale up industrial productionBacteriaMicroorganism based processesBiotechnologyTyrosine
The invention discloses a method for improving production efficiency of D-tyrosine, which belongs to the technical field of bioengineering. A double-plasmid co-expression recombinant bacterium is constructed through a molecular biological means, efficient expression of three enzymes is achieved at the same time, L-tyrosine is converted into D-tyrosine, NADP<+> is converted into NADPH through a coupling coenzyme regeneration system, and NADPH cyclic regeneration and conversion can be efficiently conducted, the efficient production of D-tyrosine is realized by optimizing whole-cell transformation conditions. The yield of the D-tyrosine produced by the method can reach 8.4 g / L, and the conversion rate reaches 93%.
Owner:JIANGNAN UNIV
Opioid receptor partial agonist supported sustained release microsphere as well as preparation method and application thereof
ActiveCN108704137AHigh drug loading rateGuaranteed repeatabilityOrganic active ingredientsNervous disorderDrugPartial agonist
The invention discloses an opioid receptor partial agonist supported sustained release microsphere as well as a preparation method and application thereof. The opioid receptor partial agonist supported sustained release microsphere has a drug embedding rate of higher than 80%, the initial burst release of the drug is lower than 20% within half an hour, and the drug can realize sustained release ata constant speed for 1-15 days. The preparation method disclosed by the invention is simple in process, the prepared product is uniform in particle size, the repeatability of each batch of products is excellent, industrial production is easily realized, the repeatability of the product is ensured, the curative effect of the drug is stable, the prepared microsphere has excellent re-suspending property in water, and industrial production cost is saved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Crystal form B of Apixaban and preparation method thereof
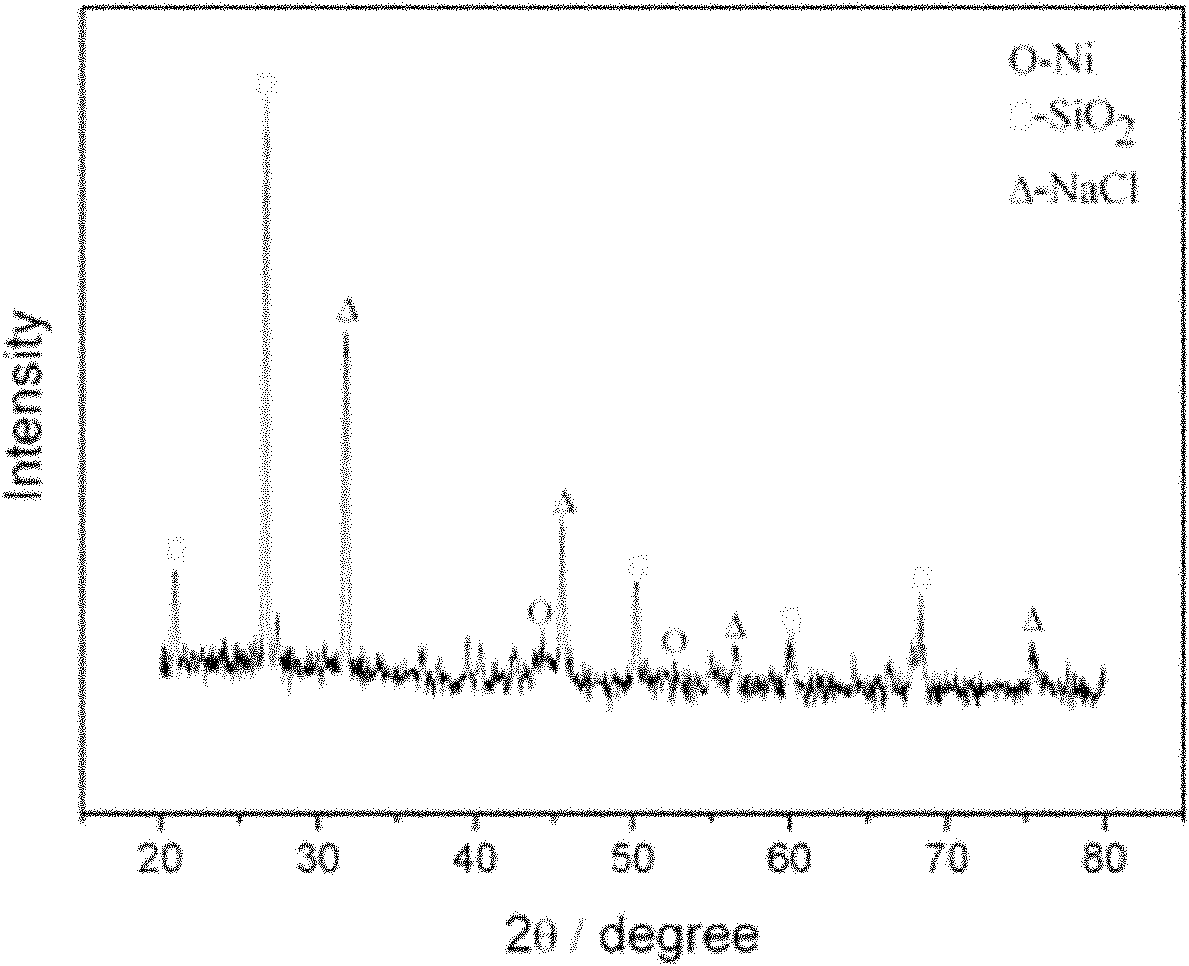
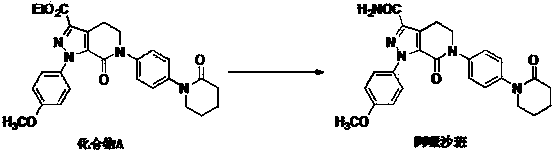
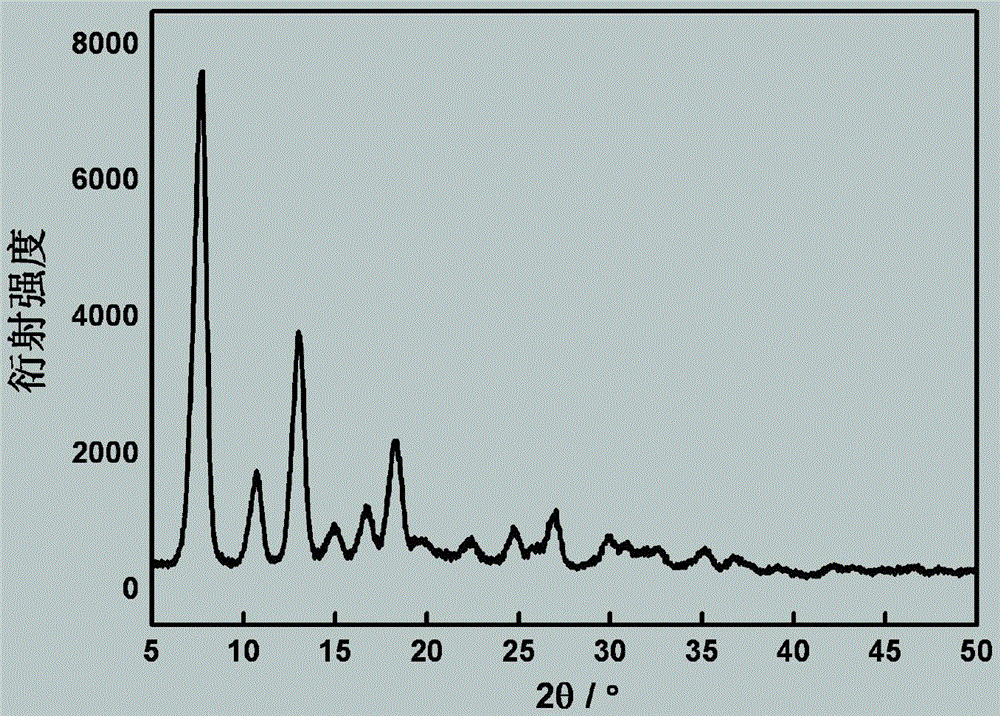
InactiveCN103833755AEasy to prepareEase of industrial productionOrganic chemistryPhenyl groupFormamides
The invention relates to a crystal form B of 4, 5, 6, 7-tetrahydro-1-(4-methoyxl phenyl)-7-oxo-6-[4-(2-oxo-1-piperidyl) phenyl]-1H-pyrazolo[3,4] pyridine-3-formamide (apixaban). The X-powder diffraction pattern of the crystal form is shown in the figure. The crystal form is good in solubleness and can be prepared into a formulation which is qualified to dissolve out without controlling the granularity in a micronizing manner. Meanwhile, the invention further provides two methods for preparing the crystal form. One method comprises the following step: carrying out ammonolysis on ester groups of a compound A under the effect of formamide and sodium alkoxide under a proper organic solvent condition to obtain crystal form B of Apixaban. The preparation method is simple and convenient and easy to amplify for industrialized production. The other method comprises the step of obtaining the crystal form B of apixaban by a non-crystal form B apixaban product by way of dissolution and crystallization, so that the crystal form is convenient to convert.
Owner:CHONGQING TOPTECH PHARMA TECH
Preparation method of 2-methylimidazole and zinc complex with hierarchical porous structure
InactiveCN105837509AWide variety of sourcesLarge specific surface areaOrganic chemistryOther chemical processesMicrosphereEngineering
A preparation method of a 2-methylimidazole and zinc complex with a hierarchical porous structure comprises the following steps: 1, mixing arranged soluble polystyrene microspheres adopted as a template with methanol and 2-methylimidazole, and stirring to obtain a solution a; and 2, dissolving zinc nitrate hydrate in methanol to obtain a solution b, adding the solution b to the solution a in a dropwise manner 5min later, stirring the solution a and the solution b at room temperature for 24h, centrifuging the obtained solution mixture, washing the obtained material to obtain a white solid, and removing the arranged soluble polystyrene microsphere template at 80DEG C by using N,N-dimethyl formamide or toluene to obtain the 2-methylimidazole and zinc complex with a hierarchical porous structure. The preparation method has the advantages of simplicity, wide sources of raw materials, low cost and easy industrial amplified production; and the above material finally prepared through the method has the advantages of high specific surface area, facilitation of enhancement of the hydrophobicity and diffusion of organic solvents due to macro-pores, meso-pores and micro-pores, large adsorption quantity of the organic solvents, and certain hydrophobicity.
Owner:NANKAI UNIV
Process for producing lignin diesel
InactiveCN102181311ASimple processSimple and efficient operationLiquid carbonaceous fuelsChemistryAqueous solution
The invention discloses a process for producing lignin diesel. The method is characterized by comprising the following steps of: uniformly mixing the components of an emulsifier in a ratio, forcibly dispersing the uniformly mixed emulsifier and diesel by a physical means to obtain a stable dispersing system, then adding assistant emulsifier and an oil modifier in a certain ratio, injecting decolored alkali lignin aqueous solution from which impurities are removed into the system at a constant speed, continuously dispersing cyclically for certain times, taking out and naturally settling for 1 to 2 days, and filtering to obtain supernate, namely the lignin diesel. In the process, the lignin diesel is prepared from the waste lignin used as raw materials instead of partial diesel. The process has the advantages of simplicity, easiness in amplification, and industrialization and the like.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com