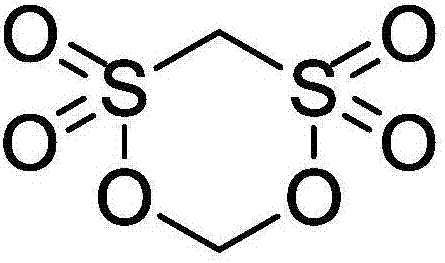
Methylene methanedisulfonate synthesis method
A technology of methylene disulfonate and methane disulfonic acid, which is applied in the direction of organic chemistry to achieve the effects of improving efficiency, reducing pollution and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 176 g of methanedisulfonic acid and 30 g of paraformaldehyde into a 1 L reaction flask, and stir mechanically for 15 minutes. Then 120 g of anhydrous magnesium sulfate was added to the above system, the temperature was raised to 100° C., and the reaction was carried out at 100° C. for 5 hours. After the reaction, the system was cooled, and the solid was extracted with 200 mL×3 methylene chloride, the insoluble matter was removed by filtration, and the filtrate was spin-dried to obtain 120 g of white methylene disulfonate product, with a yield of 64%. 1 H NMR (CDCl 3 ,400M), 4.83(s,2H), 5.97(s,2H).
Embodiment 2
[0023] Add 176 g of methanedisulfonic acid and 33 g of paraformaldehyde into a 1L reaction flask, and stir mechanically for 15 minutes. Then 144 g of anhydrous magnesium sulfate was added to the above system, the temperature was raised to 80° C., and the reaction was carried out at 80° C. for 6 hours. After the reaction was completed, the system was cooled, and the solid was extracted with 200 mL×3 methylene chloride, the insoluble matter was removed by filtration, and the filtrate was spin-dried to obtain 143 g of white methylene disulfonate product, with a yield of 76%. 1 H NMR (CDCl 3 ,400M), 4.83(s,2H), 5.97(s,2H).
Embodiment 3
[0025] Add 176 g of methanedisulfonic acid and 45 g of paraformaldehyde into a 1 L reaction flask, and stir mechanically for 15 minutes. Then 240 g of anhydrous magnesium sulfate was added to the above system, the temperature was raised to 70° C., and the reaction was carried out at 70° C. for 8 hours. After the reaction, the system was cooled, and the solid was extracted with 200 mL×3 methylene chloride, the insoluble matter was removed by filtration, and the filtrate was spin-dried to obtain 96 g of white methylene disulfonate product, with a yield of 51%. 1 H NMR (CDCl 3 ,400M), 4.83(s,2H), 5.97(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com