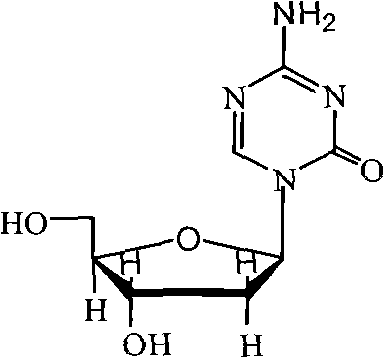
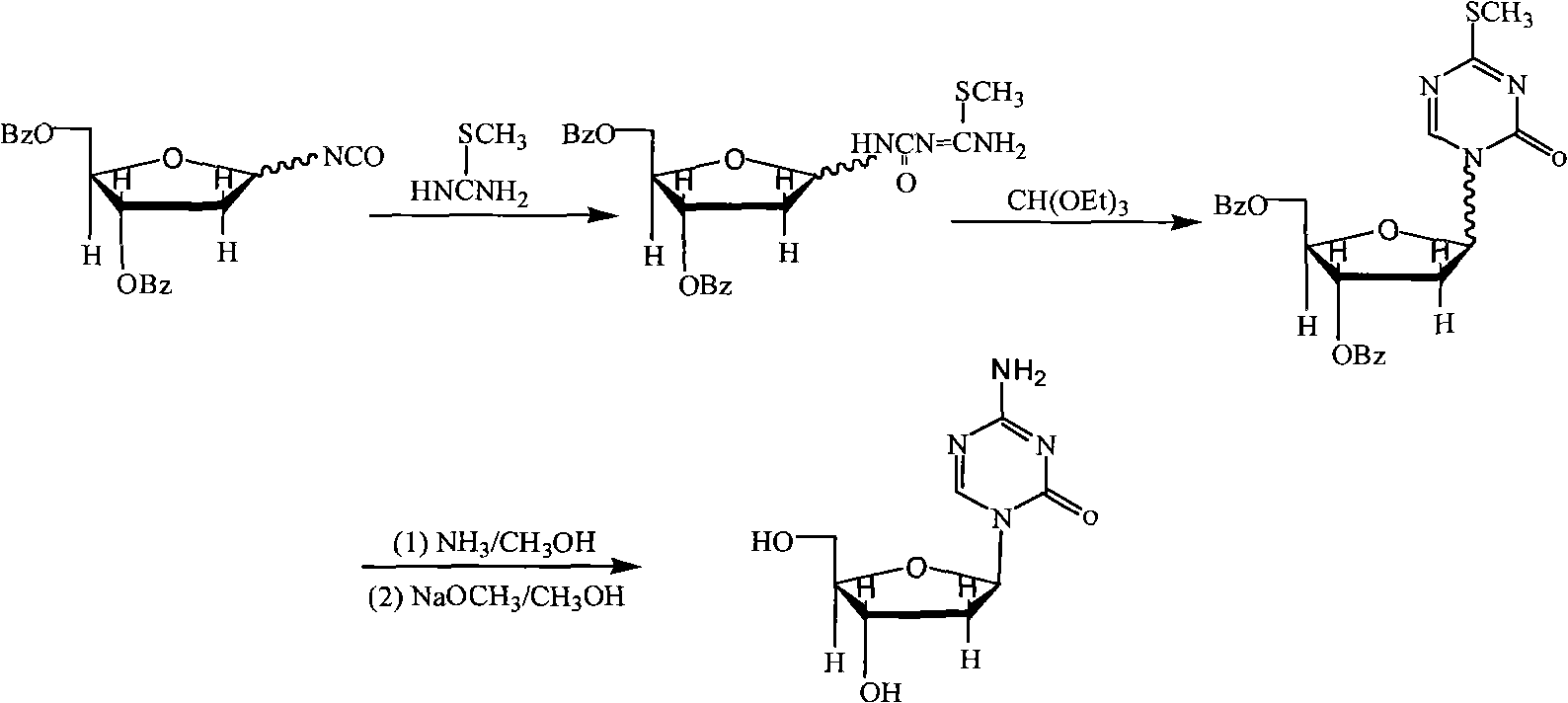
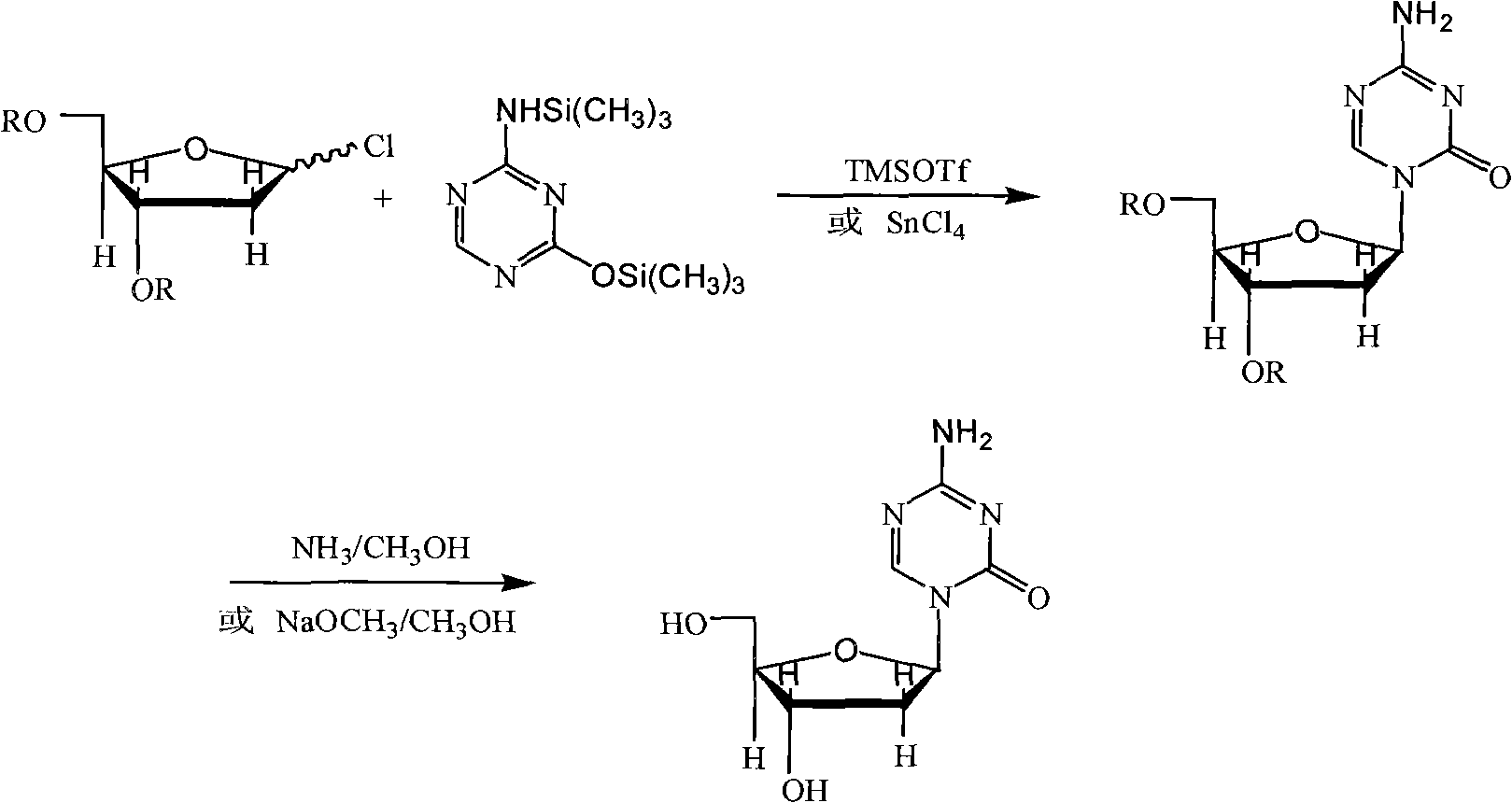
Industrialized production method for high-purity decitabine
A decitabine and production method technology, applied in the field of medicinal chemistry, can solve the problems of difficult scale-up and repeated experiments in column chromatography purification operations, complicated intermediate quality control, and long production cycle, and achieve easy industrial scale-up production, operators and The effect of small environmental impact and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 decitabine
[0045] (1) Preparation of 2,4-bis(trimethylsilyl)-5-azacytosine (intermediate A)
[0046] Add 10 g (0.089 mol) of 5-azacytosine and 120 g of hexamethyldisilazane into the reaction flask, stir evenly, add 9.7 g (0.089 mol) of trimethylchlorosilane, heat to 100°C, and react for 6 hours Afterwards, filtered and evaporated to dryness under reduced pressure at 80°C to obtain 22.6 g of a white solid, ie Intermediate A, with a yield of 99.2%.
[0047](2) Preparation of 1-(3,5-di-O-p-chlorobenzoyl-2-deoxy-β-D-ribofuranose)-5-azacytosine (intermediate B) crude product
[0048] Add 22.6g (0.088mol) of intermediate A and 30.2g (0.07mol) of 1-chloro-3,5-di-O-p-chlorobenzoyl-deoxy-D-ribofuranose to the reaction flask, add dichloromethane 600g, add 14.7g (0.066mol) of trimethylsilyl trifluoromethanesulfonate (TMSOTf), stir and react at 25°C for 12 hours, add 200ml of water to wash the reaction solution, let stand to separate layers, and ...
Embodiment 2
[0064] Embodiment 2 stability test
[0065] Get the samples of Example 1 (batch number: 100102 batches) and comparative example 1 (090501 batches), put them in a petri dish, spread them into a thin layer less than or equal to 5mm thick, place them in high temperature (60°C, 40°C), high humidity (25°C) ℃RH92.5%, 25℃RH75%±5%) for 10 days, samples were taken on the 5th and 10th day respectively. The results are shown in Table 1 below.
[0066] Table 1 The results of the decitabine influencing factors test
[0067]
[0068] Conclusion: The samples of Example 1 and Comparative Example 1 were placed at high temperature 60°C, 40°C and high humidity RH75%, 92.5% for 5 and 10 days respectively, the appearance and α isomerization of the sample of Example 1 (batch number: 100102) There is no significant change in the indicators such as body, related substance maximum single impurity and total impurity, content; the sample related substance maximum single impurity and total impurity ...
Embodiment 3
[0069] The preparation of embodiment 3 decitabine
[0070] (1) Preparation of 2,4-bis(trimethylsilyl)-5-azacytosine (intermediate A)
[0071] Add 50 g (0.446 mol) of 5-azacytosine and 1 kg of hexamethyldisilazane into the reaction flask, stir evenly, add 72.7 g (0.669 mol) of trimethylchlorosilane, heat to 30°C, and react for 10 hours Afterwards, filtered and evaporated to dryness under reduced pressure at 80°C to obtain 112.5 g of a white solid, ie Intermediate A, with a yield of 98.5%.
[0072] (2) Preparation of 1-(3,5-di-O-p-chlorobenzoyl-2-deoxy-β-D-ribofuranose)-5-azacytosine (intermediate B) crude product
[0073] Add 112g intermediate A (0.438mol) and 1-chloro-3,5-di-O-p-chlorobenzoyl-deoxy-D-ribofuranose 188.1g (0.438mol) to the reaction flask, add 1,2- Dichloroethane 9.4kg, add trimethylsilyl trifluoromethanesulfonate (TMSOTf) 97.7g (0.438mol), stir and react at 10 ℃ for 15 hours, add 3kg water to wash the reaction solution, let stand to separate layers, take The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com