Nano iron fluoride-based composite material, and preparation method thereof
An iron fluoride-based, positive electrode material technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of expensive graphene, harsh reaction conditions, and small specific surface area, and achieve easy industrial scale-up production and mild preparation conditions , the effect of uniform distribution of elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of nanometer iron fluoride-based composite material
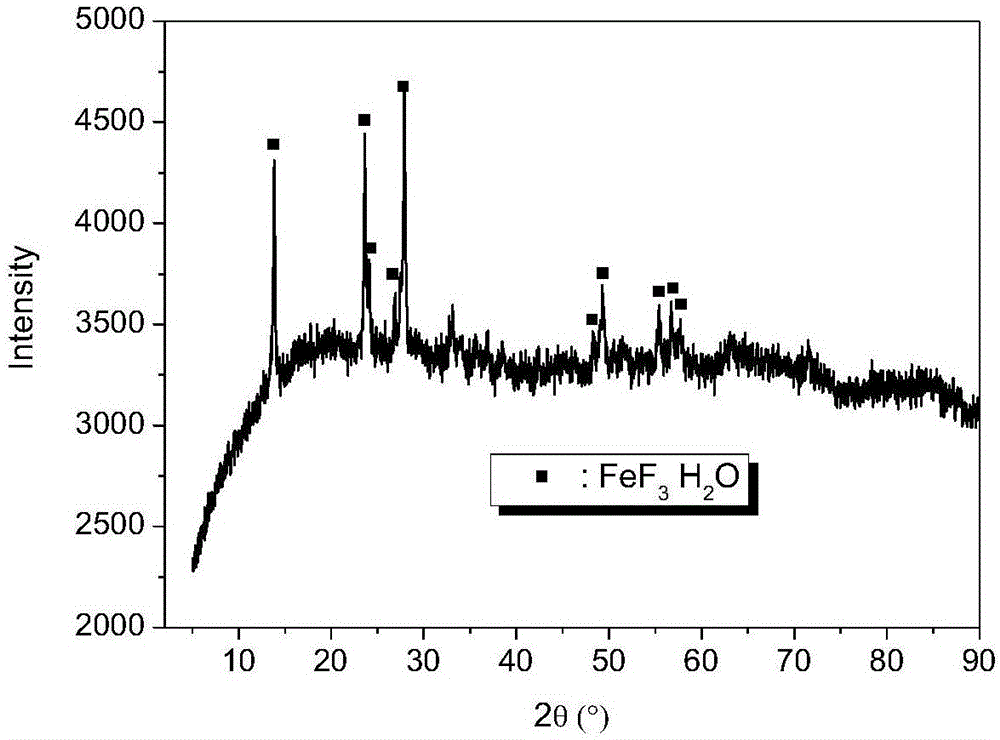
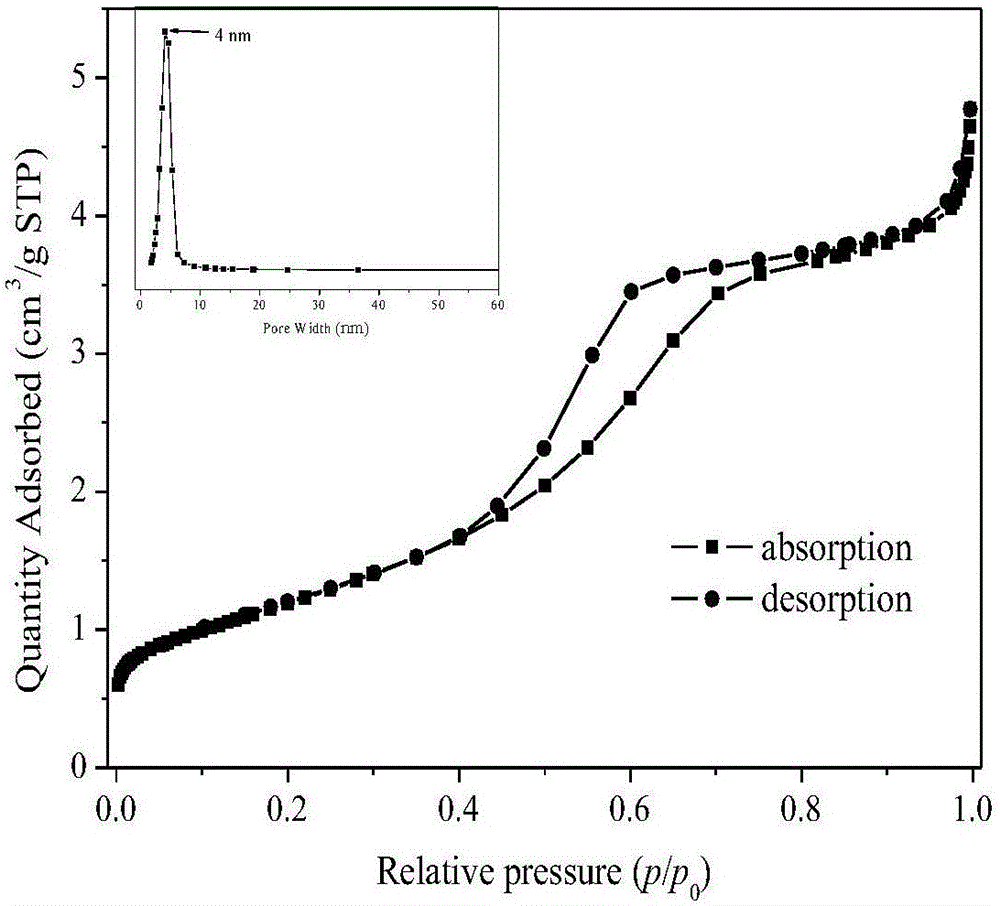
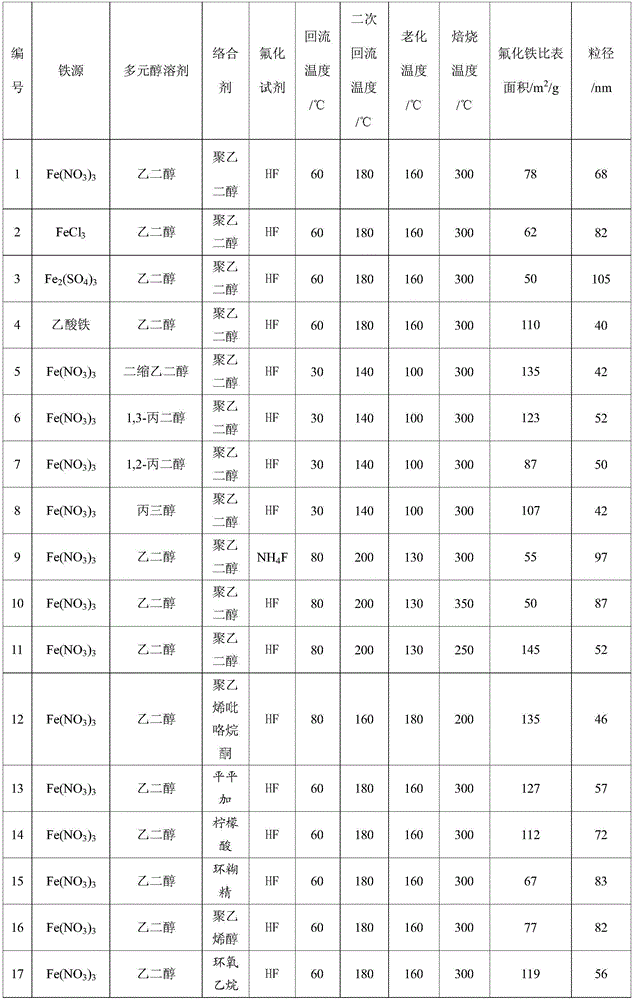
[0026] Dissolve 1.0M iron source in 50mL of polyol solvent, reflux at 30-80℃ for 6h, then add fluorinated reagent dropwise to the above solution under stirring, the dropwise addition time is 30min, after the dropwise addition is completed Reflux and stir at 140-200°C for 6 hours to obtain a suspension; then statically age at 100-160°C for more than 24 hours, and then wash and filter to obtain a solid; finally, calcinate at 200-350°C for more than 4 hours in an air atmosphere to prepare A nanocrystalline iron fluoride matrix composite material was obtained. The texture properties of iron fluoride prepared under different iron sources, polyol solvents, complexing agents, fluorinating reagents and calcination temperatures are shown in Table 1.
[0027] Table 1 Results of physicochemical properties of iron fluoride in Example 1
[0028]
Embodiment 2
[0029] Example 2: Preparation of nanometer iron fluoride-based composite material
[0030] Dissolve 1.0 M ferric nitrate and polyethylene glycol in 50 mL of ethylene glycol, reflux at 60 °C for 6 h with stirring, and then add HF aqueous solution (40 wt.%) dropwise to the above solution with stirring. The dropwise addition time is 30min, after the dropwise addition was completed, reflux and stirring at 160°C for 6h to obtain a suspension; then statically aged at 160°C for more than 24h, washed and filtered to obtain a solid; finally, calcined at 300°C for more than 4h to obtain a nanoparticle Crystalline iron fluoride. The ferric fluoride textures obtained with different amounts of polyethylene glycol are shown in Table 2.
[0031] Table 2 The results of physical and chemical properties of iron fluoride in Example 2
[0032]
Embodiment 3
[0033] Example 3: Preparation of nanometer iron fluoride-based composite material
[0034] Dissolve 1.0M ferric nitrate and polyethylene glycol (the mass ratio of Fe / complexing agent is 1:3) in 50 mL of ethylene glycol, reflux for 6 h under stirring at 60 °C, and then add HF aqueous solution dropwise to the mixture under stirring. In the above solution, the dropwise addition time was 30min. After the dropwise addition was completed, the solution was refluxed and stirred at 160°C for 6h to obtain a suspension; then statically aged at 160°C for more than 24h, washed and filtered to obtain a solid; finally, the solid was obtained at 300°C. Under roasting for more than 4h, nanocrystalline iron fluoride is obtained. The textures of iron fluoride prepared with different HF concentrations are shown in Table 3.
[0035] Table 3 The results of physical and chemical properties of iron fluoride in Example 3
[0036]
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com