New method for preparing polyaspartic acid hydrogels
A technology for polyaspartic acid and aspartic acid is applied in the field of preparing polyaspartic acid hydrogels, which can solve the problems of complicated process, pollute the environment, difficult recovery, etc. High capacity, avoid the effect of polluting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
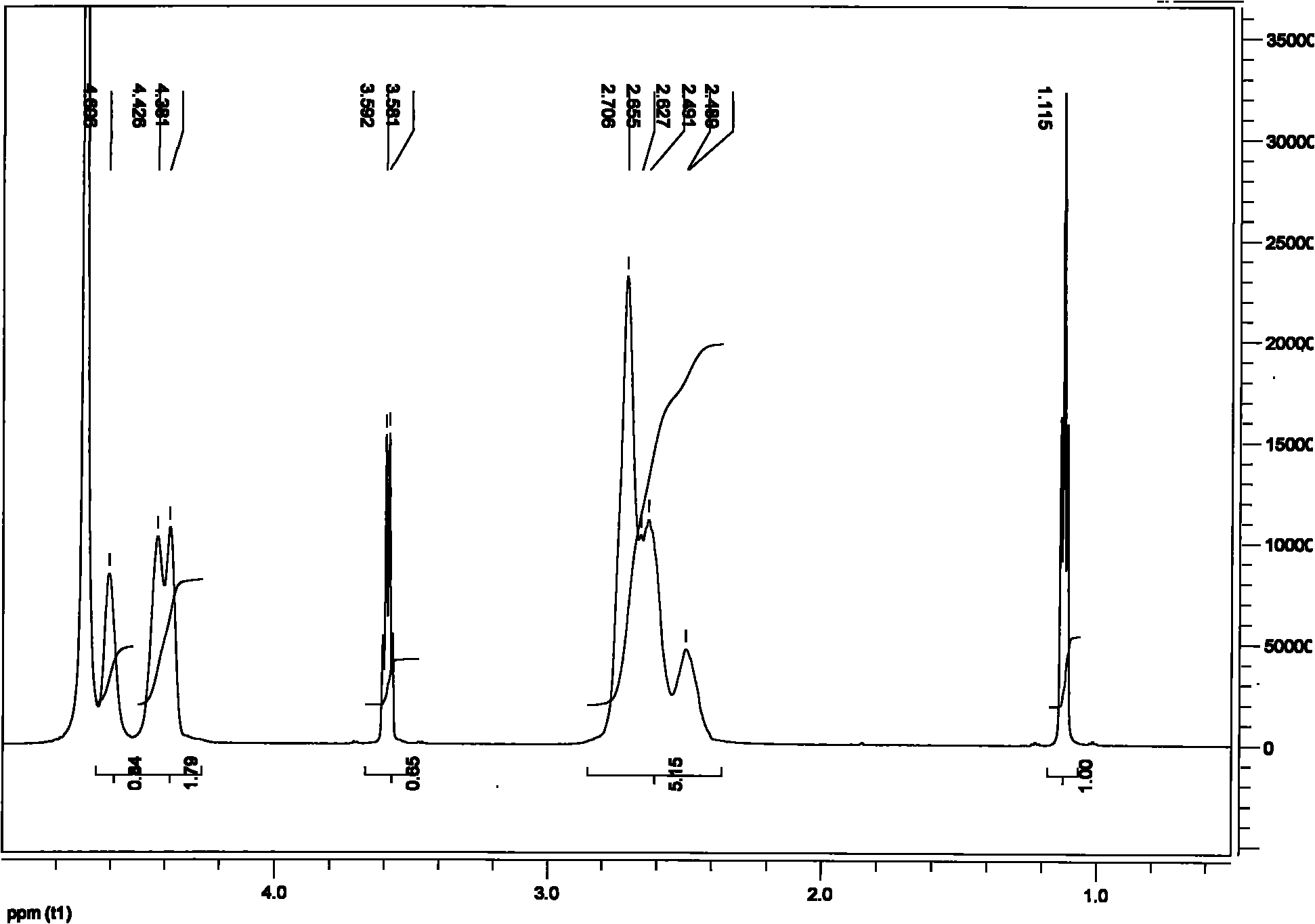
[0037] Add 1.000g of polysuccinimide with a number average molecular weight of 30,000 Daltons and 40ml of water to a 250ml beaker, and add 4.0ml of hexamethylenediamine and NaOH with a molar ratio of 1:9 at 25°C at a speed of 200rpm. Mixture. After 0.5 hour, 4.0 ml of hexamethylenediamine and NaOH in a molar ratio of 1:9 were added. After 0.5 hours, 1.7 ml of a mixture of hexamethylenediamine and NaOH with a molar ratio of 1:9 was added, and the concentration of the two solutions was 1 mol / L. The reaction stopped after 24 h of reaction.
[0038] The hydrogel after the reaction was put into a blast oven at 60° C. for 48 hours, and then taken out to obtain a white or light yellow solid, that is, a cross-linked PASP hydrogel. It is measured that the swelling ratio of the polyaspartic acid hydrogel in deionized water is 100 g / g, and the swelling ratio in 0.9 wt % NaCl aqueous solution is 20 g / g. According to electron microscope photos, it can be seen that the prepared hydrogel ...
Embodiment 2
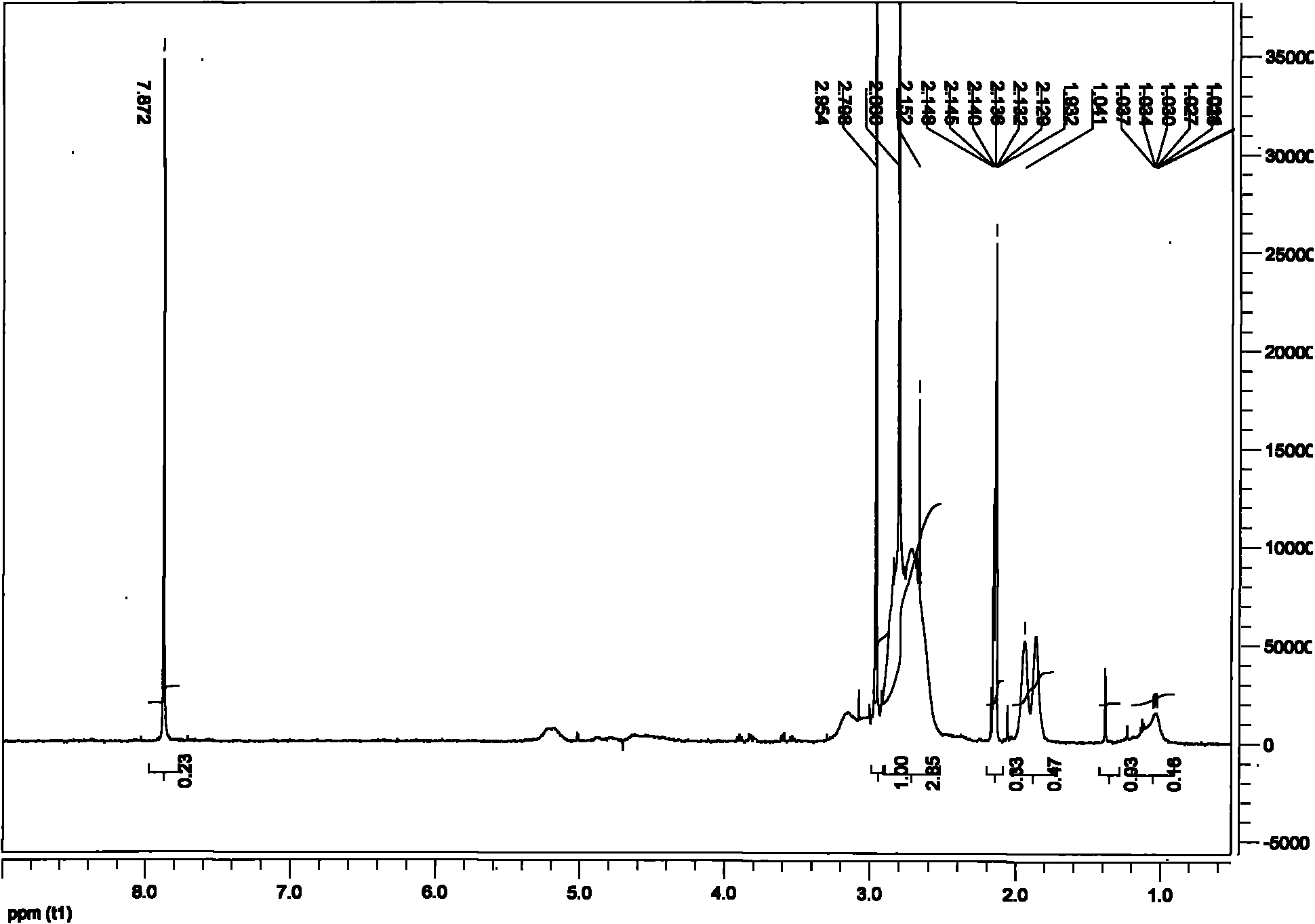
[0040] What is different from Example 1 is that when preparing cross-linked polyaspartic acid, the molar ratio of hexamethylenediamine and NaOH mixed solution is 2:8, and the concentration of both solutions is 1mol / L; the mixed solution is divided into 4 times , added at intervals of 0.5 hours, adding 2.42ml each time. It is measured that the swelling ratio of the polyaspartic acid hydrogel in deionized water is 150 g / g, and the swelling ratio in 0.9 wt % NaCl. aqueous solution is 35 g / g. According to electron micrographs, it can be seen that the prepared polyaspartic acid hydrogel is a multi-level porous network structure with a certain density gradient, and the connection between network units at all levels is a multi-chain network connection, which is better than that of the polyaspartic acid hydrogel prepared in Example 1. The network structure of the amino acid hydrogel is denser and has better continuity. Therefore, its color is transparent, and its water absorption cap...
Embodiment 3
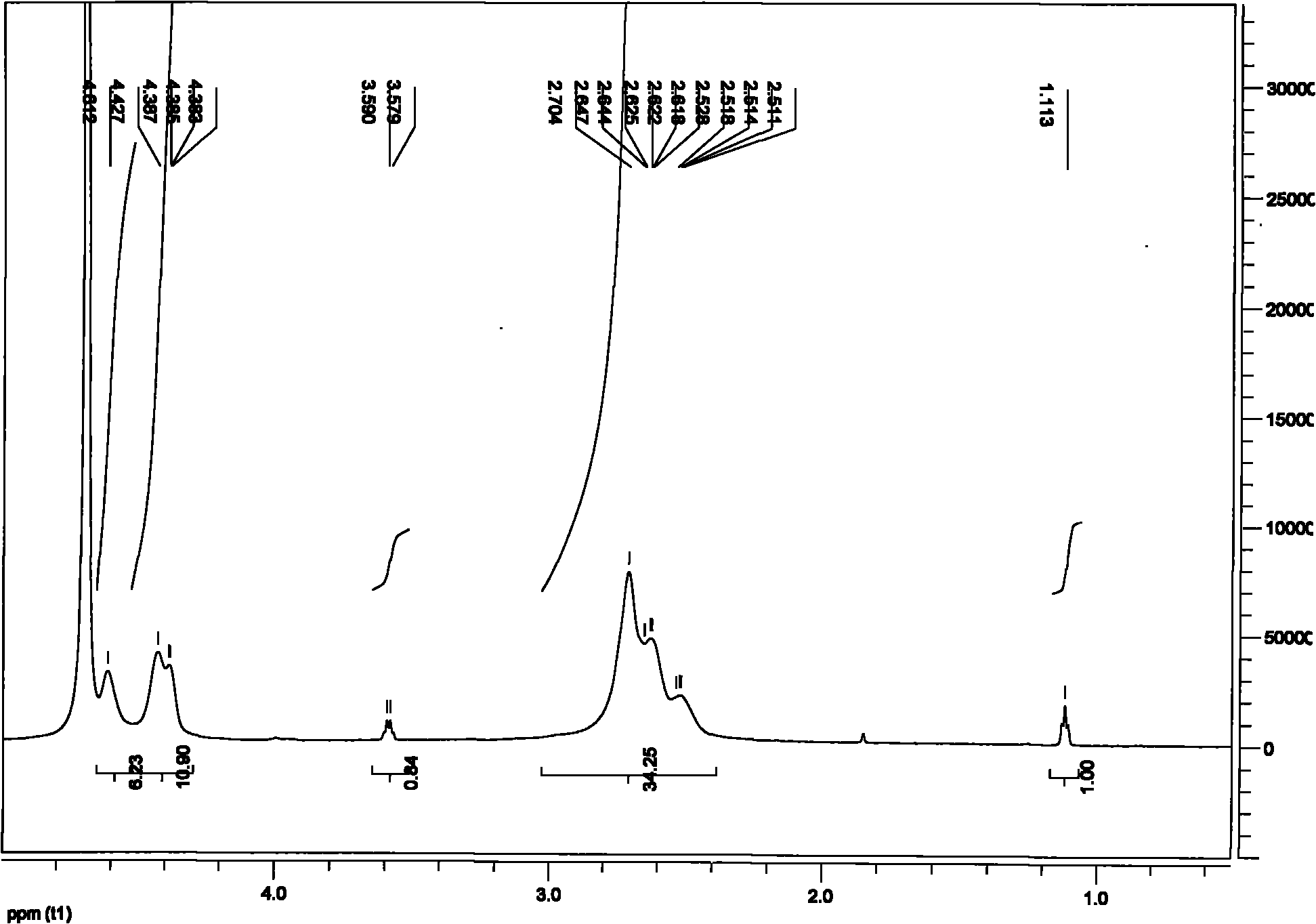
[0042] The difference from Example 1 is that when preparing cross-linked polyaspartic acid, the molar ratio of hexamethylenediamine and NaOH mixed solution is 3:7, and the concentration of both solutions is 1mol / L. The mixed solution was added in 2 times at intervals of 1 hour, and 4.9 ml was added each time. The reaction was completed in 72 hours. It is measured that the swelling ratio of the polyaspartic acid hydrogel in deionized water is 160 g / g, and the swelling ratio in 0.9 wt % NaCl. aqueous solution is 40 g / g. According to electron micrographs, it can be seen that the prepared polyaspartic acid hydrogel is a multi-level porous network structure with a certain density gradient, and the connection between network units at all levels is a multi-chain network connection, which is better than that of the polyaspartic acid hydrogel prepared in Example 1. The network structure of the amino acid hydrogel is denser and has better continuity. Therefore, its color is transparen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com