Butylphthalide synthesis method and purification technology
A butylphthalide and process technology, applied in the field of medicine, can solve problems such as unsuitable production, unsuitable process for industrialized production, etc., and achieve the effects of low cost, improved process safety, industrial practicability, and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Synthesis of butylphthalide
[0053]
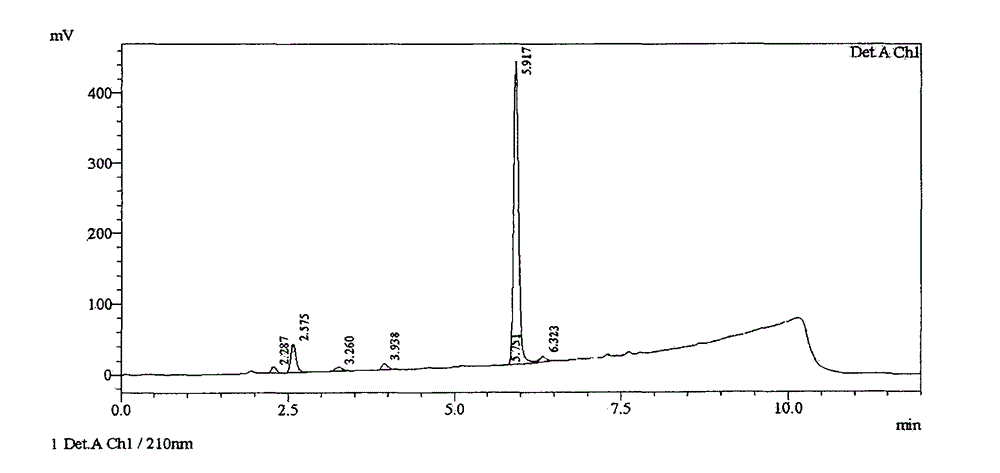
[0054] Put o-carboxybenzaldehyde (15.0kg) and anhydrous tetrahydrofuran (60.0kg) into a clean reactor, stir to dissolve, cool to 0°C, add n-BuMgCl (2.0M, 102.0kg) slowly, and control the temperature at 0~10°C, after the addition, HPLC detects that the raw materials disappear, add dropwise 10% ammonium chloride aqueous solution (60.0kg), and then add concentrated hydrochloric acid (20.0kg), extract with ethyl acetate, and depressurize the organic phase Concentrate to dryness to obtain crude butylphthalide.
[0055] figure 1 Represent the HPLC purity result of the obtained butylphthalide crude product, and the specific data are shown in the following table 1:
[0056] Peak#
Ret. Time
Area
Area%
Height
Resolution
1
2.287
46749
1.776
8899
0.000
2
2.575
227539
8.645
40224
1.853
3
3.260
41459
1.575
6086
3.793
4
3.938
...
Embodiment 2
[0065] Embodiment 2: purify butylphthalide
[0066]
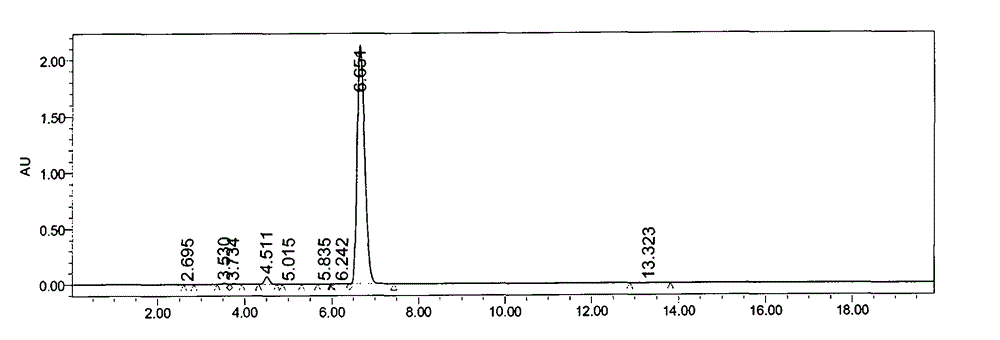
[0067] Add methanol and water to the butylphthalide crude product (24kg) obtained by the method of Example 1, add lithium hydroxide (8.4kg) under stirring, heat up to reflux reaction, evaporate methanol under reduced pressure, then add water and citric acid aqueous solution ( 50.0kg), centrifuged, and the solid was rinsed with water; Figure 5 Represent the HPLC purity result of the butylphthalide intermediate after acid and base adjustment for the first time (5.107min is the butylphthalide intermediate, and 6.310min is the butylphthalide), and the specific data are shown in the following table 5:
[0068] Peak#
Ret. Time
Area
Area%
Height
Resolution
1
3.241
18253
0.297
3983
0.000
2
3.368
104996
1.707
22327
0.906
3
5.107
5666245
92.094
1100046
12.253
4
6.310
363164
5.903
67726
7.905
Total
615265...
Embodiment 3
[0075] Embodiment 3: purify butylphthalide
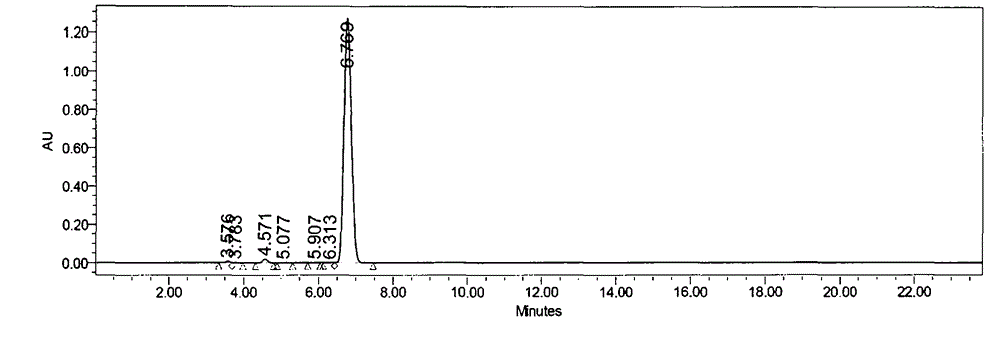
[0076] The butylphthalide crude product (12Kg) obtained by the method of Example 1 was added butanol and water, sodium hydroxide (7.1kg) was added under stirring, the temperature was raised to reflux reaction, butanol was distilled off under reduced pressure, and then water and tartaric acid aqueous solution ( 28.0kg), centrifuged, and the solid was washed with water; the purity of the butylphthalide intermediate obtained after adjusting the acid and base for the first time was 94.5%.
[0077] Add water and sodium hydroxide (1.6kg) to the above centrifuged solid, stir to dissolve, add activated carbon (0.6kg), stir for 2h, filter, add tartaric acid aqueous solution (28.0kg) to the filtrate, centrifuge, and rinse the solid with water; The HPLC purity of the butylphthalide intermediate after acid and base adjustment is 97%.
[0078] Add water and sodium hydroxide (1.6kg) to the centrifuged solid, stir to dissolve, add tartaric acid a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com