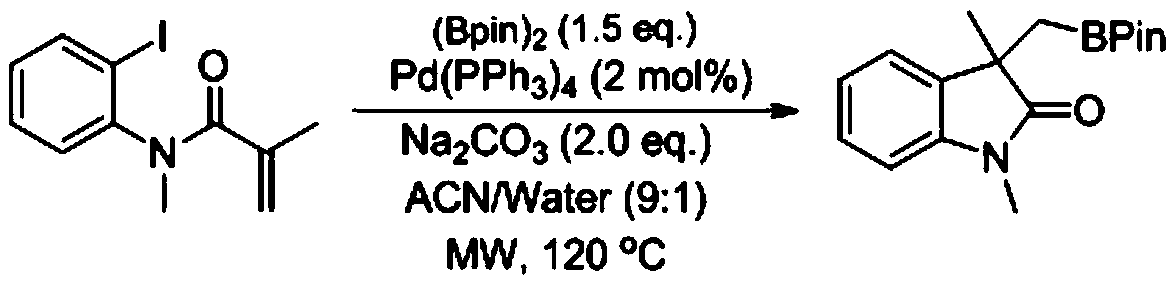Preparation method of boron-containing indolinone derivative
A technology of indolinone and its derivatives, which is applied in the field of catalytic synthesis, and achieves the effects of simple operation, simple and easy-to-obtain raw materials, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add o-iodoaniline derivative 1a (72.6mg, 0.2mmol), cuprous iodide (3.8mg, 10mol%), triphenylphosphine (10.4mg, 20mol%), lithium tert-butoxide (16mg , 1.0eq.), diboronic acid pinacol ester (101.3mg, 2.0eq.), dichloroethane (2.0mL, 0.1M), the reaction mixture was reacted at 40°C for 5min, and passed through the rotary evaporator directly after the reaction After removing the solvent, the target compound 1 was obtained by column chromatography (petroleum ether: ethyl acetate = 15:1) with a yield of 98%.
[0025] 1H NMR (500MHz, CDCl3) δ7.32 (t, J = 1.7Hz, 1H), 7.30 (dd, J = 11.8, 4.0Hz, 3H), 7.25 (dd, J = 9.3, 5.7Hz, 2H), 7.19 (ddd, J=8.2,4.2,1.2Hz,1H),7.08–7.03(m,1H),6.86(d,J=7.6Hz,1H),3.20(s,3H),1.92(dd,J=58.5 ,15.2Hz,2H),0.96(s,6H),0.86(s,6H).
Embodiment 2
[0027] Add o-iodoaniline derivative 2a (88.2mg, 0.2mmol), cuprous bromide (5.6mg, 20mol%), tricyclohexylphosphine (22.4mg, 40mol%), potassium tert-butoxide (44.8 mg, 2eq.), pinacol diboronate (76.0mg, 1.5eq.), dichloromethane (2.0mL, 0.1M), the reaction mixture was reacted at 60°C for 15min, and removed directly by a rotary evaporator after the reaction After the solvent, the target compound 2 was obtained by column chromatography (petroleum ether: ethyl acetate = 10:1) with a yield of 66%.
[0028] 1H NMR (500MHz, CDCl3) δ7.32–7.27(m,4H),7.25–7.20(m,2H),7.18(d,J=7.9Hz,1H),7.03(d,J=1.6Hz,1H) ,3.19(s,3H),1.91(dd,J=44.7,15.4Hz,2H),1.01(s,6H),0.91(s,6H).
Embodiment 3
[0030] In the reaction tube, add o-iodoaniline derivative 3a (86.2mg, 0.2mmol), cuprous chloride (1.9mg, 10mol%), 1,10-phenanthroline (3.6mg, 10mol%), tert-butanol Sodium (28.8mg, 1.5eq.), pinacol diborate (50.6mg, 1.0eq.), toluene (2.0mL, 0.1M), the reaction mixture was reacted at 80°C for 5min, and directly evaporated by rotary evaporation after the reaction After removing the solvent, the target compound 3 was obtained by column chromatography (petroleum ether: ethyl acetate = 8:1) with a yield of 80%.
[0031] 1H NMR (500MHz, CDCl3) δ7.62 (d, J = 7.8Hz, 2H), 7.31–7.27 (m, 4H), 7.27–7.23 (m, 1H), 6.95 (d, J = 8.2Hz, 1H) ,3.24(s,3H),1.99–1.91(m,2H),0.99(s,6H),0.89(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com