Preparation method of substituted iodobenzene with terminal double bonds
A terminal double bond, iodobenzene technology, applied in the field of preparation of substituted iodobenzene, can solve the problems of high price, difficult to obtain, etc., and achieves the effects of fast reaction speed, little environmental pollution, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The invention provides a method for preparing a substituted iodobenzene with a terminal double bond. The substituted iodobenzene with a terminal double bond has the following structural formula:
[0017]
[0018] In the formula, R 1 selected from H, substituted or unsubstituted alkyl, substituted or unsubstituted alkoxy, substituted or unsubstituted benzyl, R 2 selected from methylene or carbonyl, R 3 Selected from F, Cl, Br, I, CN, NO 2 、CF 3 , COOEt, substituted or unsubstituted hydrocarbyl, substituted or unsubstituted alkoxy, substituted or unsubstituted alkyl, R 3 It can be monosubstituted or polysubstituted, and the position on the benzene ring is not limited.
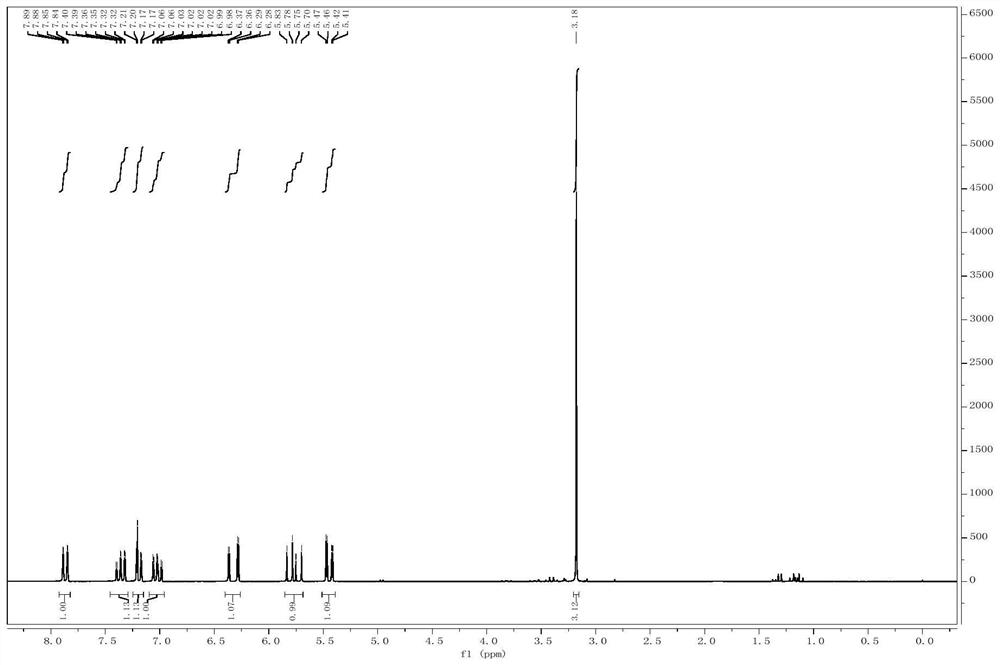
[0019] The preparation method of the substituted iodobenzene with a terminal double bond comprises: using substituted o-iodoaniline and a compound containing a terminal double bond as starting materials to react in an organic solvent to obtain the substituted iodobenzene with a terminal double bond....
Embodiment 1
[0039] Example 1 N-allyl-N-benzyl-2-iodoaniline
[0040]
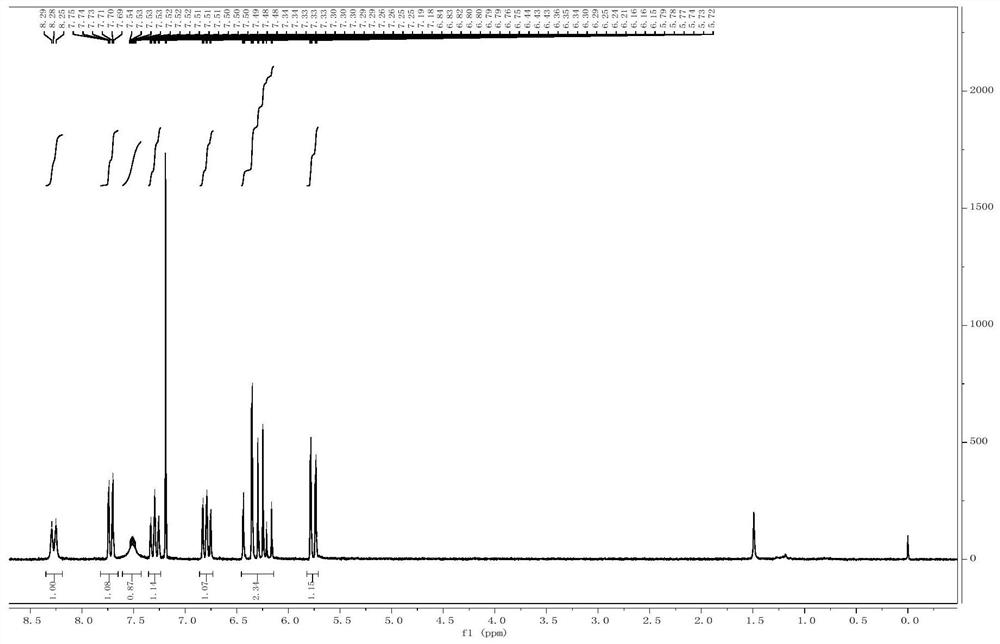
[0041] Add o-iodoaniline (50mmol) and benzaldehyde (50mmol) into the reaction flask, add 100mL of dichloroethane, then add acetic acid (100mmol) and sodium triacetoxyborohydride (150mmol), and the mixture reacts at room temperature 2 hours. After the reaction was completed, the reaction was quenched with water, extracted with dichloromethane, and the organic phases were combined and dried over anhydrous magnesium sulfate. The organic phase can be concentrated to obtain N-benzyl-2-iodoaniline. Add N-benzyl-2-iodoaniline (7mmol) to the reaction flask, add tetrahydrofuran 5mL, slowly add lithium diisopropylamide 8mL (1mol / L) at 0°C, and then add allyl bromide (14mmol) , the mixture was reacted for 2 hours and slowly raised to room temperature, and the reaction was monitored by TLC. After the reaction was completed, it was quenched with water. The organic phase was extracted with dichloromethane, combined and dried o...
Embodiment 2
[0044] Example 2 N-(2-iodophenyl)-acrylamide
[0045]
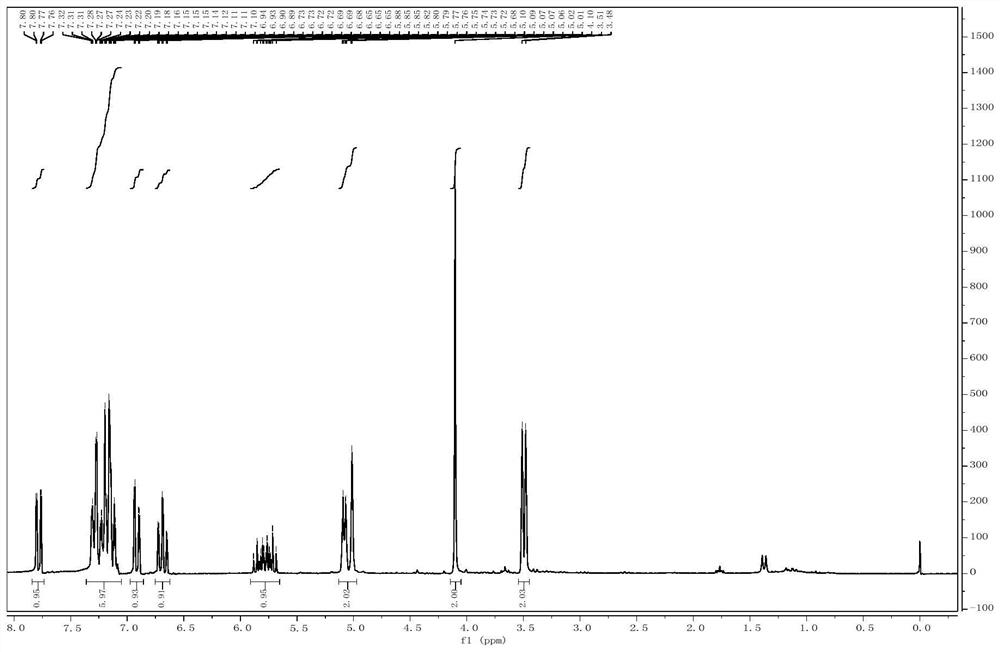
[0046] Add o-iodoaniline (10mmol) into the reaction flask, then add 5mL of dichloromethane, then add triethylamine (11mmol), then slowly add acryloyl chloride (11mmol) to the reaction solution dropwise, and the mixture is reacted at room temperature for 2 hours , the reaction was quenched with water after the reaction was complete. Extract with dichloromethane, combine the organic phases and dry with anhydrous magnesium sulfate, concentrate the organic phases to obtain N-(2-iodophenyl)-acrylamide with a yield of 75%. figure 2 It is the N-(2-iodophenyl)-acrylamide synthesized in Example 2 of the present invention 1 H NMR characterization spectrum.
[0047] 1 H NMR (200MHz, CDCl 3 ): δ (ppm) 8.27 (d, J = 8.0Hz, 1H), 7.72 (d, J = 8.0Hz, 1H), 7.52 (s, 1H), 7.66 (t, J = 8.0Hz, 1H), 6.79 (t,J=8.0Hz,1H),6.44-6.15(m,2H),5.76(d,J=10.0Hz,1H).
[0048] HRMS(ESI)m / z[M+H] + Calcd for C 9 h 9 INO(273.9723), found: 273.9729...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com