Preparation method of fluoro indole carboxylic acid compound
A technology of fluoroindole carboxylic acid and indole carboxylic acid, which is applied in the field of preparation of fluoroindole carboxylic acid compounds, can solve the problems of unsuitability for industrial scale production, potential safety hazards, lack of synthesis routes and the like, and achieves The effect of reducing production costs and potential safety hazards, simplifying purification difficulties, and improving reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
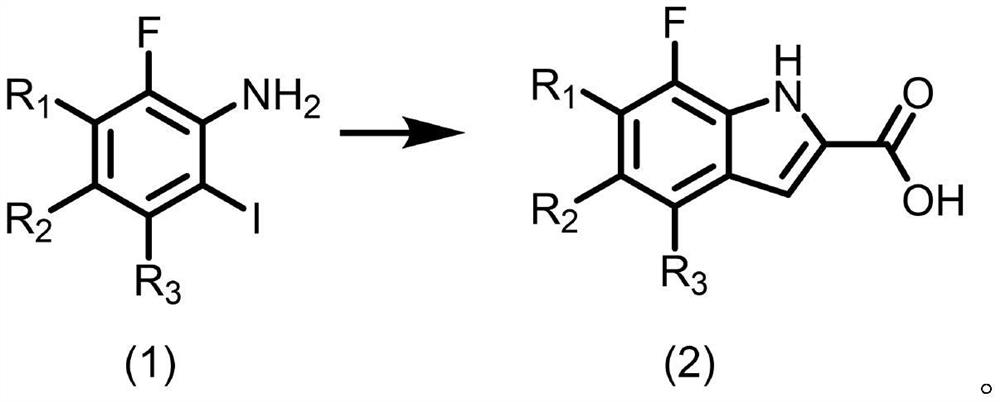
[0031] A kind of preparation method of fluoroindole carboxylic acid compound, R in compound (2) 1 = H, R 2 = F, R 3 When =H, the preparation method comprises the following specific steps:
[0032] (1) Synthesis of 2,4-difluoro-6-iodoaniline
[0033]
[0034] Add 400ml acetic acid in the reaction bottle, then add 2,4-difluoroaniline (100g, 0.775mol, 1eq), then add N-iodosuccinimide (183g, 0.813mol, 1.05eq) in batches, control Stir at 25°C for 1 hour. After the reaction is complete, pour into 600ml of ice water, extract with 1000ml of ethyl acetate, wash with 500ml of 5% aqueous sodium sulfite, wash with 500ml of 5% aqueous sodium bicarbonate, wash with 500ml of saturated saline, dry and concentrate , and then distilled under reduced pressure to obtain 183.91 g of compound 2,4-difluoro-6-iodoaniline, with a purity of 99.0%;
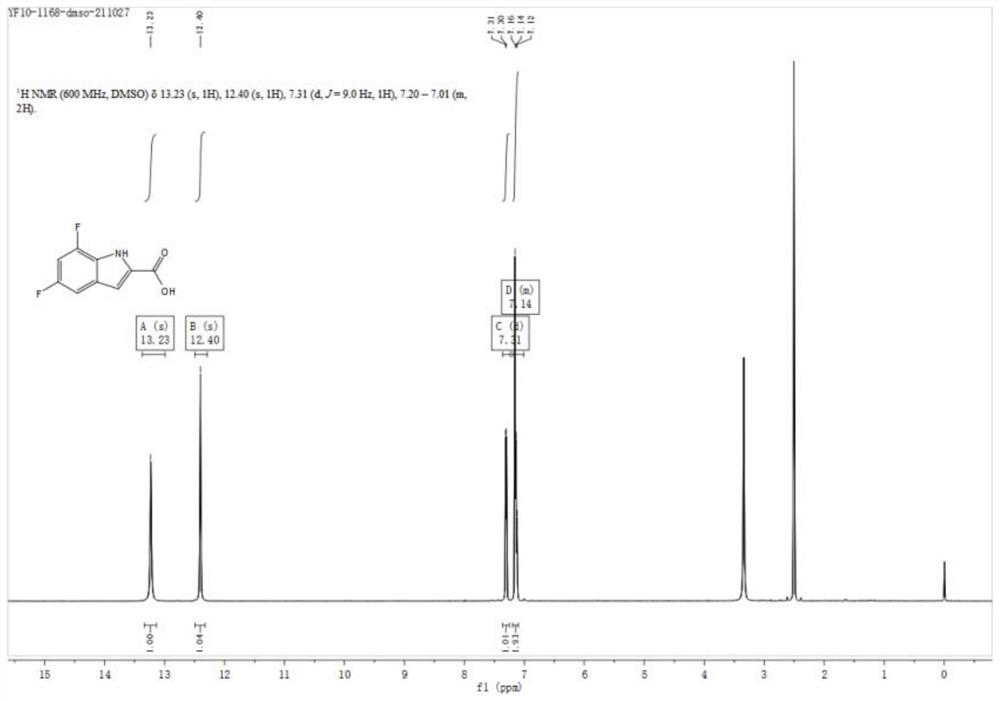
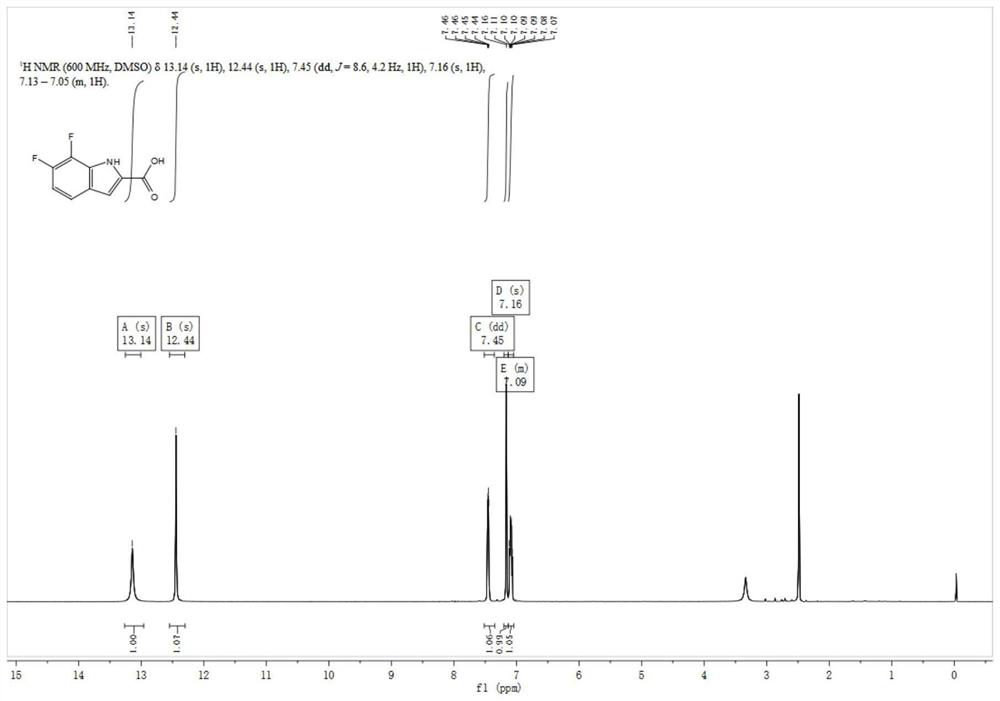
[0035] (2) Synthesis of 5,7-difluoro-1H-indole-2-carboxylic acid
[0036]
[0037] Add 30ml of N,N-dimethylformamide to the reaction flask, then a...
Embodiment 2
[0039] A kind of preparation method of fluoroindole carboxylic acid compound, R in compound (2) 1 = H, R 2 = F, R 3 When =H, the preparation method comprises the following specific steps:
[0040] (1) Synthesis of 2,4-difluoro-6-iodoaniline
[0041]
[0042] Add 8L acetic acid in the reaction flask, then add 2,4-difluoroaniline (2kg, 15.5mol, 1eq), then add N-iodosuccinimide (3.6kg, 16.26mol, 1.05eq) in batches, Control the temperature at 25°C and stir for 2 hours. After the reaction is complete, pour into ice water, extract with ethyl acetate, wash with 5% aqueous sodium sulfite solution, wash with 5% aqueous sodium bicarbonate solution, wash with saturated brine, dry and concentrate, and then depressurize The compound 2,4-difluoro-6-iodoaniline was distilled to 3.63 kg with a purity of 98.2%;
[0043] (2) Synthesis of 5,7-difluoro-1H-indole-2-carboxylic acid
[0044]
[0045] Add 9LN,N-dimethylformamide to the reaction flask, then add 2,4-difluoro-6-iodoaniline (3...
Embodiment 3
[0047] A kind of preparation method of fluoroindole carboxylic acid compound, R in compound (2) 1 = H, R 2 = F, R 3 When =H, the preparation method comprises the following specific steps:
[0048] (1) Synthesis of 2,4-difluoro-6-iodoaniline
[0049]
[0050] Add 450ml of acetic acid in the reaction flask, then add 2,4-difluoroaniline (110g, 0.85mol, 1eq), then add N-iodosuccinimide (229.5g, 1.02mol, 1.2eq) in batches, Control the temperature at 25°C and stir for 1 hour. After the reaction is complete, pour into 600ml of ice water, extract with 1000ml of ethyl acetate, wash with 600ml of 5% aqueous sodium sulfite, wash with 600ml of 5% aqueous sodium bicarbonate, wash with 600ml of saturated saline, and dry Concentrate, then distill under reduced pressure to obtain 199.39 g of compound 2,4-difluoro-6-iodoaniline, with a purity of 97.0%;
[0051] (2) Synthesis of 5,7-difluoro-1H-indole-2-carboxylic acid
[0052]
[0053] Add 30ml of N,N-dimethylformamide to the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com