Preparation method of boscalid
A technology of boscalid and chloronicotinyl chloride, applied in the field of compound preparation, can solve the problems of unindustrialization, high price of o-iodoaniline, difficult recovery and the like, and achieve the effects of convenient recycling and saving catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The raw materials and reagents used in the preparation method of the novel fungicide boscalid provided by the present invention can be purchased from the market.
[0030] The progress of the reaction was monitored by thin-layer chromatography, and the spot was detected with a UV lamp or with Ce(NH 4 ) 2 (NO 3 ) 6 (0.5g) and (NH 4 ) 6 Mo 7 o 24 4H 2 O (24.0g) in 6% H 2 SO 4 (500 mL) of a yellow solution developed. The proton nuclear magnetic resonance spectrum of reaction product is measured by Varian mercury plus400MHz nuclear magnetic resonance instrument (TMS is internal standard), and ESI mass spectrum is determined by ESQUIRE Ion Trap LC / MS liquid chromatography / mass spectrometry analysis, the mixture of the reaction end and target product The purity was analyzed by Agilent1260 high performance liquid chromatography (Kromasil C 18 Stainless steel column, 250mm×4.6mm, methanol-water mixture solvent, 65:35V / V as mobile phase, flow rate 1.0mL / min, photodio...
Embodiment 1
[0032] The preparation of embodiment 1 4'-chloro-2-acetamidobiphenyl
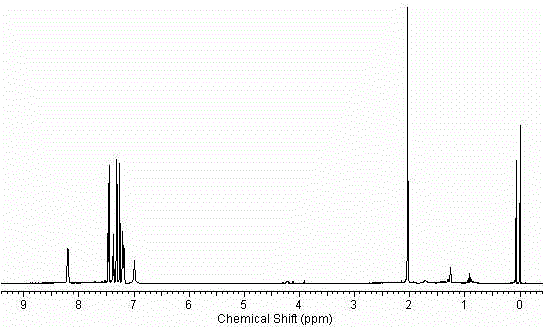
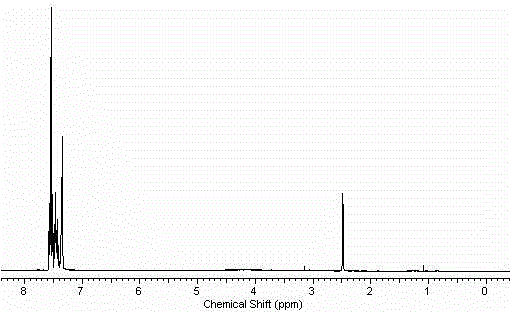
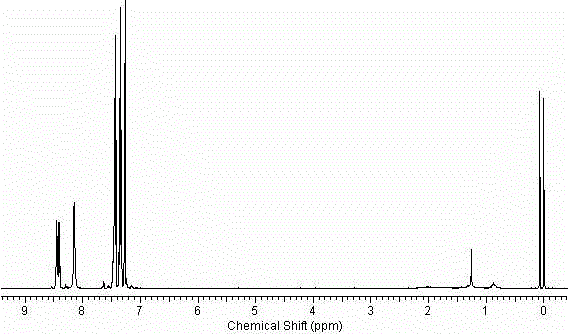
[0033] 2-Acetamidobromobenzene (21.4g, 0.1mol), p-chlorophenylboronic acid (18.7g, 0.12mol), 10% Pd(OH) 2 / C(0.14g, 1mmol of Pd(OH) 2 ), tetrabutylammonium bromide (6g) (or other quaternary ammonium salts of the same equivalent), potassium phosphate 7 water (64g) (or other inorganic bases of the same equivalent) and DMF (or other solvents) (100mL) in the set Stir at a certain temperature, stop the reaction after TLC monitors that the reaction is complete, filter, dilute the filtrate with ethyl acetate (500mL), wash with 1M NaOH solution (300mL), and saturated brine (3×500mL), wash with After drying with anhydrous sodium sulfate, the solvent was evaporated to obtain 24.2 g of the product, with a yield of 99%. 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=6.4Hz, 1H), 7.46(d, J=6.4Hz, 2H), 7.41-7.36(m, 1H), 7.31(d, J=6.4Hz, 2H), 7.24- 7.16 (m, 2H), 6.90 (s, br, 1H, NH), 2.04 (s, 3H). ESI-MS, m / z, [M+H] + : 246.2. ...
Embodiment 2
[0040] Example 2 Pd(OH) 2 / C recycling
[0041] 2-Acetamidobromobenzene (21.4g, 0.1mol), Pd(OH) containing p-chlorophenylboronic acid (21.4g, 0.1mol), Example 1 filtered out 2 / C, tetrabutylammonium bromide (6g), K 3 PO 4 ·7H 2 O (64g) and DMF (100mL) were reacted at 125°C until the point of 2-acetamidobromobenzene monitored by TLC disappeared, and the aftertreatment was the same as above. The results are shown in Table 2.
[0042] Table 2 Pd(OH) 2 / C recycling results
[0043]
[0044]
[0045] The results in Table 2 show that the activity of the catalyst has not changed substantially in the first two cycles, that is, the Suzuki reaction can be completed after 11 hours of reaction. From the third cycle, the activity has decreased, and the reaction time needs to be increased to complete reaction. When cycling to the 6th time, it takes 14 hours to substantially complete the reaction. But in general, Pd(OH) 2 / C can be used repeatedly when synthesizing 4'-chloro-2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com