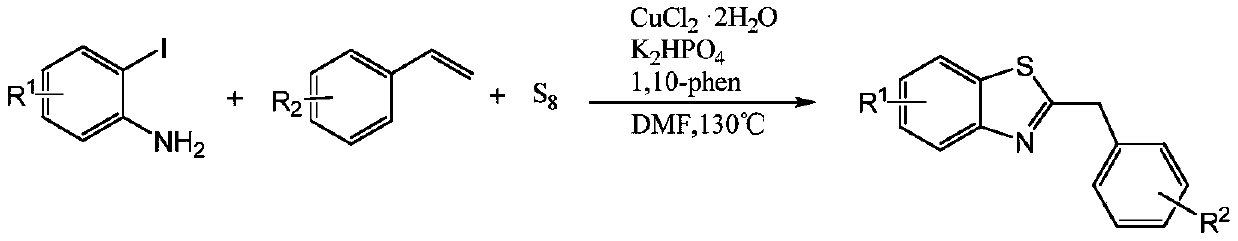
Method for synthesizing 2-substituted benzothiazole by one-pot method
A technology for benzothiazoles and compounds, which is applied in the field of one-pot synthesis of 2-substituted benzothiazoles, can solve the problems of restricted reaction conditions in the reaction process, expensive catalysts, complex reaction conditions, etc., and achieve the conversion rate of the substrate and the product The effect of high yield, expanding industrial application, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
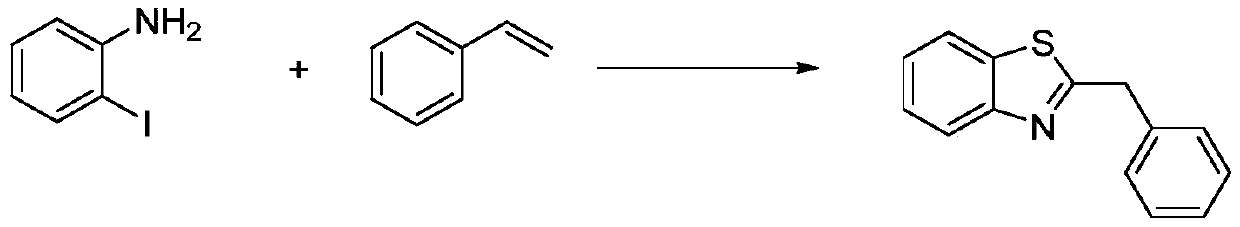
[0026] This embodiment is the synthesis of 2-benzylbenzothiazole, with o-iodoaniline, styrene, sulfur powder, K 2 HPO 4 , CuCl 2 2H 2 O, 1,10-phen, N,N-dimethylformamide are used as raw materials, and the reaction formula is as follows:
[0027]
[0028] Preparation method: add 0.5mmol o-iodoaniline, 1.0mmol styrene, 1.50mmol sulfur powder, 1.50mmol K 2 HPO 4 , 0.1mmol CuCl 2 2H 2 O, 0.1 mmol 1,10-phen, and 2.5 mL N,N-dimethylformamide were added, and stirred at 130°C for 12 hours under a nitrogen atmosphere. After the reaction was completed, the TLC plate detected that a product was formed.
[0029] The reaction solution was cooled, extracted, dried, distilled under reduced pressure, column chromatography and filtered to obtain a brown oil.
[0030] Yield 78%, this 2-benzylbenzothiazole H NMR 1 H NMR (300MHz, Chloroform-d) δ8.01(d, J=8.1Hz, 1H), 7.79(d, J=7.9Hz, 1H), 7.46(t, J=7.7Hz, 1H), 7.36(dd ,J=7.3,3.7Hz,6H),4.45(s,2H).
Embodiment 1-1
[0032] This embodiment is the synthesis of 2-benzylbenzothiazole, with o-iodoaniline, styrene, sulfur powder, K 2 HPO 4 , CuCl 2 2H 2 O, 1,10-phen, N,N-dimethylformamide are used as raw materials, and the reaction formula is as follows:
[0033]
[0034] Preparation method: Add 0.5mmol o-iodoaniline, 0.5mmol styrene, 1.50mmol sulfur powder, 1.50mmol K 2 HPO 4 , 0.1mmol CuCl 2 2H 2 O, 0.1 mmol 1,10-phen, and 2.5 mL N,N-dimethylformamide were added, and stirred at 130°C for 12 hours under a nitrogen atmosphere. After the reaction was completed, the TLC plate detected that a product was formed.
[0035] The reaction solution was cooled, extracted, dried, distilled under reduced pressure, column chromatography and filtered to obtain a brown oil.
[0036] Yield 55%, this 2-benzylbenzothiazole NMR 1H NMR (300MHz, Chloroform-d) δ8.01(d, J=8.1Hz, 1H), 7.79(d, J=7.9Hz, 1H), 7.46(t, J=7.7Hz, 1H), 7.36(dd ,J=7.3,3.7Hz,6H),4.45(s,2H).
Embodiment 1-2
[0038] This embodiment is the synthesis of 2-benzylbenzothiazole, with o-iodoaniline, styrene, sulfur powder, K 2 HPO 4 , CuCl 2 2H 2 O, 1,10-phen, N,N-dimethylformamide are used as raw materials, and the reaction formula is as follows:
[0039]
[0040] Preparation method: Add 0.5mmol o-iodoaniline, 1.5mmol styrene, 1.50mmol sulfur powder, 1.50mmol K 2 HPO 4 , 0.1mmol CuCl 2 2H 2 O, 0.1 mmol 1,10-phen, and 2.5 mL N,N-dimethylformamide were added, and stirred at 130°C for 12 hours under a nitrogen atmosphere. After the reaction was completed, the TLC plate detected that a product was formed.
[0041] The reaction solution was cooled, extracted, dried, distilled under reduced pressure, column chromatography and filtered to obtain a brown oil.
[0042] Yield 59%, this 2-benzylbenzothiazole H NMR 1 H NMR (300MHz, Chloroform-d) δ8.01(d, J=8.1Hz, 1H), 7.79(d, J=7.9Hz, 1H), 7.46(t, J=7.7Hz, 1H), 7.36(dd ,J=7.3,3.7Hz,6H),4.45(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com