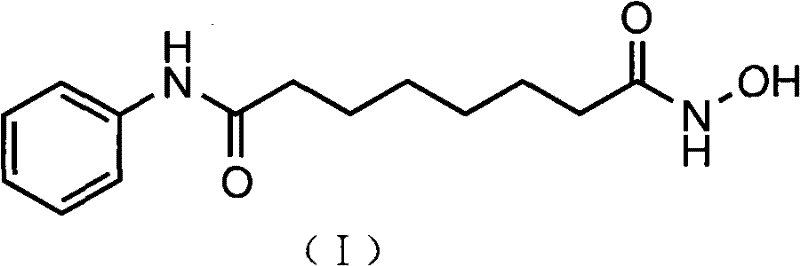
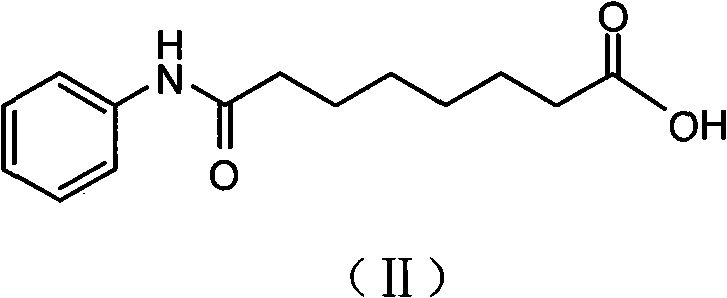
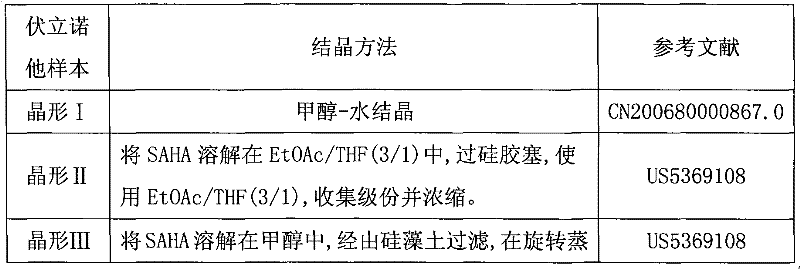
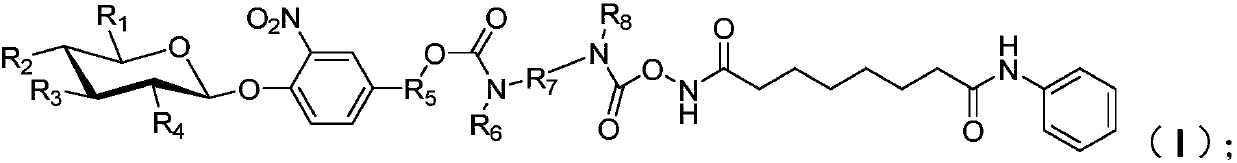
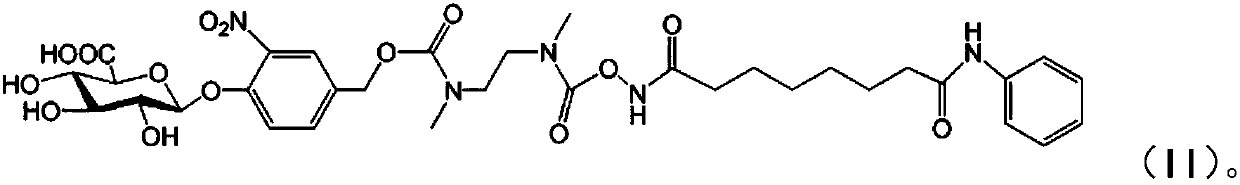
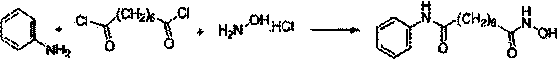Patents
Literature
60 results about "Vorinostat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
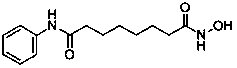
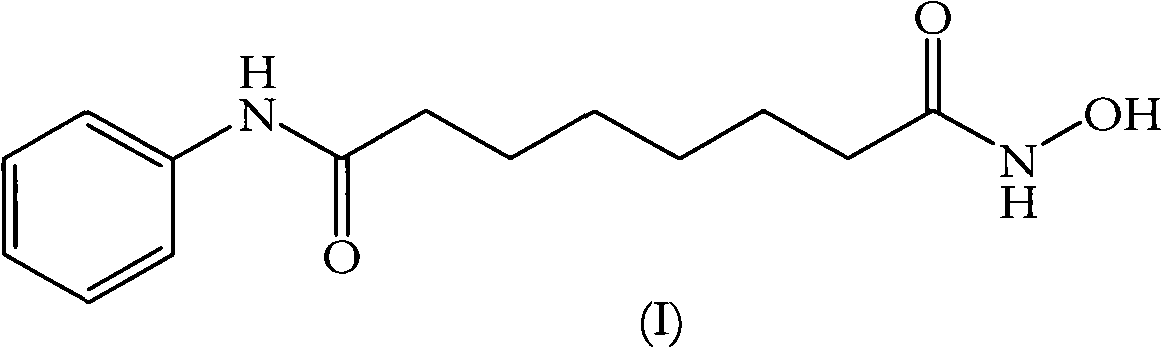
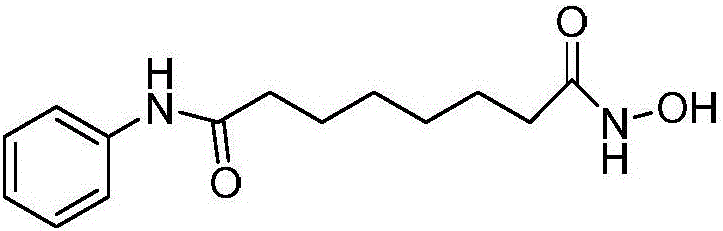
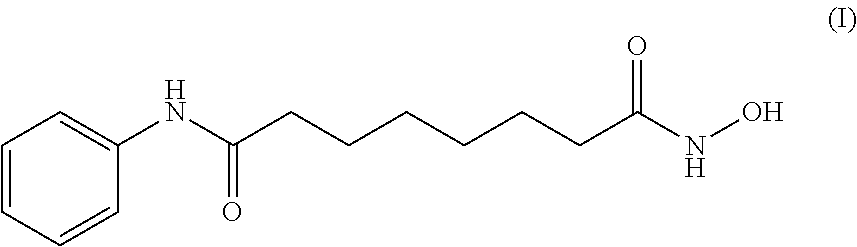
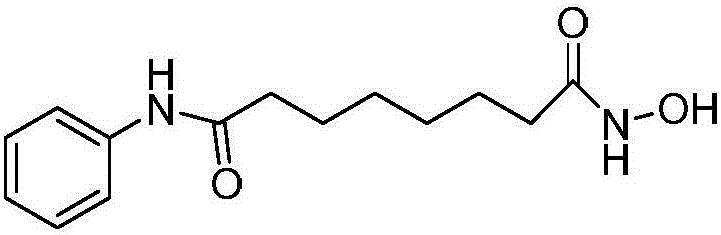
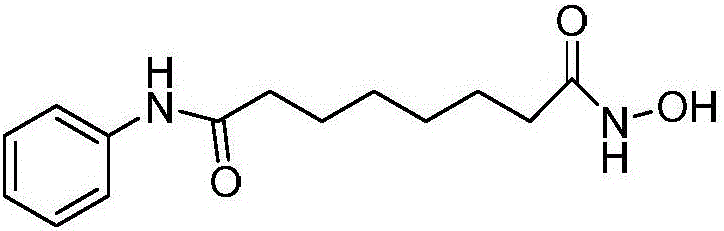
Vorinostat is used to treat a certain type of cancer (CTCL-cutaneous T-cell lymphoma).
Composition for activating latent HIV virus and application thereof
ActiveCN109620954AFully activatedHigh induction activationAntiviralsAntibody ingredientsSide effectValproic Acid
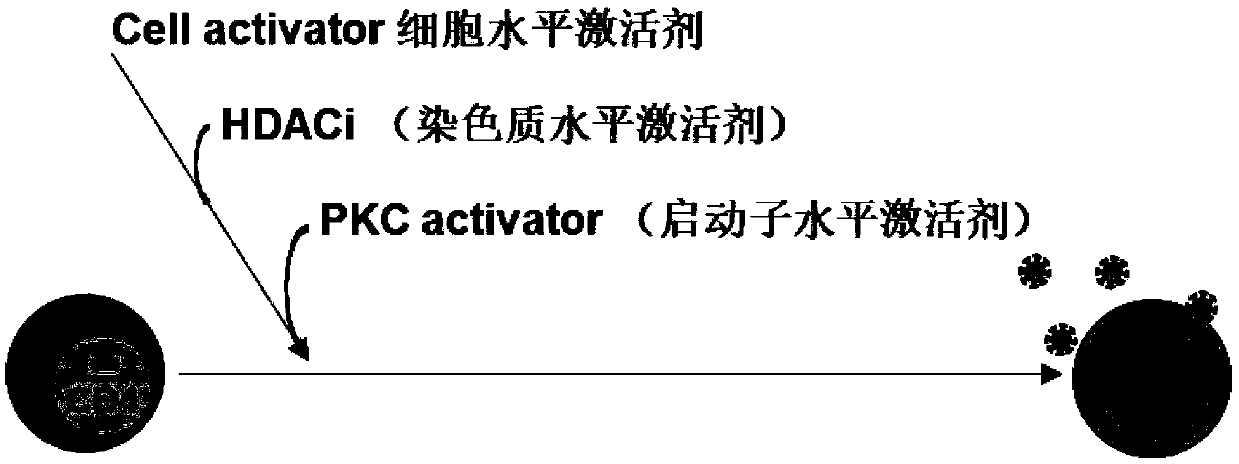
The invention relates to the field of medicine, in particular to a composition for activating an HIV latent virus and application thereof. The composition consists of a monoclonal antibody drug, a histone deacetylase inhibitor and a PKC activator, wherein the monoclonal antibody drug is selected from an anti-human CD3 monoclonal antibody and an anti-human CD28 monoclonal antibody, the histone deacetylase inhibitor is selected from at least one of vorinostat and valproic acid, and a PKC activator is 12-deoxyphorbol-13-acetic acid (Prostratin). The main obstacle to cure the HIV is that the HIV establishes a hidden 'reservoir' of viruses in the body at the very early stage of infection. The composition has the effect of significantly activating latent HIV-infected CD4+ T cells, can activate the HIV latent virus simultaneously at the cellular level, the chromatin level and the HIV-specific transcription factor level, can fully activate the HIV pre-virus in the resting CD4+ T cells withoutsignificant cytotoxic side effects and is a necessary way to HIV functional healing.
Owner:WUHAN UNIV OF SCI & TECH
Pharmaceutical composition containing a hypomethylating agent and a histone deacetylase inhibitor
InactiveUS20110129521A1Good curative effectBiocideCarbohydrate active ingredientsTransdermal patchHistone methylation
A pharmaceutical composition for induction therapy which has a hypomethylating agent and a histone deacetylase inhibitor (“HDAC inhibitor”); wherein the hypomethylating agent is a DNA and histone methylation inhibitor such as cladribine and the HDAC inhibitor is, for example, entinostat, panobinostat, vorinostat, and / or romedepsin; further wherein the hypomethylating agent and the HDAC inhibitor are combined in formulations for various administrations including e.g., a continuous delivery system such as a transdermal patch of at least one reservoir or a plurality of reservoirs, oral, a fixed-dose oral combination, intravenous, and combinations thereof. This pharmaceutical composition for induction therapy is used with a monoclonal antibody in the treatment of various cancers, sarcomas, and other malignancies.
Owner:NIMBLE EPITECH
Applications of vorinostat in aspect of drugs for treating autoimmune diseases and inflammatory diseases
InactiveCN102793693AReduce infiltrationReduce morbidityOrganic active ingredientsSenses disorderImmunologic disordersAutoimmune responses
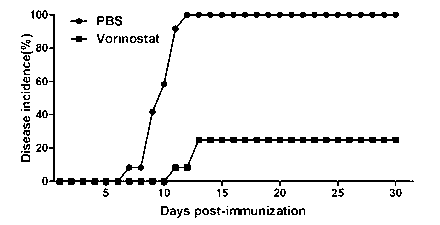
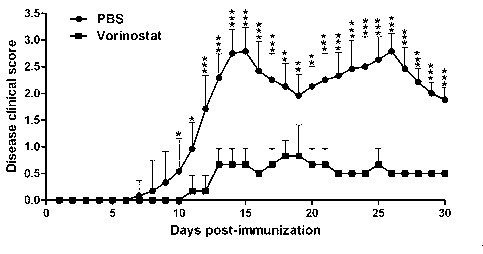
The invention discloses applications of vorinostat in aspect of drugs for treating autoimmune diseases. The experiment result disclosed by the invention shows that due to the vorinostat, the morbidity of the experimental autoimmune encephalomyelitis of mice is reudced, and also the average score, the highest score and the total score of clinical marks are reduced; spinal cord inflammatory cell infiltration and spinal cord demyelination can be alleviated; the weight percent of a cell Th1 related to IFN (Interferon)-gamma secretion and a cell Th17 cell related to IL-17A secretion accounting for positive T cells CD4 in the spleen can be reduced. Therefore the invention relates to the preparation of the drug for inhibiting the experimental autoimmune encephalomyelitis of an animal model, and the drug is expected to treat the diseases related to multiple sclerosis, ophthalmoneuromyelitis, acute disseminated encephalomyelitis and the like.
Owner:TIANJIN MEDICAL UNIV
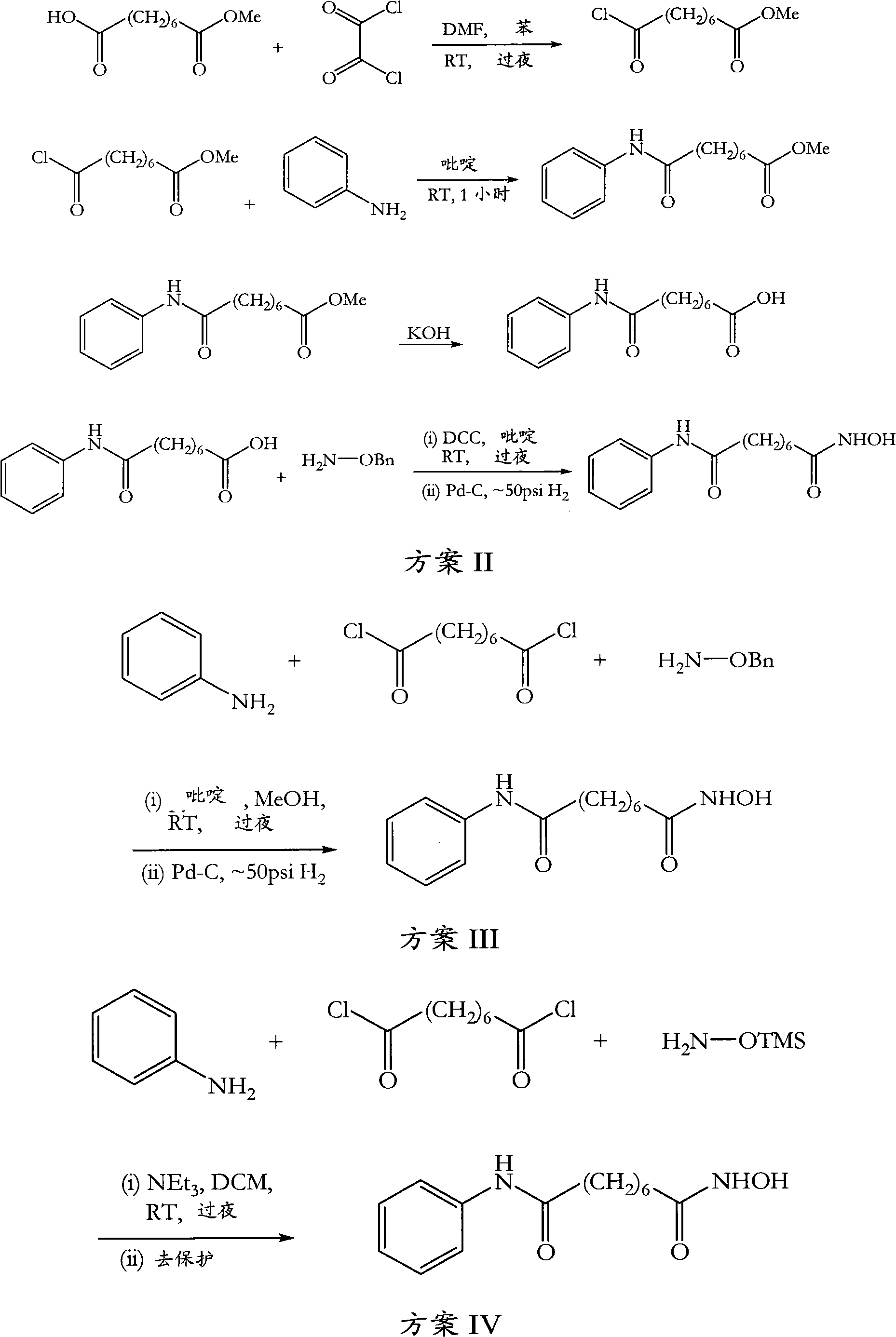
Method for synthesizing anti-cancer drug vorinostat
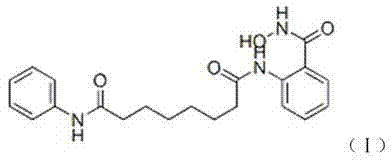
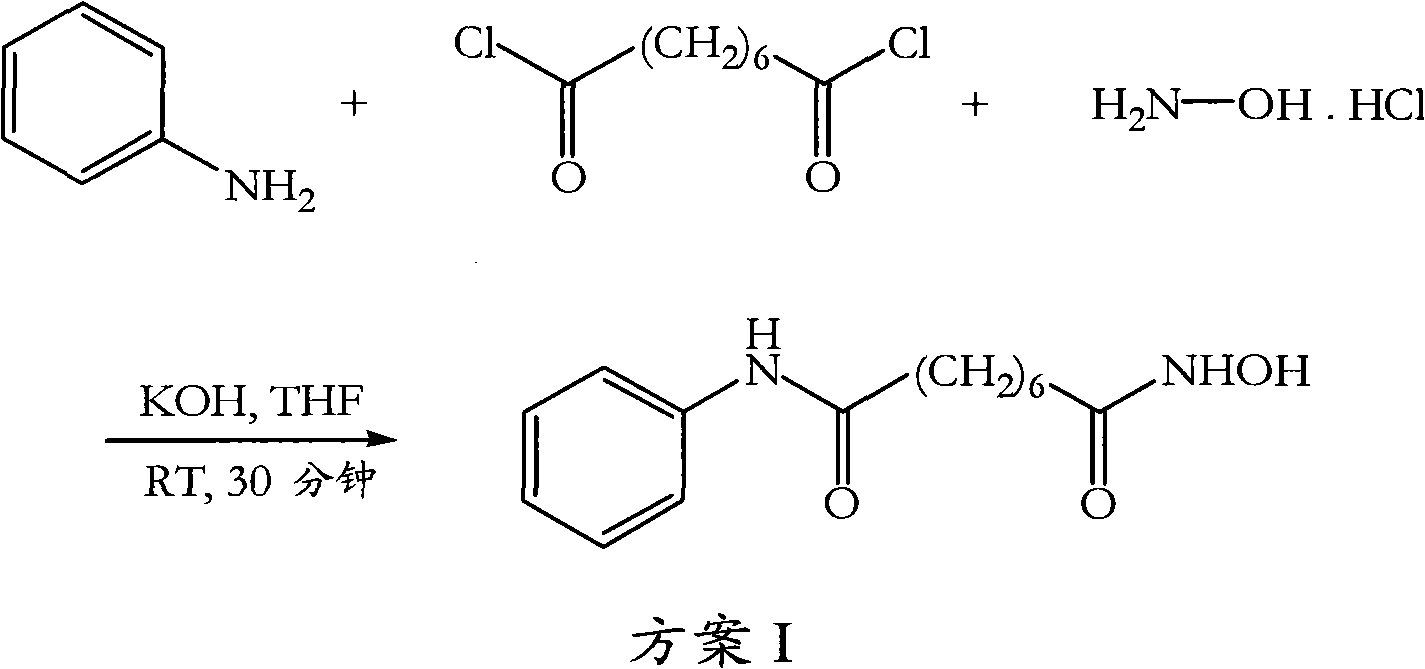
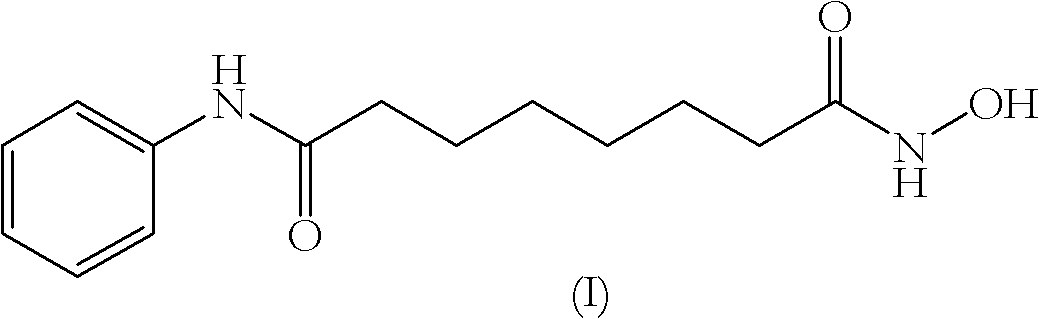
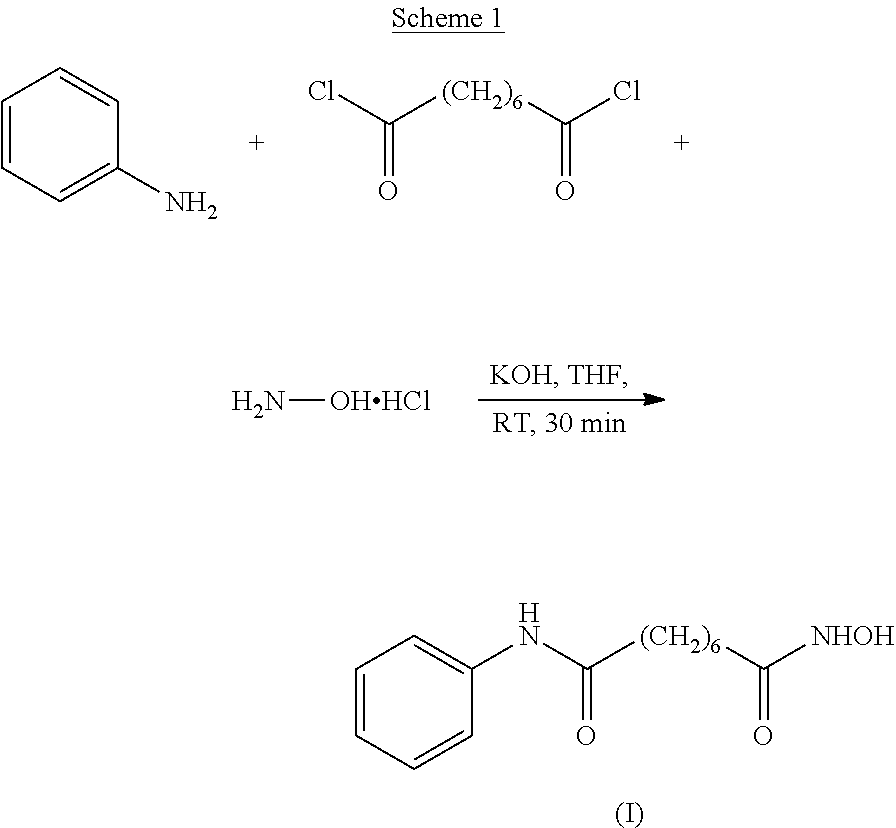
The invention relates to a method for synthesizing anti-cancer drug vorinostat and belongs to the field of drug synthesis technology. The method comprises the steps of: reacting octanedioic acid with aniline in the presence of perfluorinated sulfonic acid resin to obtain suberanilic acid; reacting the suberanilic acid with hydroxylamine in the presence of a condensation agent 1,3-dicyclohexyl carbodiimide; separating the product vorinostat. The intermediate product suberanilic acid prepared by the method is high in purity, good in yield, low in impurity content and is safe and environment-friendly, and therefore the prepared crude product vorinostat has high purity and good yield.
Owner:NANTONG FINC PHARMA CHEM
Vorinostat solid preparation
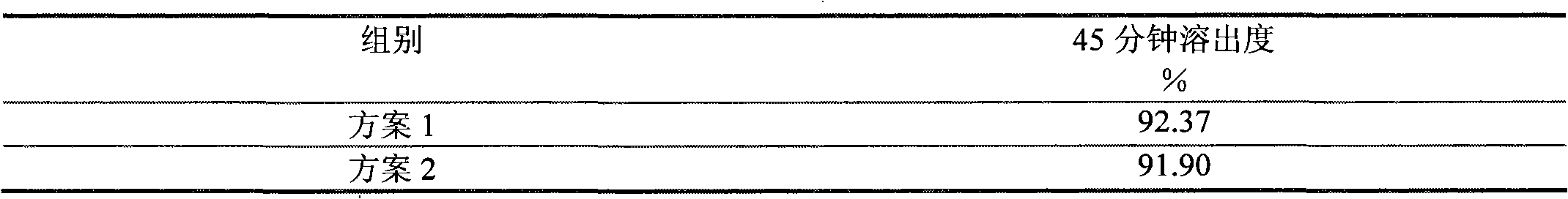
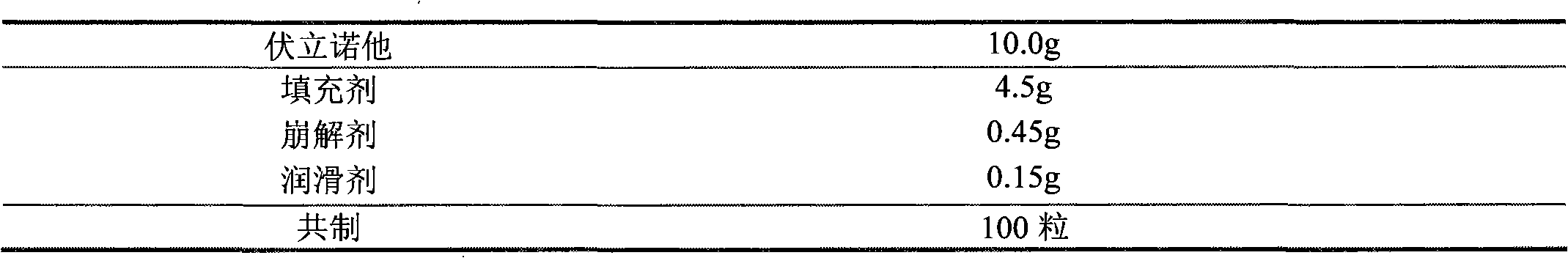
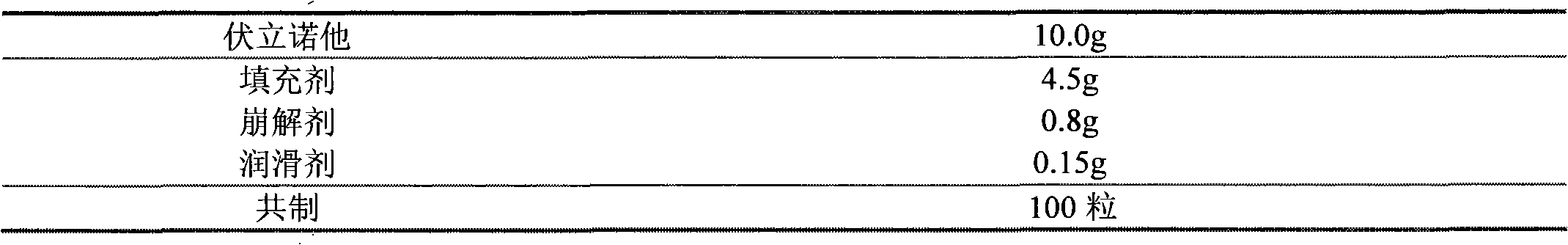
The invention discloses a Vorinostat solid preparation which is prepared from bulk drug Vorinostat and pharmaceutical adjuvant, wherein the pharmaceutical adjuvant comprises a filling agent, a disintegrating agent and a lubricating agent. The Vorinostat solid preparation is characterized in that the weight ratio of the Vorinostat to the filling agent is 1:0.9-4.5, the weight ratio of the Vorinostat to the disintegrating agent is 1:0.0834-0.4170, and the weight ratio of the Vorinostat to the lubricating agent is 1:0.0166-0.0830. The invention determines the doses of the disintegrating agent, the filling agent and the lubricating agent in the prescription of the Vorinostat solid preparation by creative experiments, and the dissolution of the Vorinostat solid preparation reaches more than 80% in 45 minutes.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
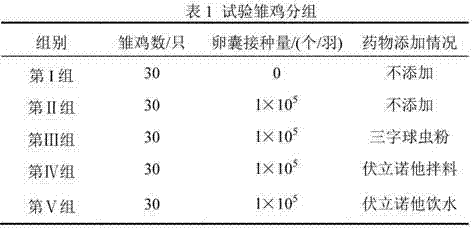
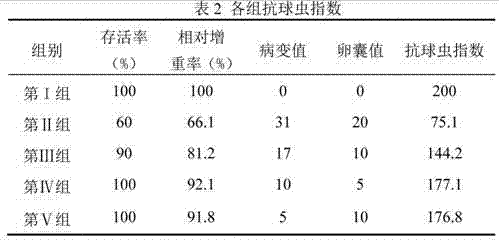
Application of Vorinostat in preparation of soft-resistant Eimeria drugs
ActiveCN102641261AGood against Eimeria tenellaOrganic active ingredientsAntiparasitic agentsBiotechnologyAnimal science
The invention discloses application of Vorinostat in preparation of soft-resistant Eimeria drugs; powder is formed by the Vorinostat and an acceptable auxiliary material, and then is added into a feed to mix uniformly so as to feed chicken by a daily feed amount; or a Vorinostat water-soluble powder is prepared by the Vorinostat, and is dissolved in water to feed chicken by a daily feed amount. Animal infection experiments confirm an anticoccidia index (ACI) of the Vorinostat is more than 175, and the Vorinostat has function of resisting soft-resistant Eimeria.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
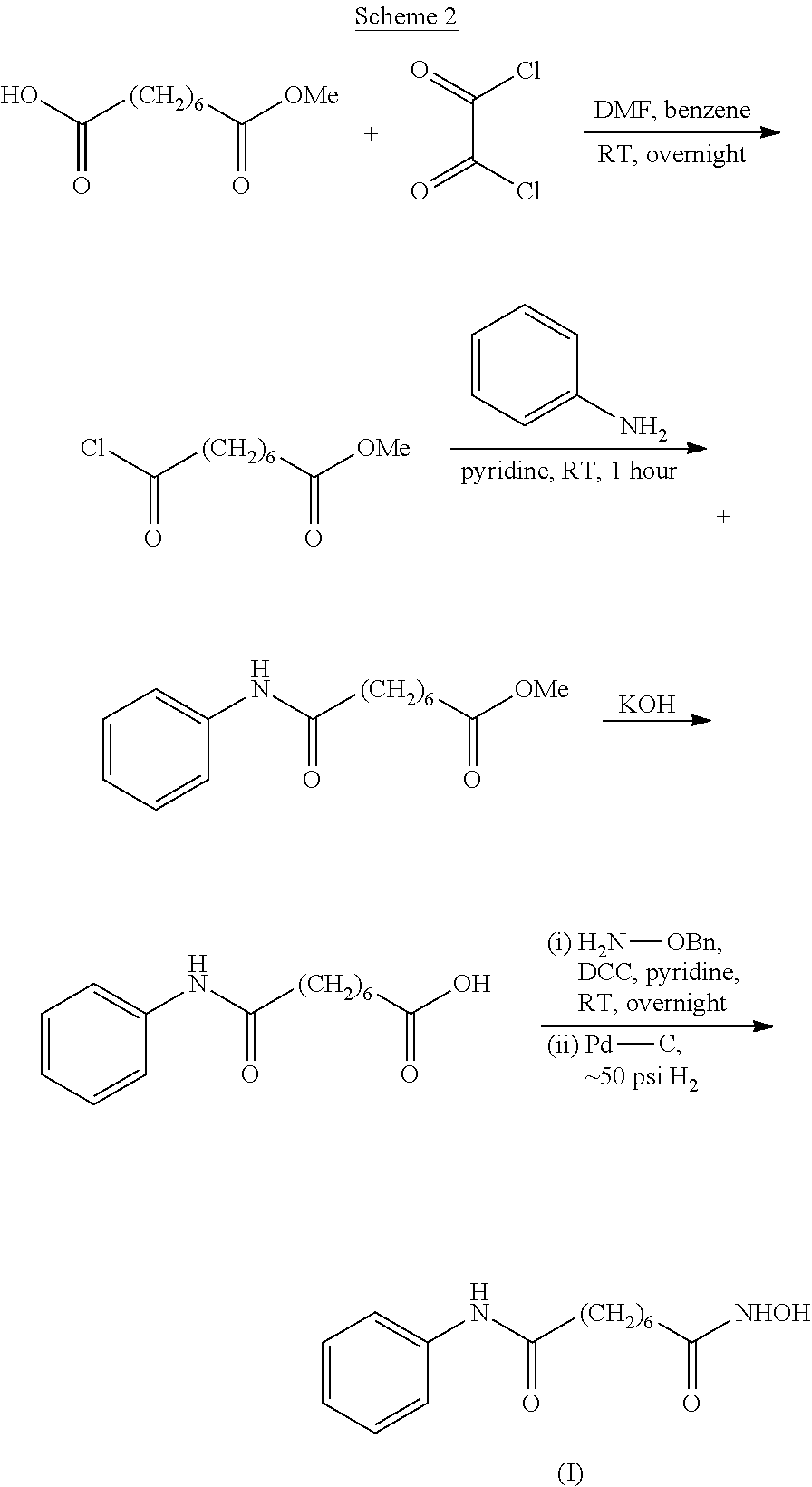
Novel process for the preparation of vorinostat
InactiveCN101939289AOrganic active ingredientsOrganic chemistryCombinatorial chemistryPharmaceutical Substances
The present invention relates to an improved process for the preparation of the active pharmaceutical ingredient, vorinostat. In particular it relates to an efficient process for the preparation of vorinostat of high purity without the requirement to isolate any synthetic intermediate compounds.
Owner:GENERICS UK LTD
Method for preparing anticancer drug--Vorinostat
InactiveCN106187818AHigh yieldMild conditionsOrganic compound preparationCarboxylic acid amides preparationHydroxylamine HydrochlorideAniline
The invention discloses a method for preparing an anticancer drug--Vorinostat. The method comprises the following steps: 1) subjecting a hydrophilic substrate and octanedioic acid to contacting and carrying out self-assembling so as to obtain an octanedioic acid-substrate self-assembled film; 2) in the presence of 1,3-dicyclohexylcarbodiimide, subjecting the octanedioic acid-substrate self-assembled film to contacting with aniline in THF, after a reaction is completed, adding a 4M HCl solution and carrying out a reaction under stirring, and carrying out extraction with dichloromethane so as to obtain octanedioic acid monoanilide; and 3) subjecting octanedioic acid monoanilide and hydroxylamine hydrochloride to a reaction so as to obtain Vorinostat. The preparation method for Vorinostat provided by the invention provides a novel synthetic route for Vorinostat. By adopting the method for preparing Vorinostat provided by the invention, mild conditions and good selectivity are achieved; the time of the reaction, specifically the time of the aniline amidation reaction, is greatly reduced; meanwhile, the yield of Vorinostat is greatly improved.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
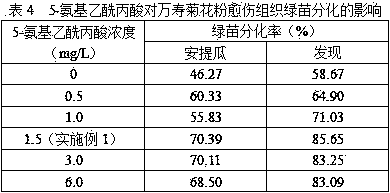
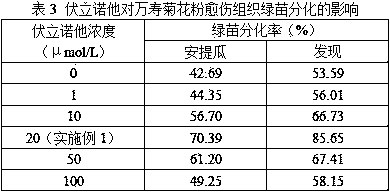
Marigold pollen differential medium and differentiation culture method
ActiveCN110547200AImproved growth and development characteristicsPromote differentiationHorticulture methodsPlant tissue cultureBetaineVitamin A Retinol
The invention discloses a marigold pollen differential medium and a differentiation culture method. The medium comprises KNO3, NH4NO3, KH2PO4.H2O, MgSO4.7H2O, CaCl2.2H2O, MnSO4.4H2O, ZnSO4.7H2O, H3BO3, KI, Na2MoO4.2H2O, CuSO4.5H2O, CoCl2.6H2O, ferrous glycine, inositol, thiamine hydrochloride, pyridoxine hydrochloride, niacin, threonine, asparagine, retinol, an air-plant herb extraction solution,6-BA, NAA, abscisic acid, vorinostat, 5-aminolevulinic acid, maltose, betaine and agar. By means of culture using the medium and the method, the plant differentiation rate of 70% or above can be obtained, and a certain technical support is provided for establishment of a marigold pollen high-efficiency culture system.
Owner:济南易通城市建设集团股份有限公司
Preparation method and preparation of vorinostat I crystal form large grains
ActiveCN102643214AGood dissolution effectHigh commercial application valueOrganic active ingredientsOrganic chemistryMedicineCutaneous T-cell lymphoma
The invention provides a preparation method of vorinostat I crystal form large grains as a medicament for treating cutaneous T cell lymphoma and a preparation prepared from vorinostat obtained by the preparation method. The preparation method can crystallize in place once, is simple, effective and suitable for large-scale industrial production and further has greater commercial application value. The obtained preparation is good in dissolution effect.
Owner:杭州容立医药科技有限公司
Method for refining histone deacetylase (HDAC) inhibitor vorinostat
The invention provides a method for refining a medicine vorinostat for treating cutaneous T-cell lymphomas. The method, which allows an impurity suberanilic acid to be rapidly removed and high purity vorinostat to be prepared and has the advantages of simple operation and high yield, is suitable for the industrialization production.
Owner:杭州容立医药科技有限公司
Pharmaceutical composition containing a hypomethylating agent and a histone deacetylase inhibitor
InactiveUS20120310183A1Organic active ingredientsGenetic material ingredientsTransdermal patchHistone methylation
A pharmaceutical composition for induction therapy which has a hypomethylating agent and a histone deacetylase inhibitor (“HDAC inhibitor”); wherein the hypomethylating agent is a DNA and histone methylation inhibitor such as cladribine and the HDAC inhibitor is, for example, entinostat, panobinostat, vorinostat, and / or romedepsin; further wherein the hypomethylating agent and the HDAC inhibitor are combined in formulations for various administrations including e.g., a continuous delivery system such as a transdermal patch of at least one reservoir or a plurality of reservoirs, oral, a fixed-dose oral combination, intravenous, and combinations thereof. This pharmaceutical composition for induction therapy is used with a monoclonal antibody in the treatment of various cancers, sarcomas, and other malignancies.
Owner:NIMBLE EPITECH
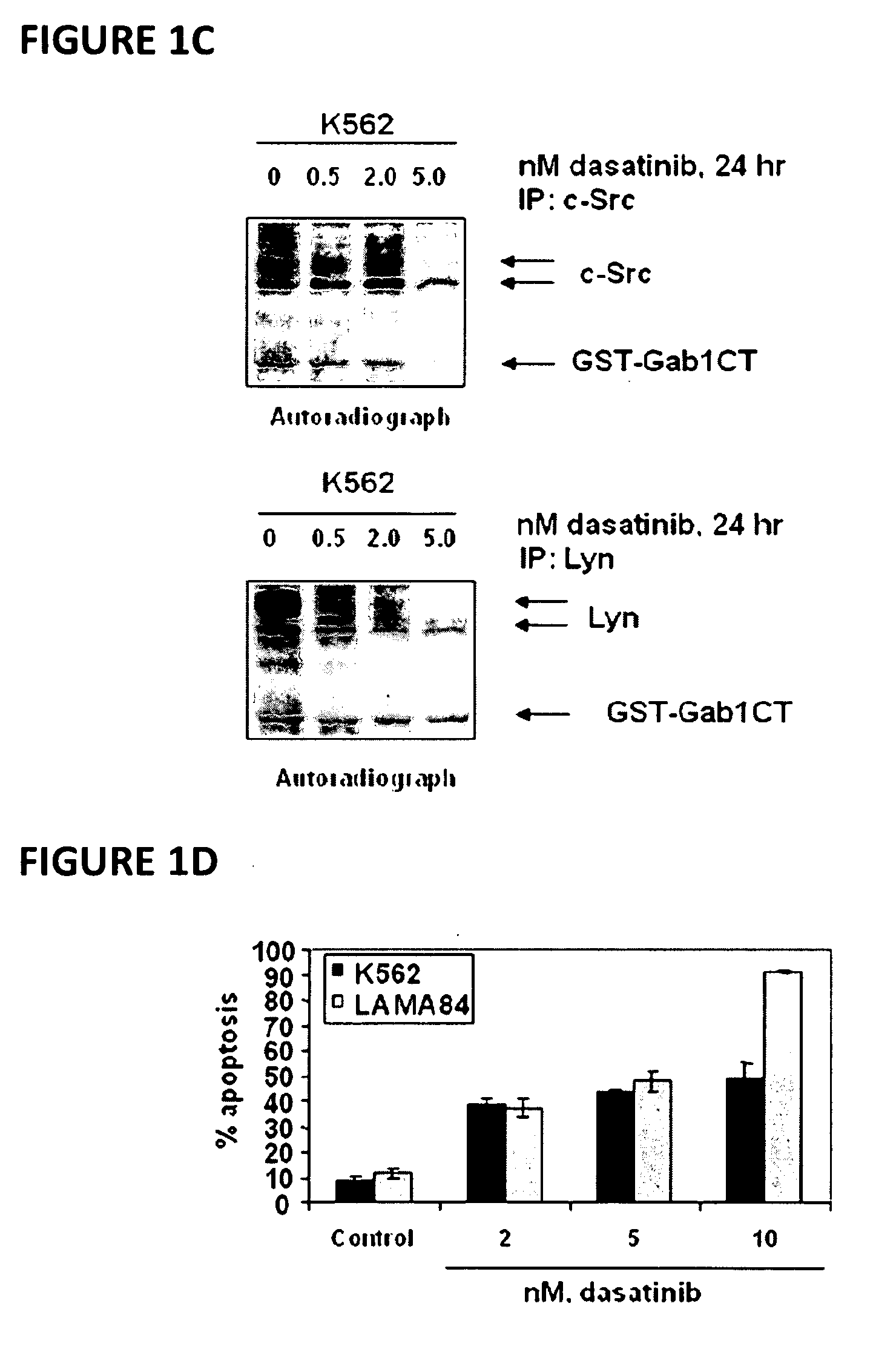
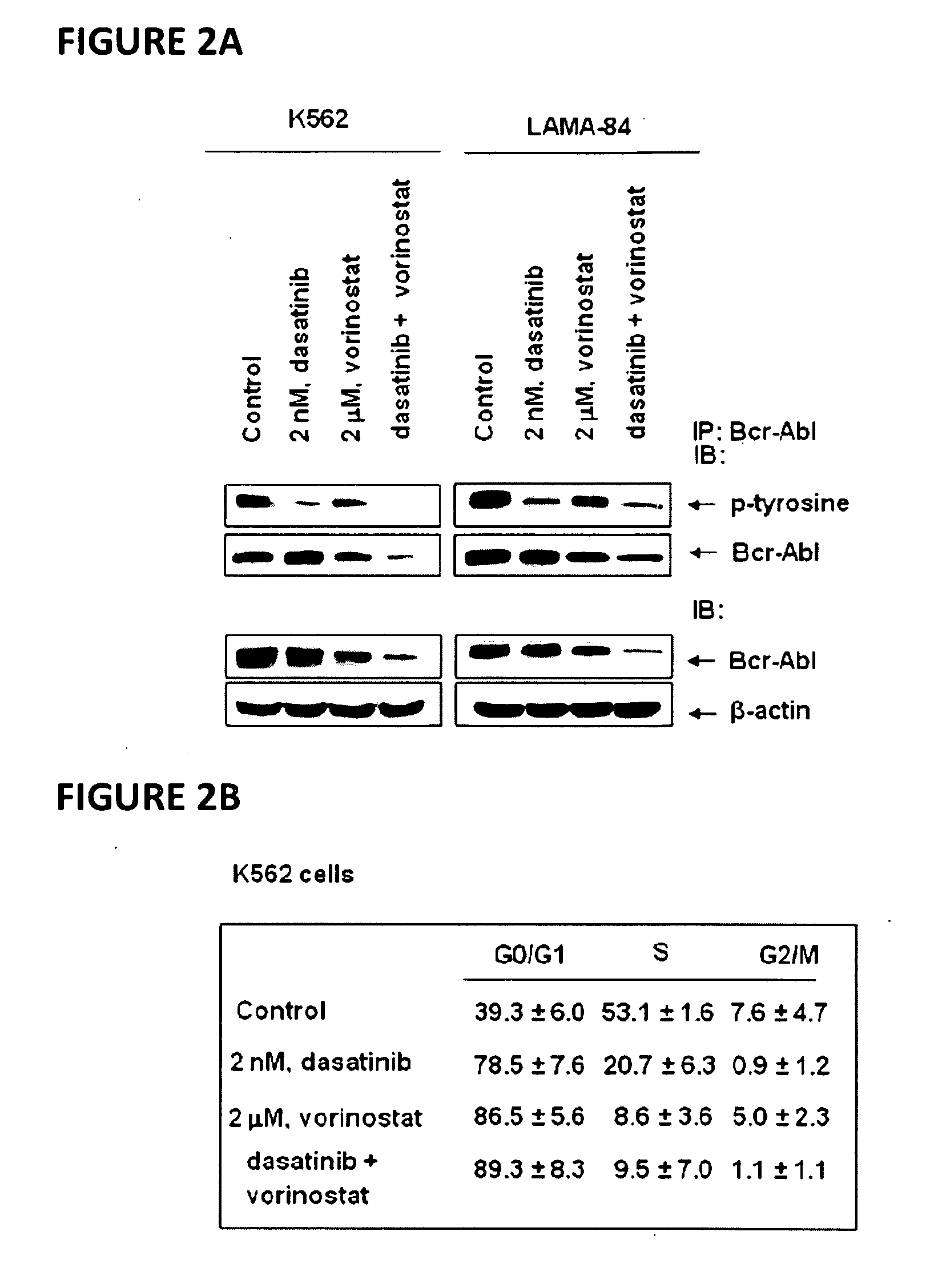
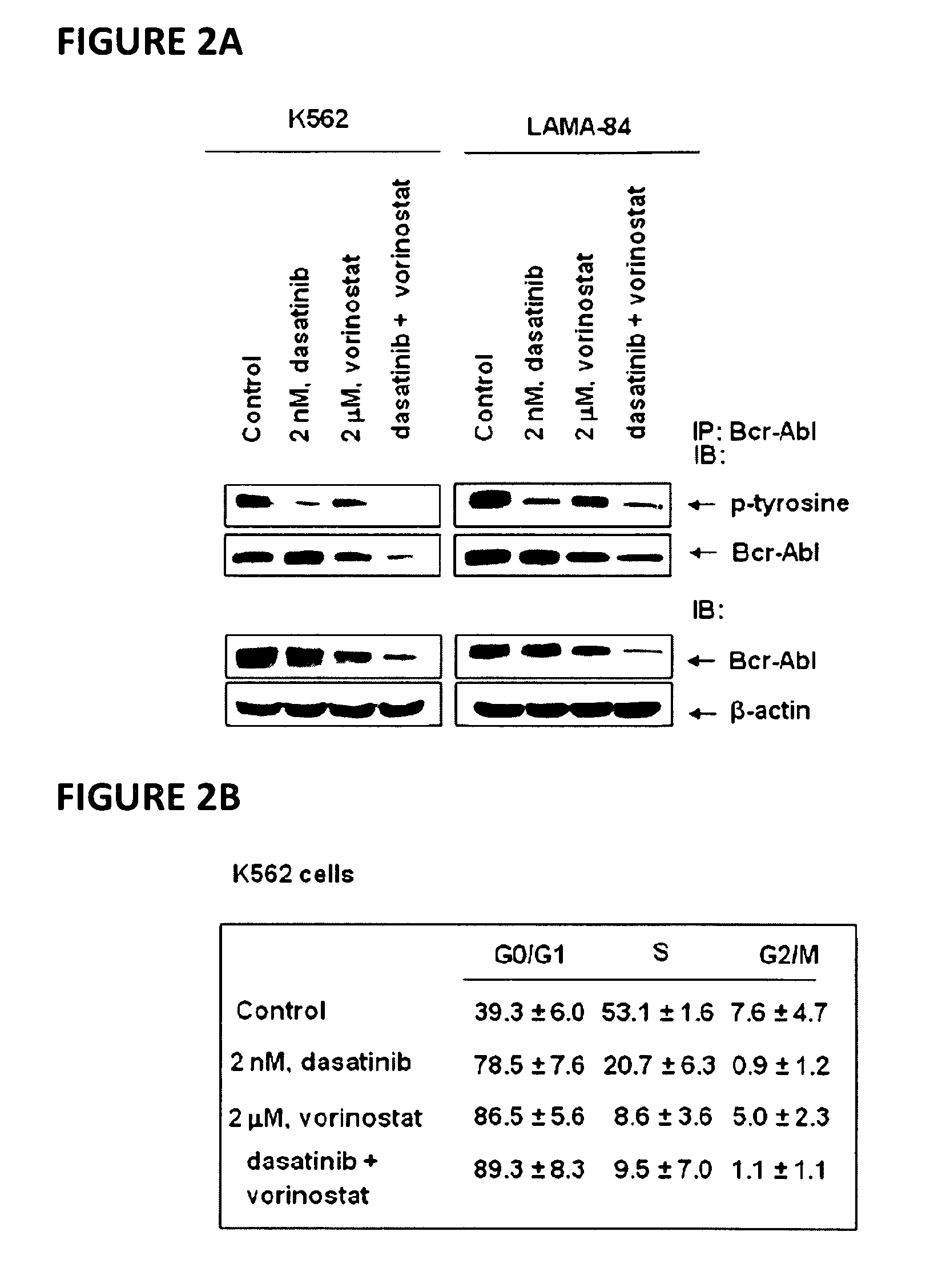
Method of treating chronic myelogenous leukemia cells
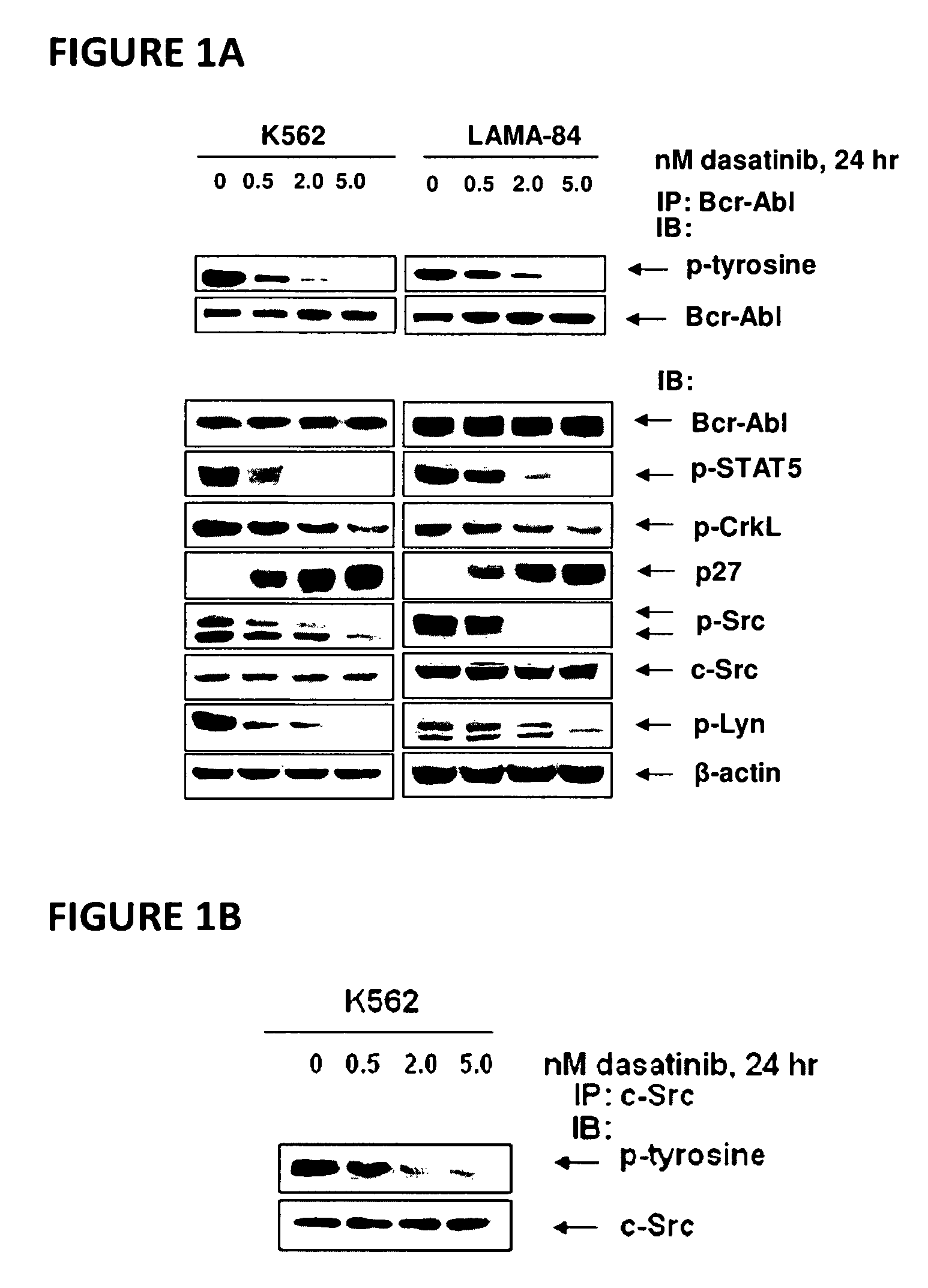
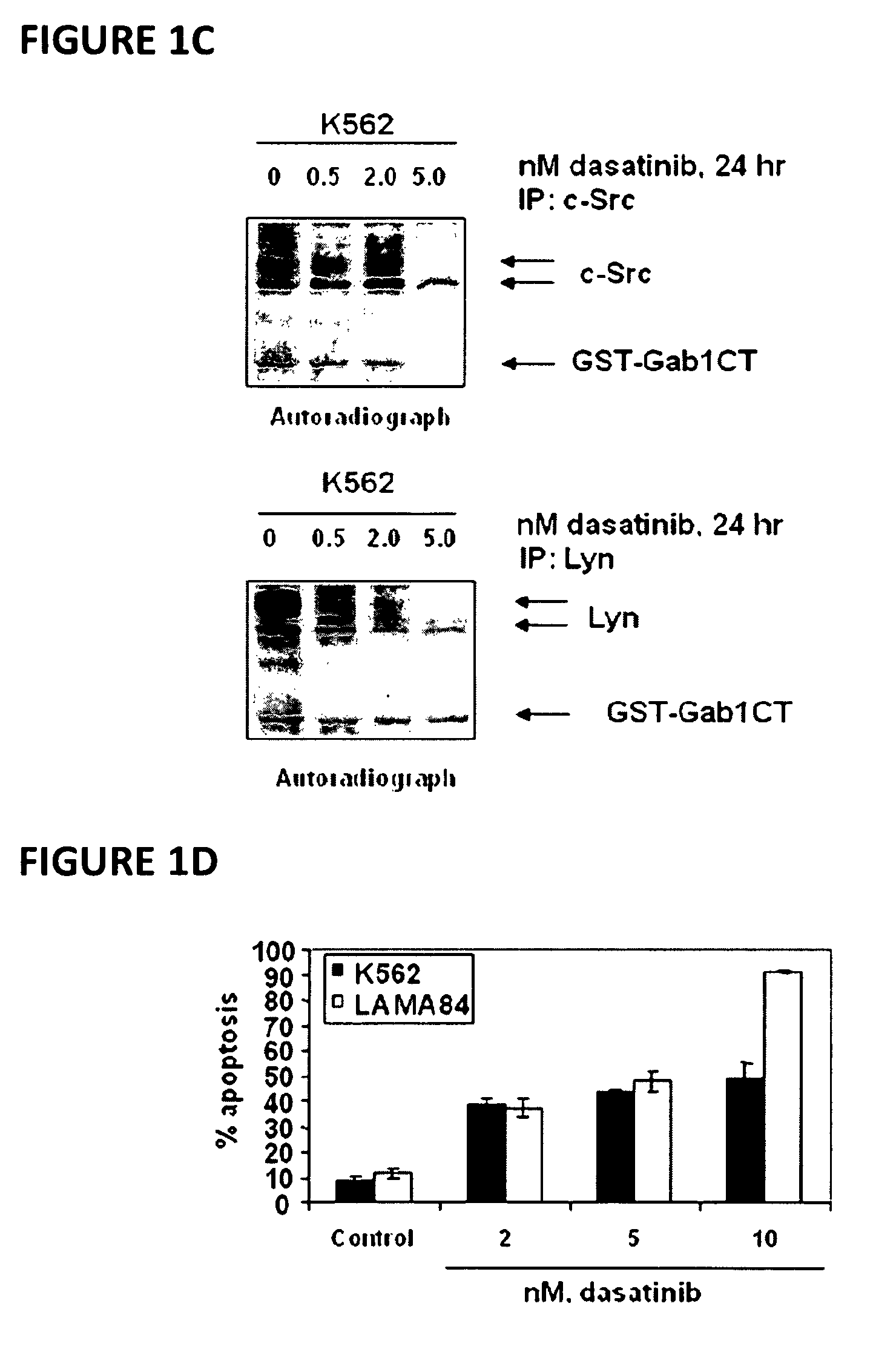
Here, the inventors disclose the treatment of imatinib mesylate resistant chronic myelogenous leukemia cells with a cotreatment of vorinostat (SAHA, suberoylanilide hydroxamic acid) and dasatinib, a dual Abl / Src kinase (TK) inhibitor. Combined treatment of cultured human CML and BaF3 cells with vorinostat and dasatinib induced more apoptosis than either agent alone, as well as synergistically induced loss of clonogenic survival, which was associated with greater depletion of Bcr-Abl, p-CrkL and p-STAT5 levels. Co-treatment with dasatinib and vorinostat also attenuated the levels of Bcr-AblE255K and Bcr-AblT315I and induced apoptosis of BaF3 cells with ectopic expression of the mutant forms of Bcr-Abl. Finally, co-treatment of the primary CML cells with vorinostat and dasatinib induced more loss of cell viability and depleted Bcr-Abl or Bcr-AblT315I, p-STAT5 and p-CrkL levels than either agent alone.
Owner:BRISTOL MYERS SQUIBB CO +1
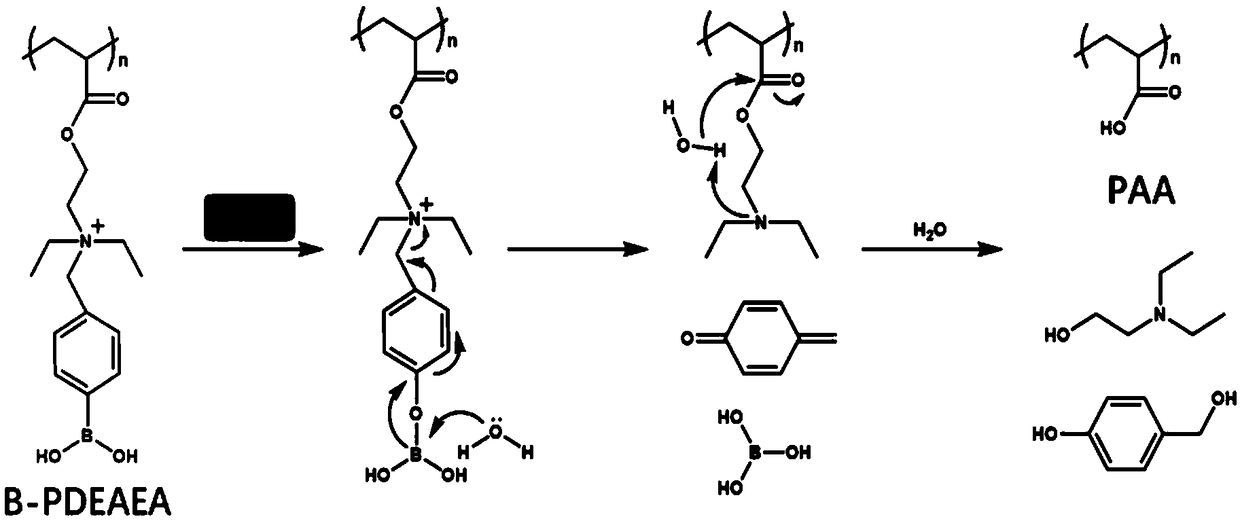
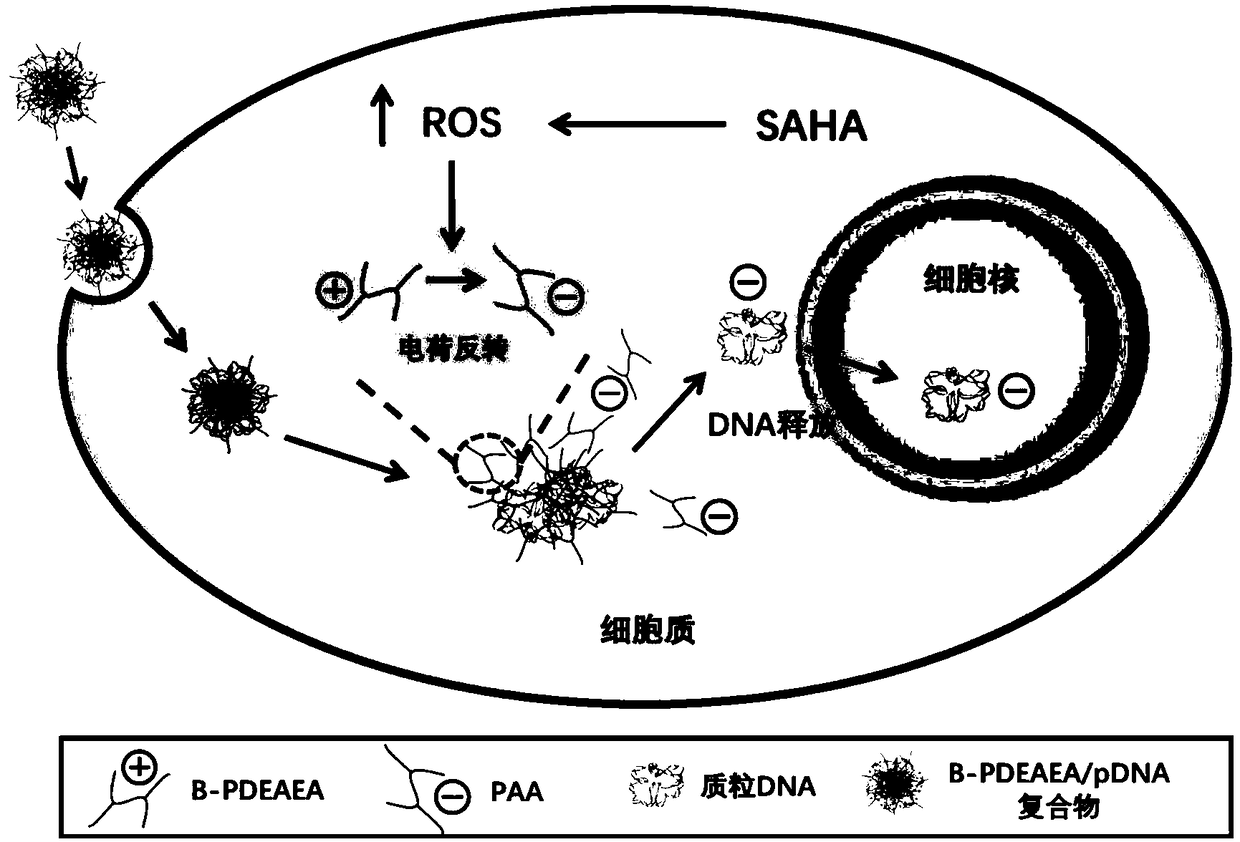
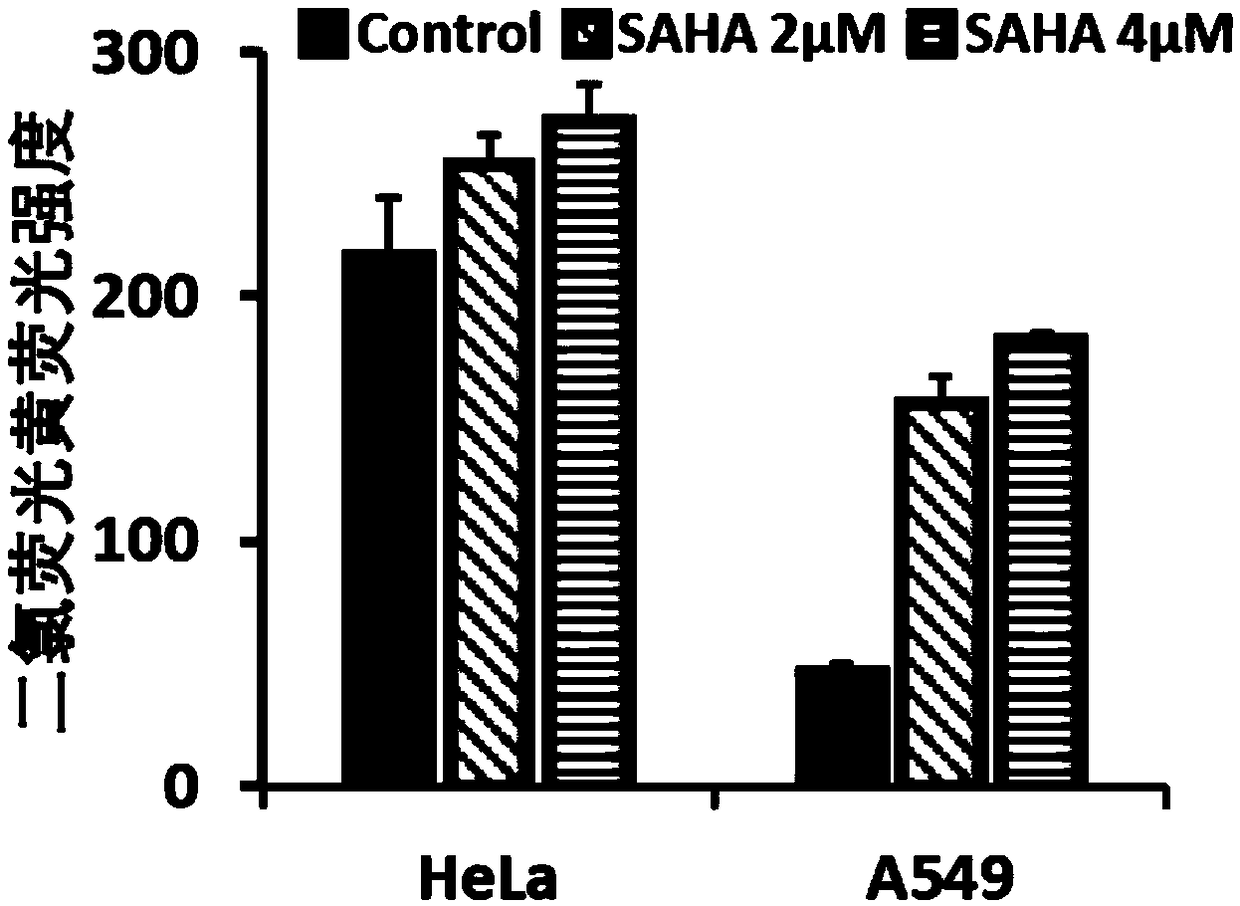
Application of vorinostat in oxidation responded cationic polymer gene vector mediated gene therapy
InactiveCN108888617AFacilitated releaseFacilitate charge reversalAmide active ingredientsAntineoplastic agentsTreatment effectRos responsive
The invention relates to a novel application of vorinostat in an oxidation responded cationic polymer gene vector mediated gene therapy. The treatment effect on tumor cells is improved obviously by being combined with a TRAIL gene. Experiments find that vorinostat can lead to up-regulation of ROS in cells at a concentration without generating cytotoxicity and can promote charge reversal of an ROSresponded cationic polymer so as to further promote release of DNA, so that the final gene transfection effect is enhanced.
Owner:ZHEJIANG UNIV
Pharmaceutical composition containing gefitinib and histone deacetylase inhibitor, lipidosome preparation of pharmaceutical composition, and pharmaceutical application of pharmaceutical composition and lipidosome preparation
ActiveCN109745326AImprove distributionIncrease savingsOrganic active ingredientsImmunological disordersLiposomeMacrophage polarization
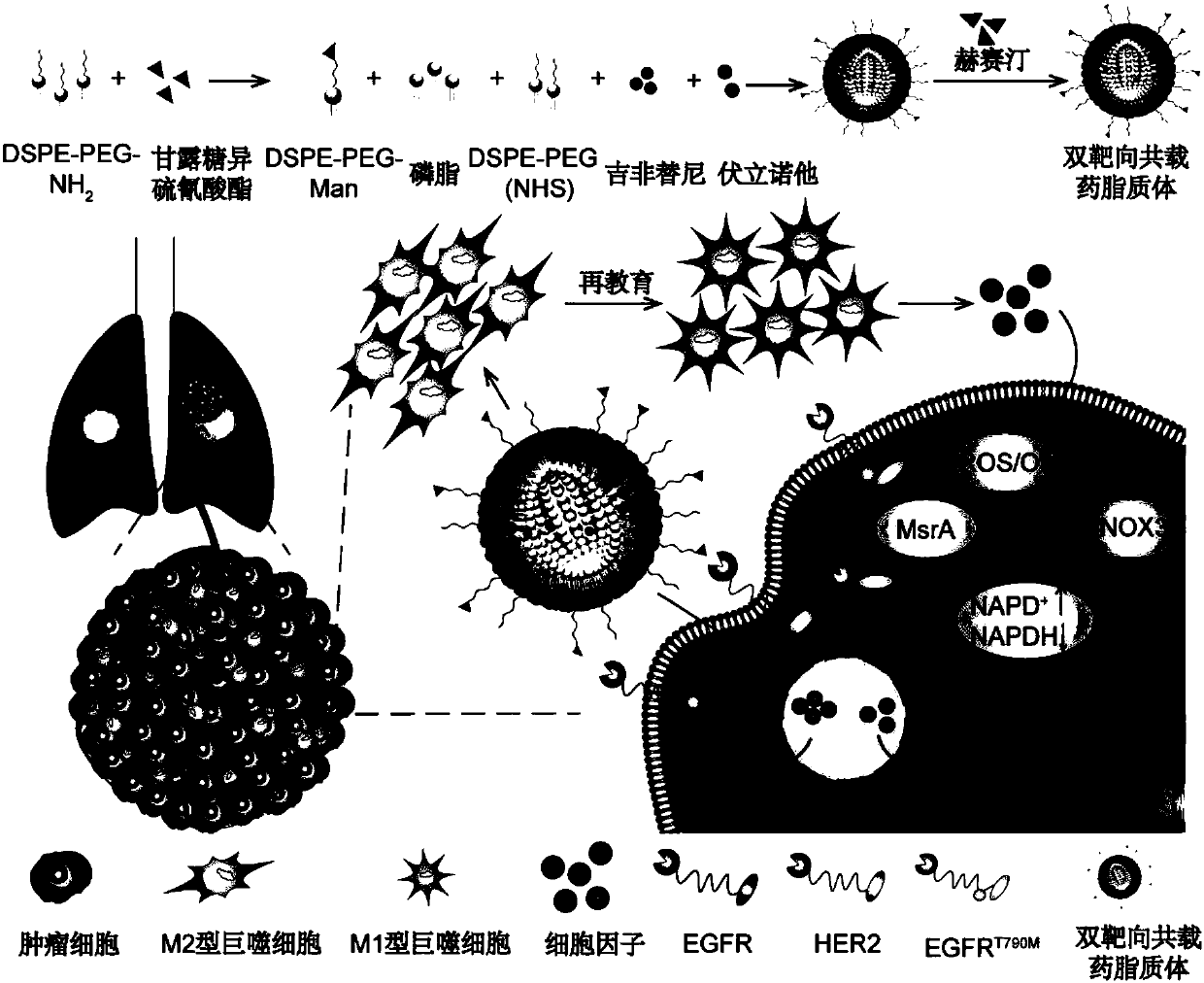
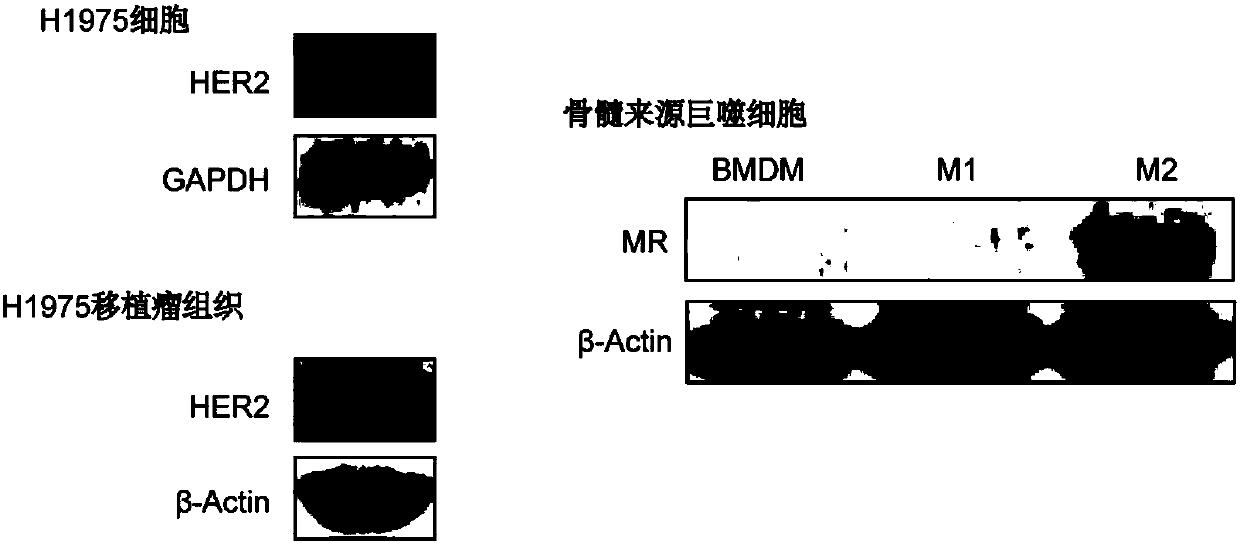
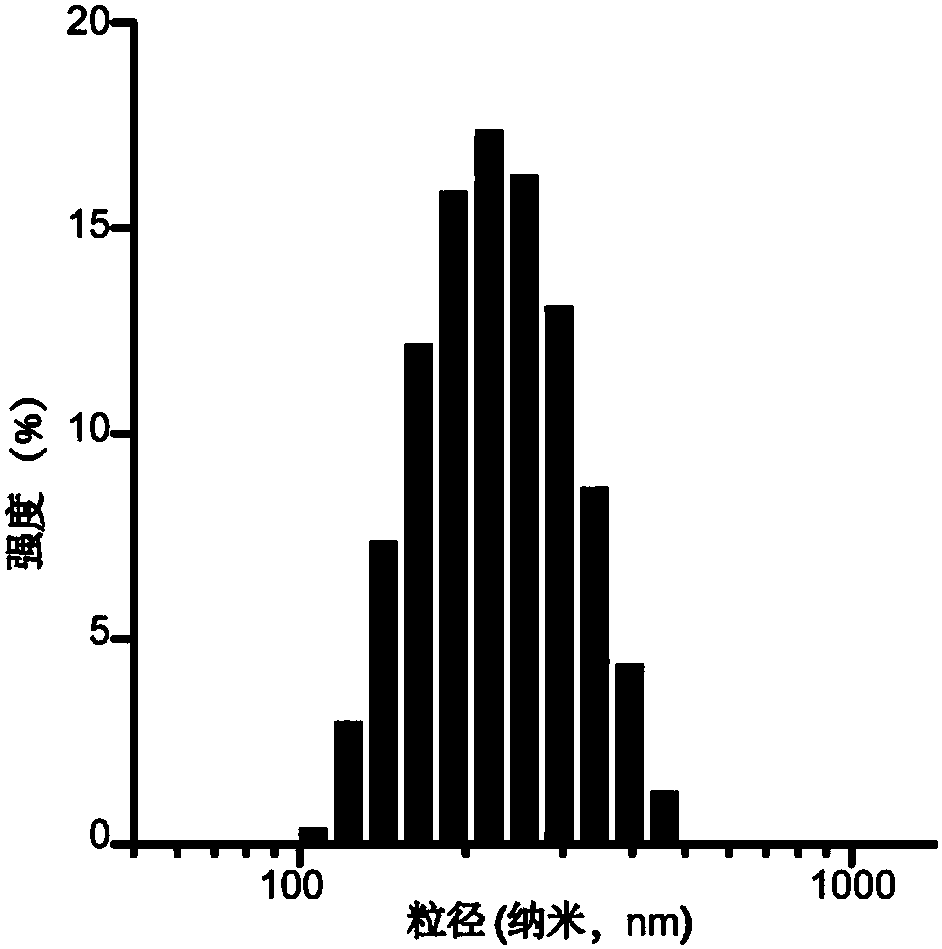
The invention relates to a pharmaceutical composition containing a gefitinib and histone deacetylase inhibitor, a lipidosome preparation of the pharmaceutical composition, and application of the pharmaceutical composition and the lipidosome preparation to preparing medicines for treating the EGFRT790M mutation drug-fast non-small cell lung cancer. The histone deacetylase inhibitor is preferentially Vorinostat and TMP195. Particularly, the invention further relates to a double-target co-carrier liposome, wherein the surface of the liposome is modified by herceptin and mannose, and the liposomecontains the pharmaceutical composition. The double-target co-carrier liposome can be used for controlling the macrophage polarization and can be used for treating the non-small cell lung cancer withEGFRT790M mutation.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
New methods and pure polymorphs
InactiveCN102282125AMild and reproducible experimental conditionsOrganic active ingredientsOrganic chemistryCombinatorial chemistryPharmaceutical Substances
The invention relates to a crystal form of vorinostat as an active pharmaceutical ingredient, a preparation method thereof and an application in a pharmaceutical composition.
Owner:GENERICS UK LTD
Process for the preparation of vorinostat
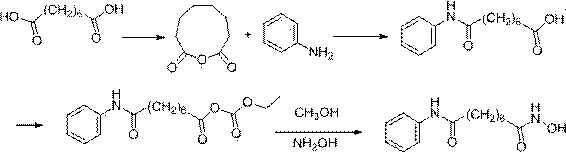
The present invention relates to an improved process for the preparation of the active pharmaceutical ingredient vorinostat. In particular it relates to a process for preparing vorinostat substantially free from impurities, involving suberic acid, aniline and hydroxylamine as starting materials.
Owner:GENERICS UK LTD
Application of combination of bortezomib and panobinostat or vorinostat in preparing drugs for treating drug-resistant MLL
ActiveCN110314222AEffective treatmentSolve the problem of drug resistanceDipeptide ingredientsAmide active ingredientsPanobinostatTreatment effect
The invention relates to application of a combination of bortezomib and panobinostat or vorinostat in preparing drugs for treating drug-resistant MLL. It is proved that panobinostat and vorinostat canrestore the sensitivity of MLL drug-resistant cells, reverse the bortezomib drug resistance in the body of a mouse, reduce the production of drug-resistant cells and prolong the lifetime. Through theapplication, solving of the drug resistance problem of MLL is facilitated, and the treatment effect on malignant tumors of this type in the personalized medical treatment era is further improved.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Inhibition effect of nobiletin as BH3 mimetic drug on small cell lung cancer and synergistic effect of nobiletin and histone deacetylase inhibitor
ActiveCN113018292AEnhanced inhibitory effectHighly synergistic withAmide active ingredientsAntineoplastic agentsBh3 mimeticAdjuvant
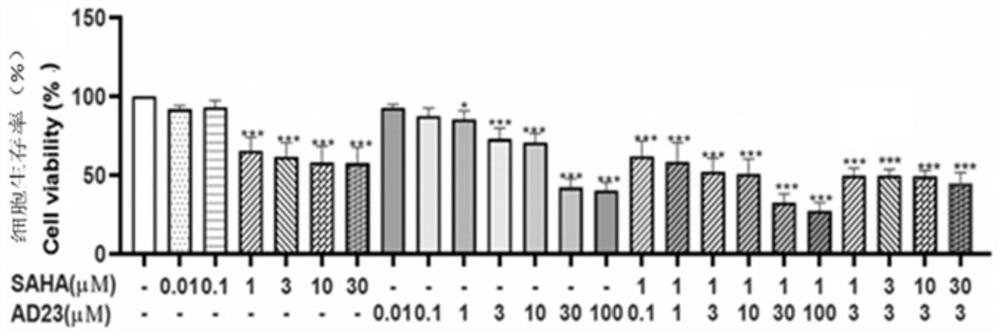
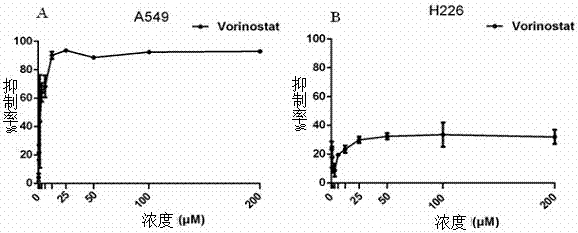
The invention provides application of nobiletin as a BH3 mimetic drug and / or a histone deacetylase inhibitor in preparation of drugs for treatment or adjuvant treatment of small cell lung cancer; or the nobiletin is used as a BH3 mimetic drug and / or a histone deacetylase inhibitor to prepare a product for inhibiting small cell lung cancer cell proliferation. Experimental results show that nobiletin shows a stronger inhibition effect on small cell lung cancer compared with non-small cell lung cancer. Moreover, according to the cell survival rate observed 24 hours after administration, the fixed vorinostat concentration is 1 [mu]M, AD23 can generate a high synergistic effect in a range of 0.1-100 [mu]M, and if the fixed AD23 is 3 [mu]M, the vorinostat has a high synergistic effect in a range of 1-30 [mu]M.
Owner:PEKING UNIV +1
Preparation method of anti-cancer drug vorinostat
The invention discloses a preparation method of an anti-cancer drug vorinostat. The method comprises the following steps that 1, a hydrophilic substrate and suberic acid make contact with each other to be self-assembled to obtain a suberic acid-substrate self-assembled membrane; 2, the suberic acid-substrate self-assembled membrane makes contact with hydroxylamine hydrochloride in THF in the presence of 1,3-dicyclohexylcarbodiimide, after reacting is finished, 4M of a HCl solution is added, reacting under stirring is conducted, and dichloromethane extraction is conducted to obtain N-hydroxyl-7-carboxyl-heptamide; 3, N-hydroxyl-7-carboxyl-heptamide reacts with aniline to obtain the vorinostat in the presence of 1,3-dicyclohexylcarbodiimide and alkali. According to the preparation method of the vorinostat, a novel synthesis way of the vorinostat is provided. By means of the preparation method of the vorinostat, the conditions are mild, the selectivity is good, the reacting time, especially the aniline amidation reacting time is greatly shortened, and meanwhile the yield of the vorinostat is greatly increased.
Owner:马腾 +1
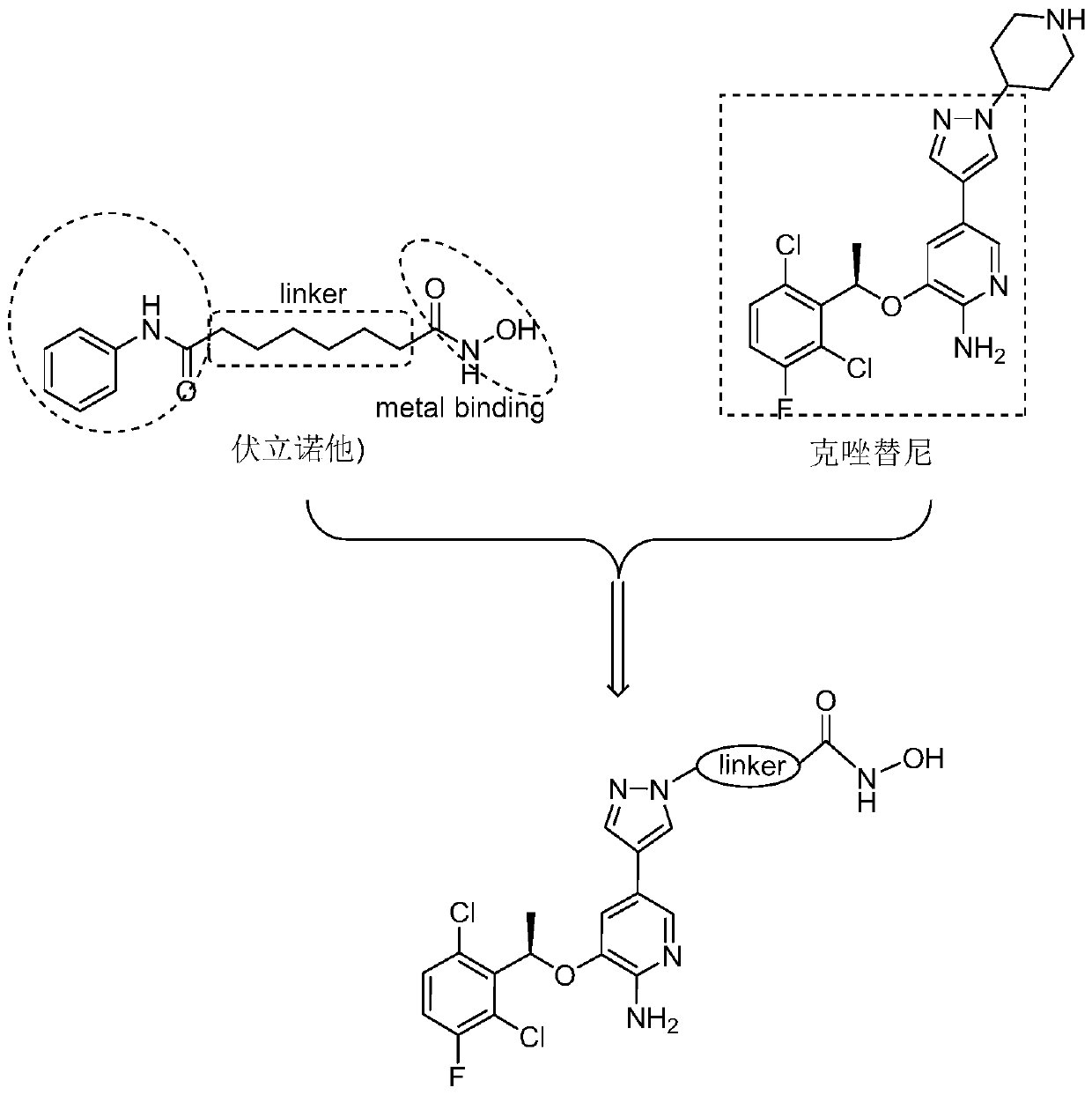
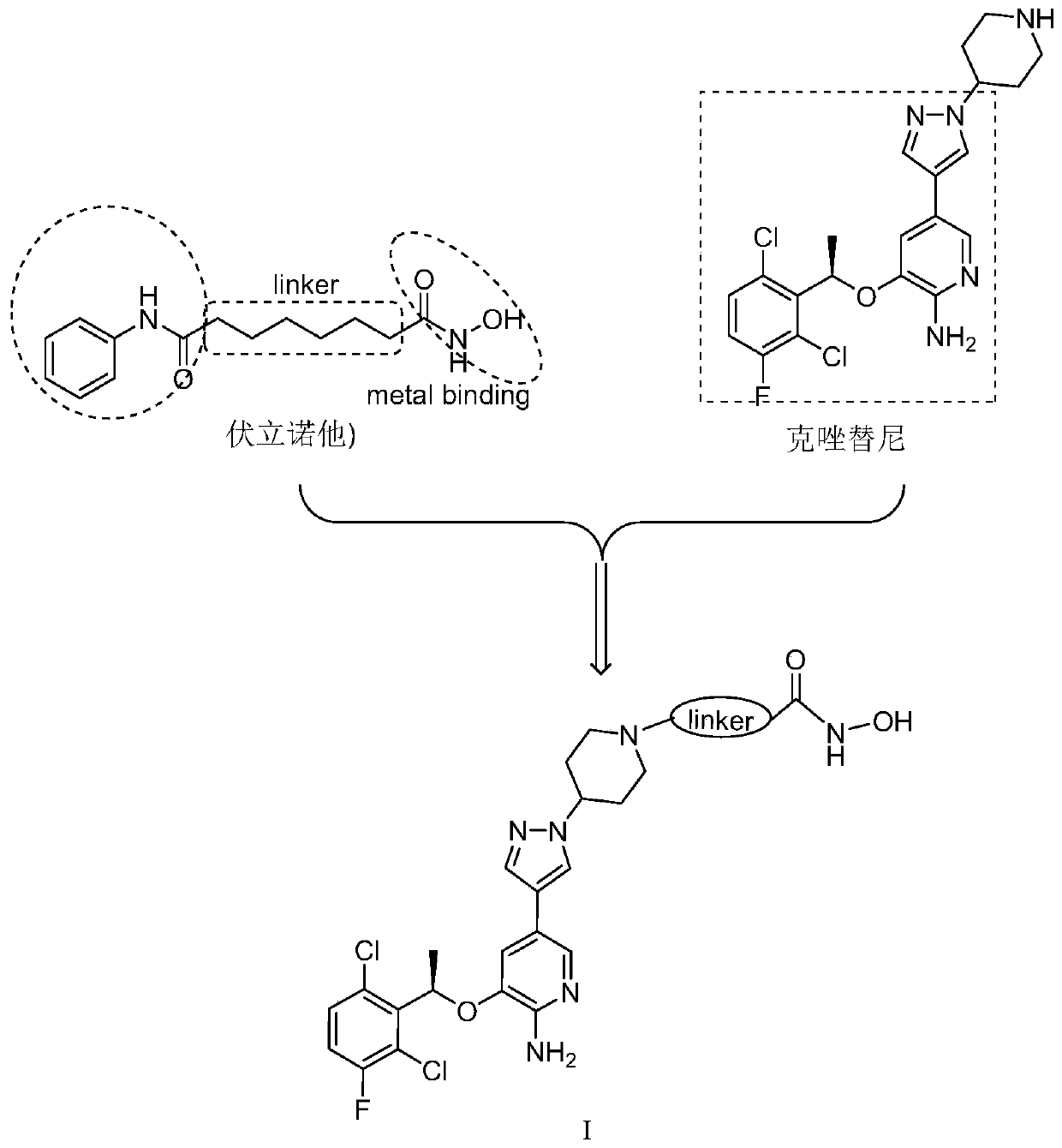
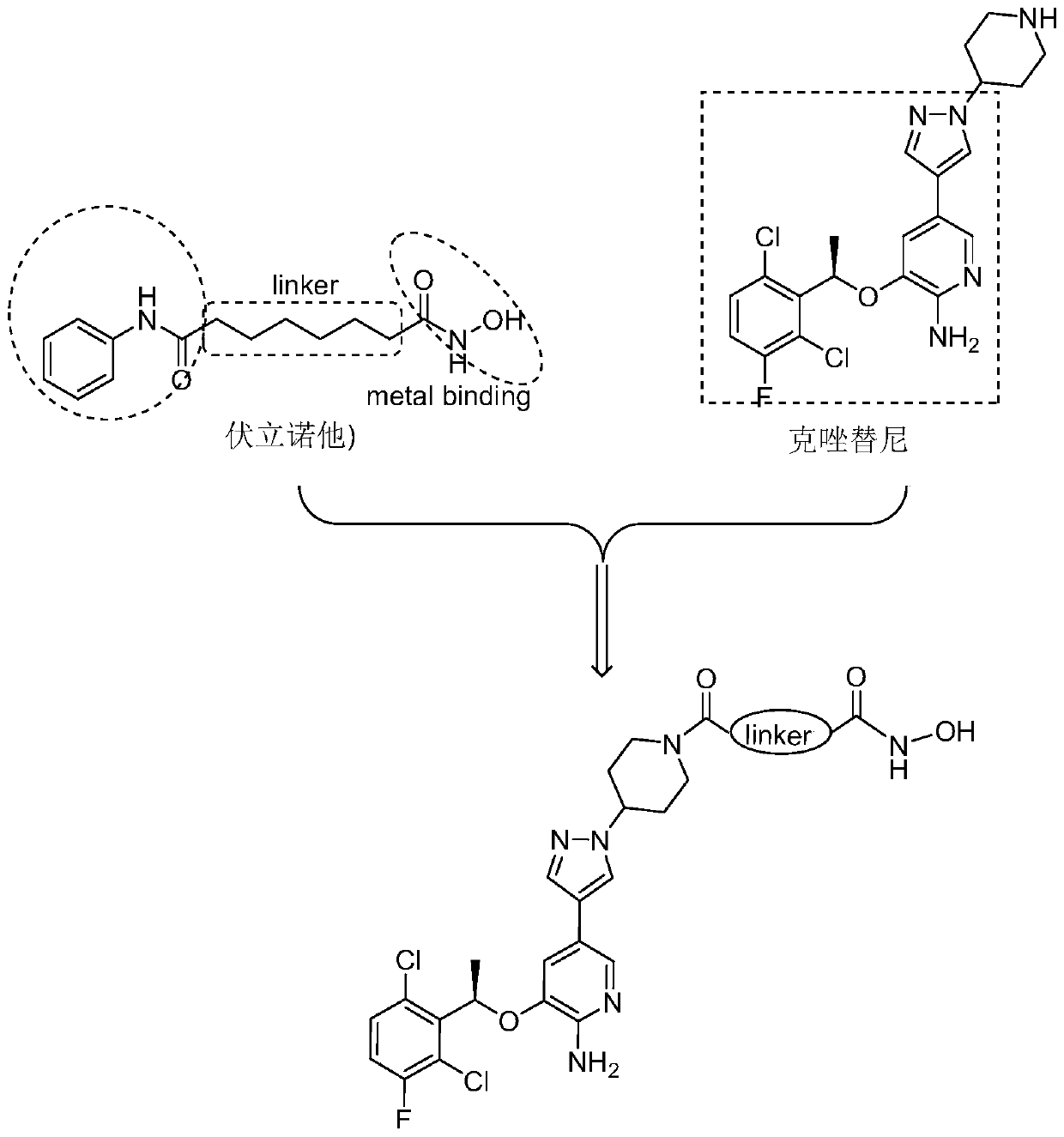
Novel c-Met/HDAC double-target inhibitor, and synthetic method and application thereof
ActiveCN110016013AEnhanced inhibitory effectImprove utilization efficiencyOrganic chemistryAntineoplastic agentsSynthesis methodsPharmacophore
The invention discloses a novel c-Met / HDAC double-target inhibitor, and a synthetic method and an application thereof. The inhibitor provided by the invention has the following advantages: with a c-Met inhibitor namely Crizotinib and an HDAC inhibitor namely Vorinostat as precursor structures, a double-target inhibitor with a single structure is designed; the Crizotinib is used to remove the structure except a piperidine ring as a cap-like structure of the HDAC inhibitor to replace an aniline acyl group in a Vorinostat structure; a hydroxamic acid structure in the Vorinostat is retained as a chelating group of a metal zinc ion; linking groups with different structures are introduced between the Crizotinib and the Vorinostat; by utilization of the principle of pharmacophore combination, thedouble-target inhibitor with the single structure is designed; and the obtained double-target inhibitor has good inhibitory effect on c-Met and HDAC, and can synergistically inhibit c-Met and HDAC.
Owner:北京凯恩梅格医药科技有限公司
Application of vorinostat to preparation of lung cancer treatment drug
InactiveCN107349191AGrowth inhibitionIncrease lethalityOrganic active ingredientsRespiratory disorderSide effectOncology
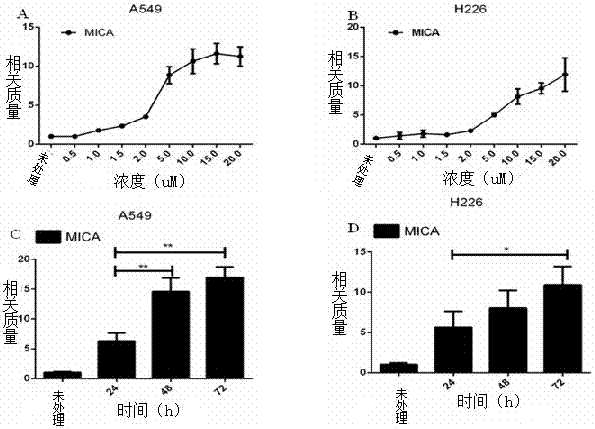
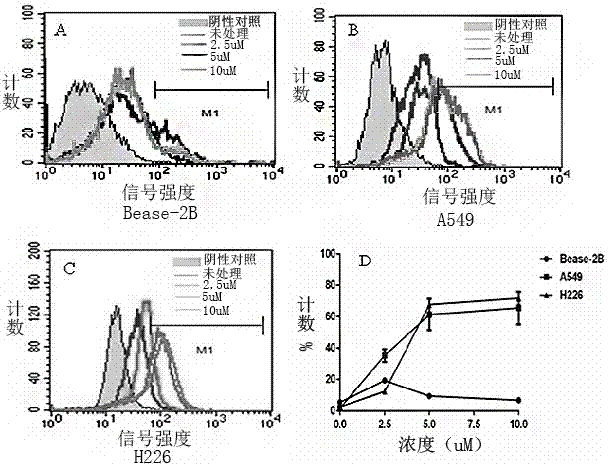
The invention provides an application of vorinostat to preparation of a lung cancer treatment drug. The drug is applied to treatment of non-small cell lung cancer, and can directly activate expression of MHCI-associated protein MICA on surfaces of tumor cells, improve killing abilities of NK cells for lung cancer cell strains and inhibit growth of tumor cells. Compared with the chemotherapeutic drugs extensively used in lung cancer treatment, the drug has small side effects; the drug is easy to quantify and standardize, safety, effectiveness, bioactive constituents and the like can be analyzed with conventional detection means, supervision is facilitated, high product cost caused by high detection cost is avoided, and good development prospect is realized.
Owner:FOSHAN MATERNAL & CHILD HEALTH CARE HOSPITAL
Method of treating chronic myelogenous leukemia cells
Here, the inventors disclose the treatment of imatinib mesylate resistant chronic myelogenous leukemia cells with a cotreatment of vorinostat (SAHA, suberoylanilide hydroxamic acid) and dasatinib, a dual Abl / Src kinase (TK) inhibitor. Combined treatment of cultured human CML and BaF3 cells with vorinostat and dasatinib induced more apoptosis than either agent alone, as well as synergistically induced loss of clonogenic survival, which was associated with greater depletion of Bcr-Abl, p-CrkL and p-STAT5 levels. Co-treatment with dasatinib and vorinostat also attenuated the levels of Bcr-AblE255K and Bcr-AblT315I and induced apoptosis of BaF3 cells with ectopic expression of the mutant forms of Bcr-Abl. Finally, co-treatment of the primary CML cells with vorinostat and dasatinib induced more loss of cell viability and depleted Bcr-Abl or Bcr-AblT315I, p-STAT5 and p-CrkL levels than either agent alone.
Owner:BRISTOL MYERS SQUIBB CO +1
Method for preparing anticarcinogen vorinostat
The invention discloses a method for preparing anticarcinogen vorinostat. The method includes: (1) subjecting suberic anhydride and aniline to contact reaction in water and 1,4-dioxane at the temperature of 5-10 DEG C in the presence of CuI, performing filtering after reaction is finished, regulating the pH of filtrate to 5-6, performing suction filtration, washing filter cake obtained after suction filtration, and drying to obtain 7-phenylcarbamoylheptanoic acid; (2) dissolving the 7-phenylcarbamoylheptanoic acid in methanol, adding cation exchange resin and ZnCl2, heating to 50-55 DEG C for reaction for 3 hours, concentrating, extracting with ethyl acetate, concentrating, washing with petroleum ether, and drying to obtain suberanilic acid methyl ester; (3) subjecting hydroxylamine hydrochloride and sodium methoxide to stirring reaction in absolute methanol for 0.5-1h, filtering prior to adding the suberanilic acid methyl ester into filtrate for reaction at the temperature of 40 DEG C for 3-5 hours, cooling to room temperature, regulating the pH to 7, performing suction filtration, washing filter cake, and performing recrystallization with ethyl alcohol to obtain the vorinostat. The method is high in yield, quick in reaction and simple to operate.
Owner:QINGDAO MUNICIPAL HOSPITAL
C-Met/HDAC double-target inhibitor, synthesis method and application thereof
InactiveCN110128411AEnhanced inhibitory effectImprove utilization efficiencyOrganic active ingredientsOrganic chemistrySynthesis methodsPharmacophore
The invention discloses a c-Met / HDAC double-target inhibitor as well as a synthesis method and application thereof. A c-Met inhibitor crizotinib and an HDAC inhibitor vorinostat are used as lead structures; a single-structure double-target inhibitor is designed, the structure of the crizotinib is used as a cap-shaped structure of an HDAC inhibitor, so that an aniline acyl group in a vorinostat structure is replaced, and a hydroximic acid structure in the vorinostat is reserved as a chelating group of metal zinc ions; the single-structure double-target inhibitor is designed by introducing linking groups with different structures between the two inhibitors and utilizing the principle of pharmacophore splicing. The obtained double-target inhibitor has a good inhibition effect on both c-Met and HDAC, and can synergistically inhibit c-Met and HDAC. The preparation method disclosed by the invention is simple, mild in condition and high in yield.
Owner:北京凯恩梅格医药科技有限公司
A c-met/hdac dual-target inhibitor based on the structure of crizotinib and its synthesis method and application
ActiveCN110003181BEnhanced inhibitory effectImprove utilization efficiencyOrganic active ingredientsOrganic chemistryPharmacophoreAcyl group
The invention discloses a c-Met / histone deacetylase (HDAC) double-target inhibitor based on a crizotinib structure, and a synthesis method and application of the double-target inhibitor. According tothe inhibitor disclosed by the invention, c-Met inhibitor crizotinib and HDAC inhibitor vorinostat are taken as a lead structure to design a double-target inhibitor with a single structure; the crizotinib structure is used as a hat-shaped structure of the HDAC inhibitor to replace aniline acyl in a vorinostat structure; a hydroximic acid structure in the vorinostat is retained as a chelating groupof metal zinc ions; connecting groups with different structures are introduced between the the crizotinib structure and the hydroximic acid structure; and the double-target inhibitor with a single structure is designed through the principle of pharmacophores combination, so that the obtained double-target inhibitor has good inhibition effects on c-Met and HDAC, and can be used for synergisticallyinhibiting the c-Met and HDAC.
Owner:北京凯恩梅格医药科技有限公司
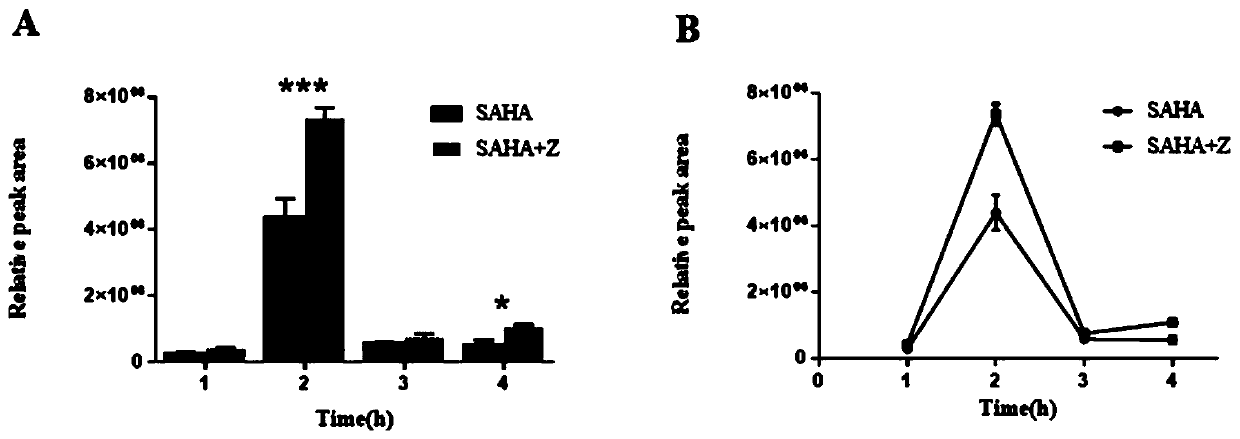
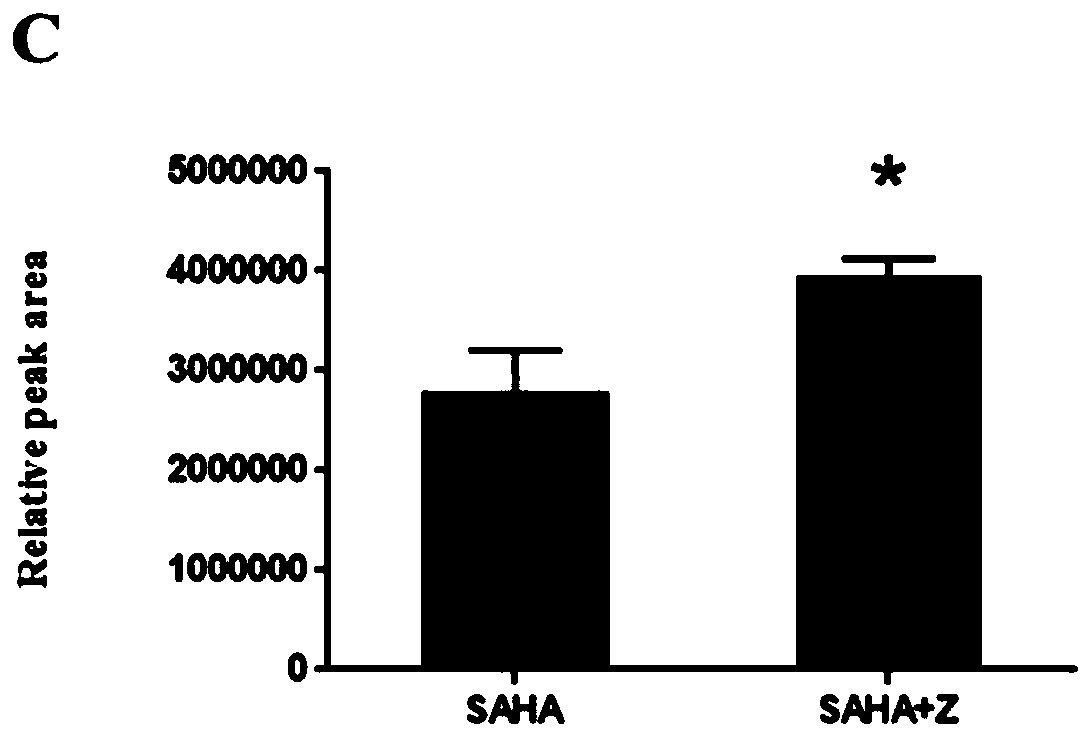
Application of Zuotai in preparation of medicine for promoting vorinostat to pass through blood brain barrier
PendingCN111202848APromote accumulationSpeed up entryOrganic active ingredientsNervous disorderBrain tissueVorinostat
The invention relates to application of Zuotai in preparation of a medicine for promoting vorinostat to pass through a blood brain barrier, belonging to the technical field of medicine preparation. According to the application of Zuotai in preparation of the medicine for promoting vorinostat to pass through the blood brain barrier, the combined administration of Zuotai and SAHA (namely vorinostat)can significantly promote the SAHA to pass through the blood brain barrier and to enter brain tissue, and can also significantly promote the accumulation of the medicine in the brain tissue.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST +1
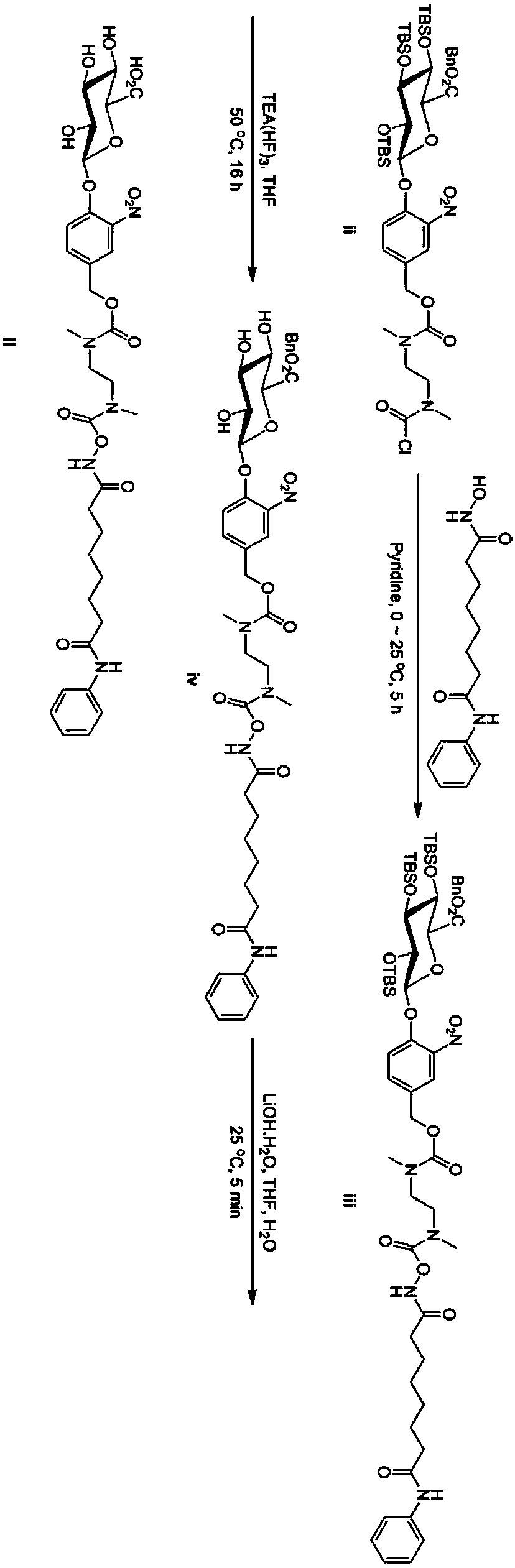
Vorinostat derivative based on lithium hydroxide, and preparation method and application thereof
ActiveCN107698631AGood water solubilityAchieve tumor suppressionOrganic active ingredientsSugar derivativesLithium hydroxideHydroxamic acid
The invention provides a vorinostat derivative based on lithium hydroxide, and a preparation method and application thereof. The vorinostat derivative based on lithium hydroxide uses vorinostat as a parent drug, and a water-soluble group is connected to the hydroxamic acid position of the mother nucleus of vorinostat to realize modification; thus, the water-solubility of the vorinostat parent drugis effectively improved, and the occurrence of adverse reactions can be effectively prevented. Meanwhile, the vorinostat derivative of the invention has good tumor inhibition effect and can be further used for preparing tumor-treating drugs. Furthermore, the invention also provides the preparation method for the lithium hydroxide-based vorinostat derivative. The preparation method has the advantages of few preparation procedures, simple operation, etc.
Owner:ZHEJIANG MEDICAL COLLEGE
Vorinostat skeleton-based anthranilamide compound as well as preparation and application thereof
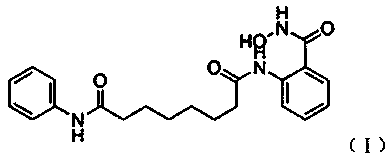
ActiveCN113200885APrevent proliferationExhibits anti-gastric cancer activityOrganic compound preparationDigestive systemCancer drugsPharmaceutical drug
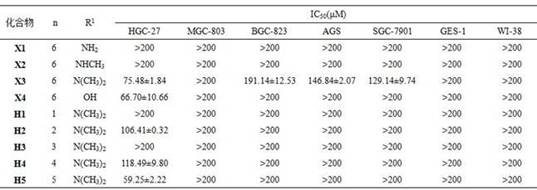
The invention provides a vorinostat skeleton-based anthranilamide compound as well as preparation and application of the anthranilamide compound. The structural formula of the o-aminobenzamide compound based on a vorinostat skeleton is shown in the specification, wherein n is equal to 1-6, and R is methylamino, dimethylamino, hydroxyl, NH2 or the like. The anthranilamide compound based on the vorinostat skeleton has the effect of inhibiting gastric cancer cell proliferation through MTT method determination, and can be used for preparing anti-gastric cancer drugs.
Owner:NANHUA UNIV
Application of Vorinostat in preparation of soft-resistant Eimeria drugs
ActiveCN102641261BGood against Eimeria tenellaOrganic active ingredientsAntiparasitic agentsBiotechnologyAnimal science
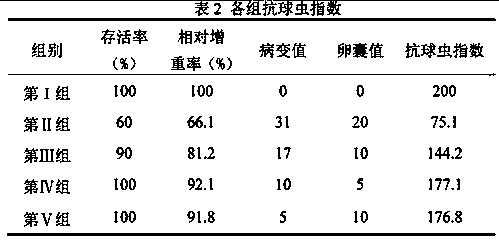
The invention discloses application of Vorinostat in preparation of soft-resistant Eimeria drugs; powder is formed by the Vorinostat and an acceptable auxiliary material, and then is added into a feed to mix uniformly so as to feed chicken by a daily feed amount; or a Vorinostat water-soluble powder is prepared by the Vorinostat, and is dissolved in water to feed chicken by a daily feed amount. Animal infection experiments confirm an anticoccidia index (ACI) of the Vorinostat is more than 175, and the Vorinostat has function of resisting soft-resistant Eimeria.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com