Application of combination of bortezomib and panobinostat or vorinostat in preparing drugs for treating drug-resistant MLL
A technology of bortezomib and panobinostat, which is applied in the field of biomedicine to solve the problem of drug resistance, effectively treat and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1 Materials and methods
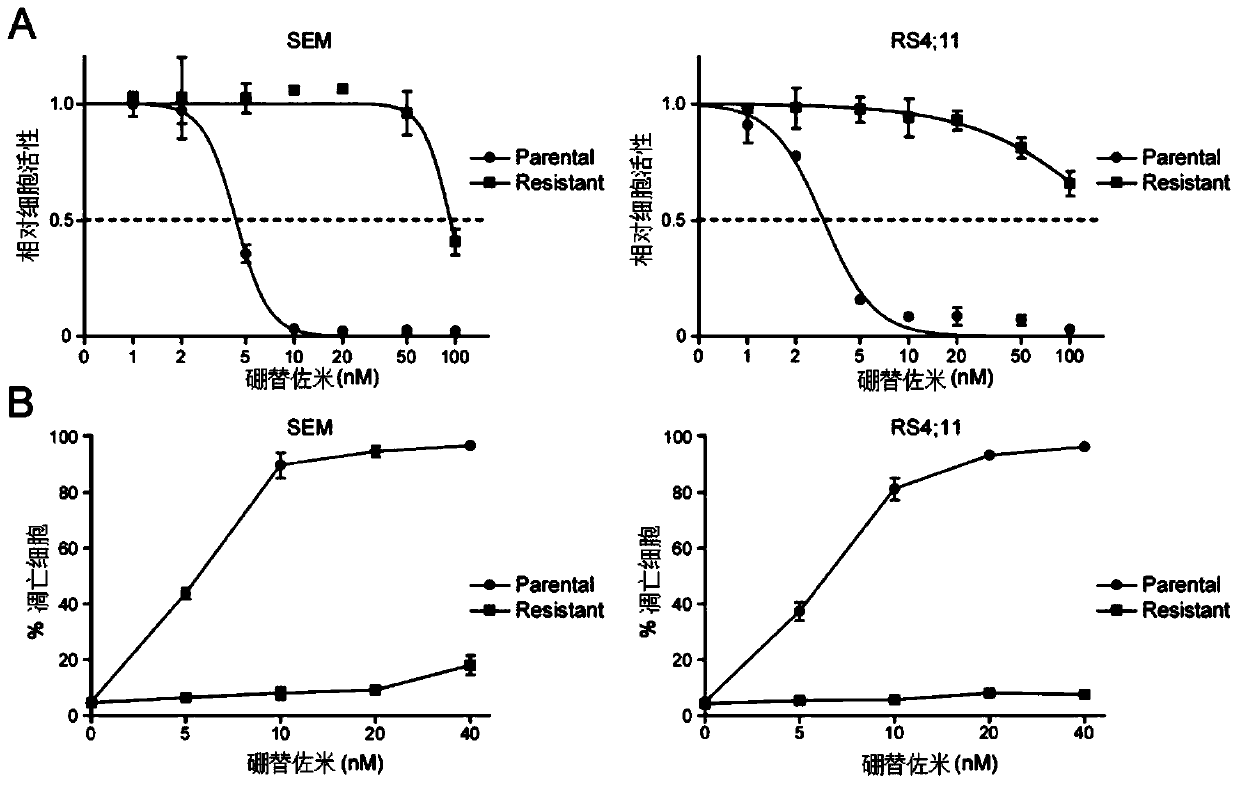
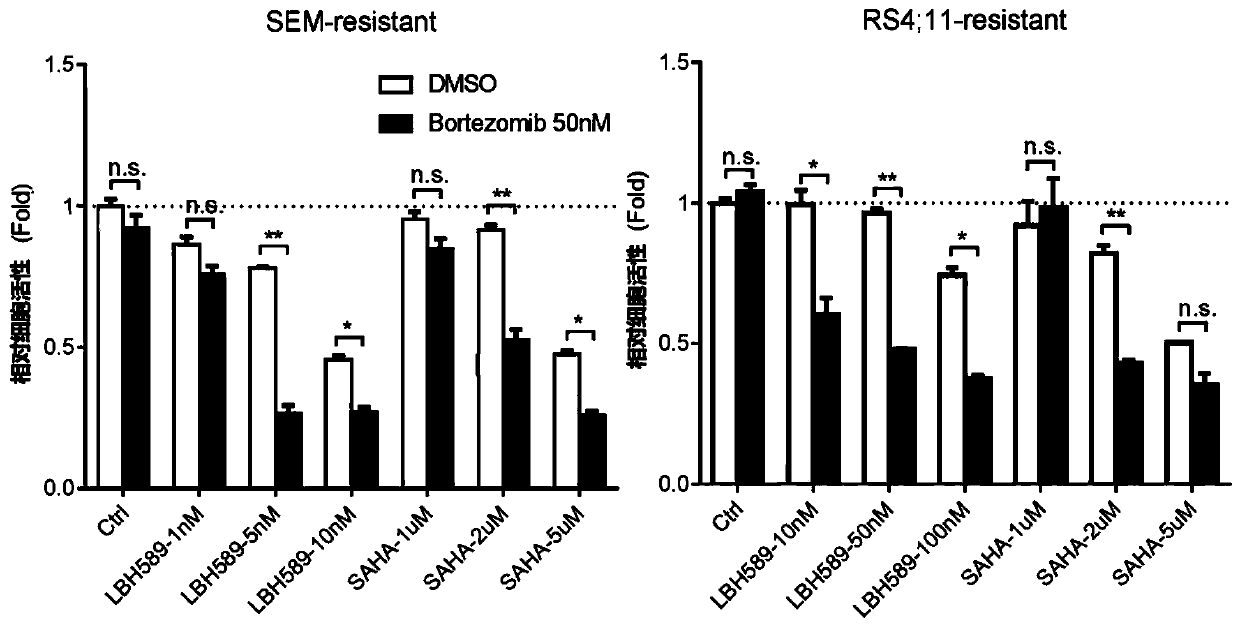
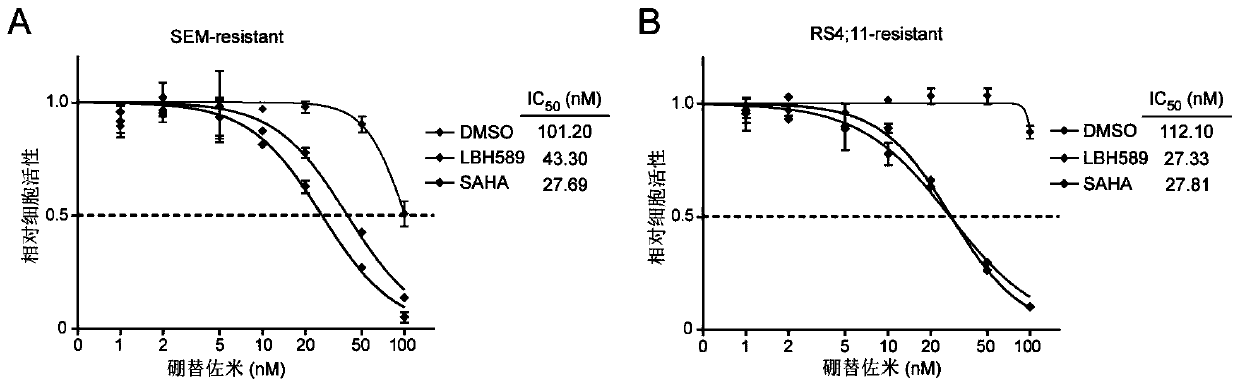
[0035] The following cell lines were used in this study: B-cell leukemia cell line RS4 with MLL ectopic rearrangement; 11 and SEM were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ). All suspension cell lines were cultured with RPMI 1640 medium containing 10% fetal bovine serum, placed at 37°C, 5% CO 2 , 95% air humidity incubator culture. The cells were replaced every other day, and the cell concentration was adjusted to 5×10 5 / ml and 2×10 6 Between pieces / ml. Proteasome inhibitor bortezomib resistant cells were obtained from parental cells treated with increasing concentrations of bortezomib for at least four weeks. The specific drug concentration is the half maximal inhibitory concentration (IC) of the drug corresponding to the parental cells. 50 ), that is, adding 5 nM bortezomib, changing the medium every three days and adding new drugs to continue the treatment for four weeks. During drug treatment, mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com