Novel process for the preparation of vorinostat
A vorinostat, stand-alone technology for the preparation of active pharmaceutical ingredients vorinostat that addresses issues that limit the feasibility of commercial production scale, reduce overall yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
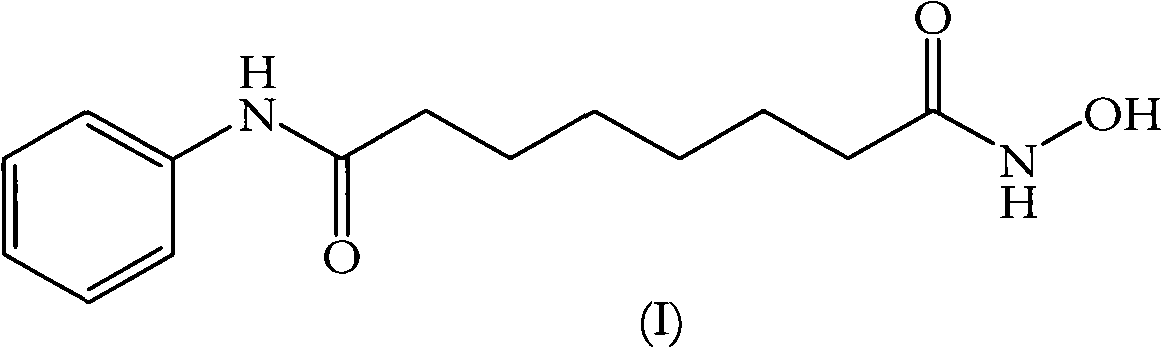
[0079] Vorinostat
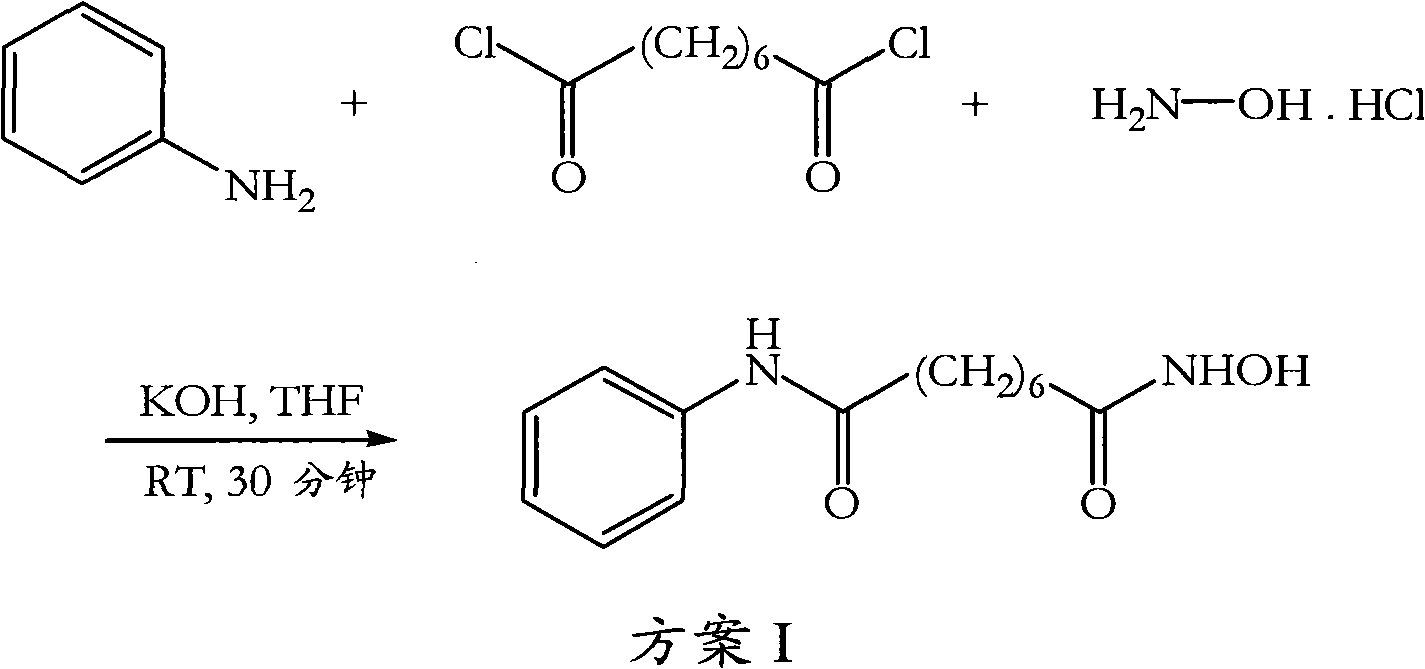
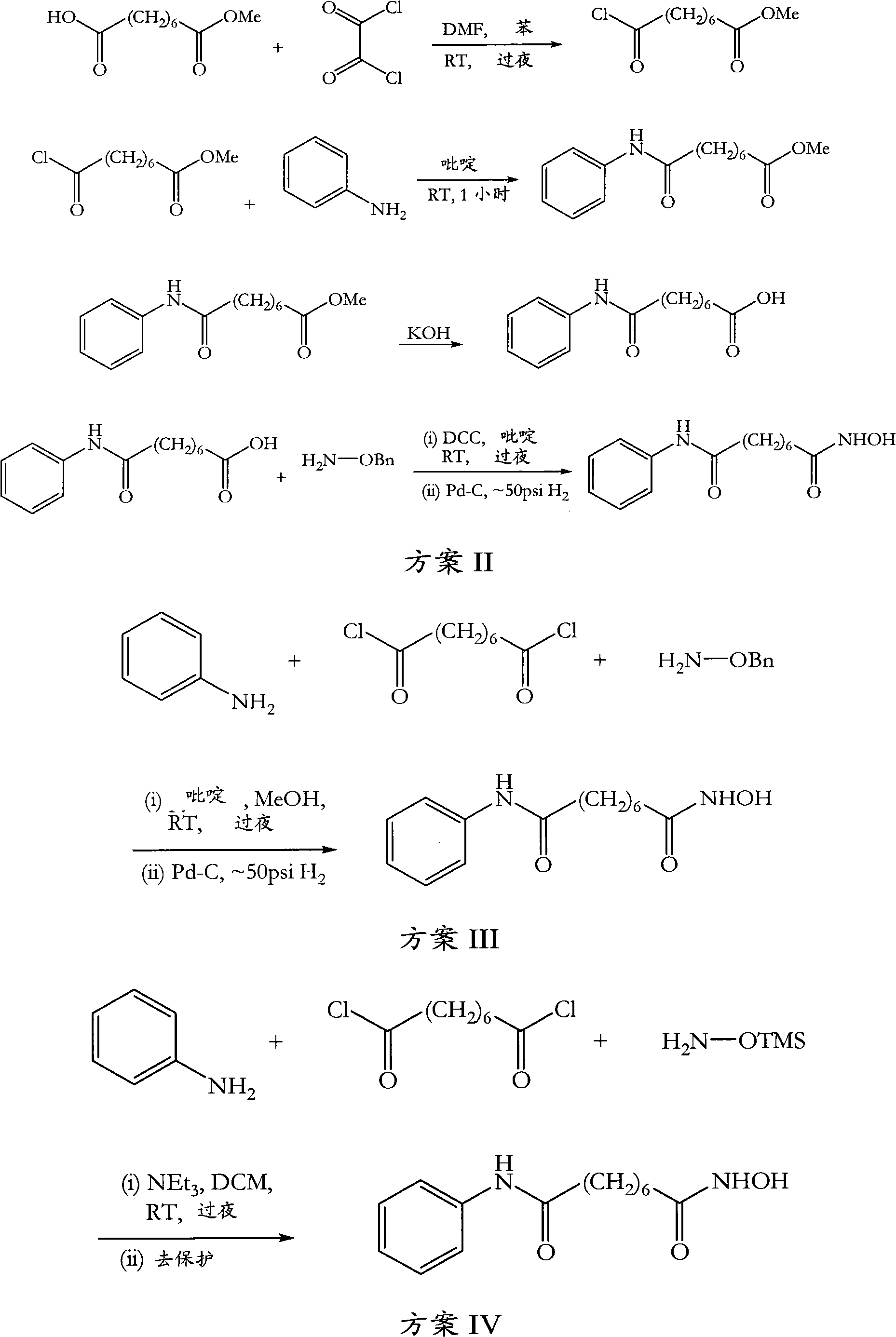
[0080]Suberic acid (1.0 eq) was dissolved in tetrahydrofuran (15 vol), and the clear solution was cooled to 0-5°C. Methyl chloroformate (1.1 eq) and triethylamine (1.1 eq) were added to the solution at the same temperature and the mixture was stirred for 15 minutes. The formed triethylamine hydrochloride was filtered off, then aniline (1 eq) was added to the reaction mixture at 0-5°C and stirring was continued for 15 minutes. Methyl chloroformate (1.1 eq) and triethylamine (1.1 eq) were added to the clear solution and stirring was continued for a further 15 minutes at 0-5°C. The cooled reaction mixture was added to fresh methanolic solution of hydroxylamine (*see below) cooled to 0-5°C and stirred at 0-5°C for 15 minutes. The solvent was removed under vacuum at 40°C, the obtained residue was taken up in dichloromethane and the organic solution was washed with water and dried over anhydrous sodium sulfate. The dichloromethane was removed under vacuum at 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com