C-Met/HDAC double-target inhibitor, synthesis method and application thereof
A synthesis method and technology of c-met are applied in the field of medicine and achieve the effects of mild conditions, improved utilization efficiency and therapeutic effect, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
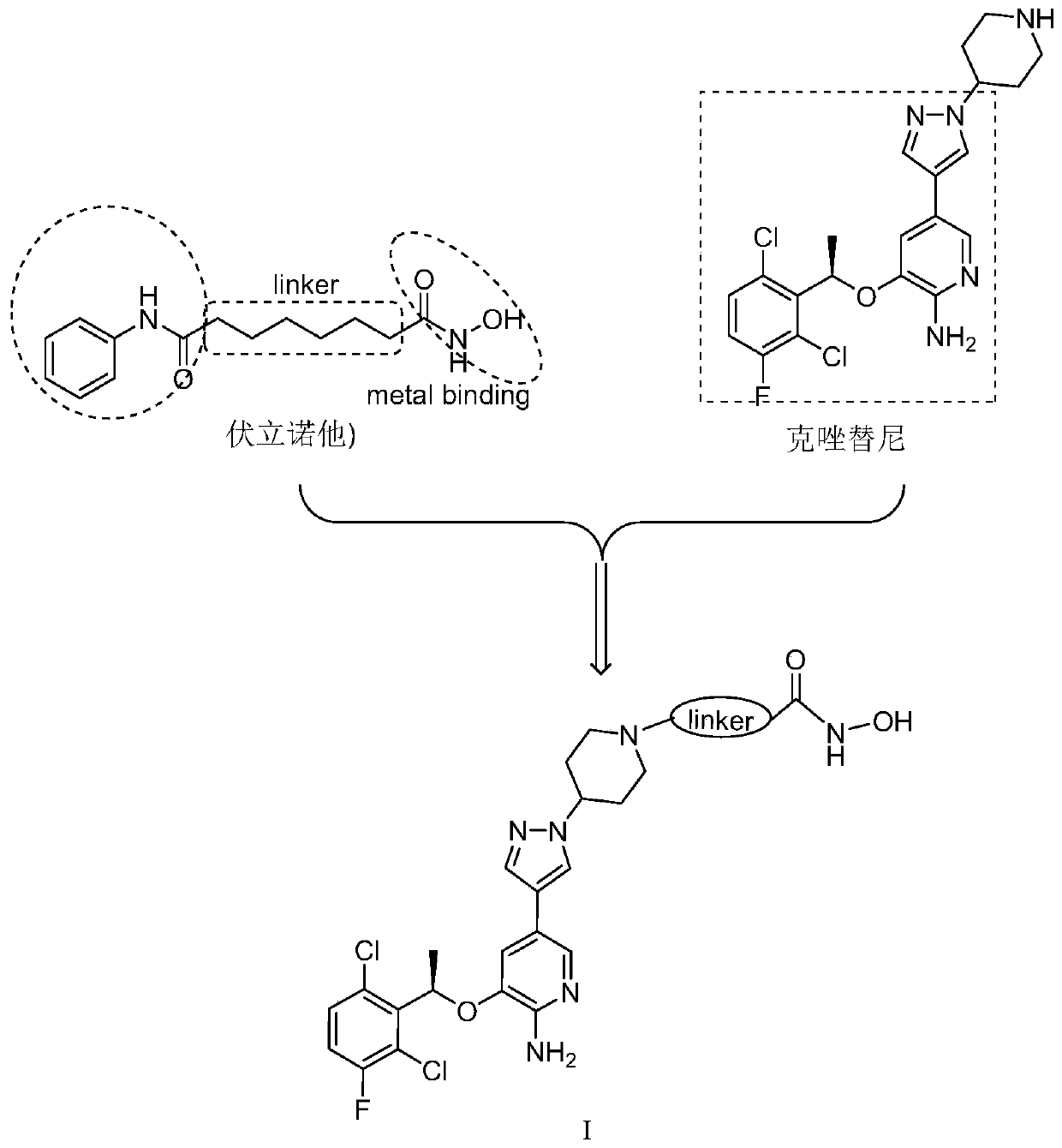
[0040] The structural formula of the c-Met / HDAC dual-target inhibitor in this example is as follows:
[0041]
[0042] Among them, the linker is n=4.
[0043] The synthesis method of the dual-target inhibitor of this embodiment is as follows:
[0044] (1) at first synthetic intermediate (a) reaction equation is as follows:
[0045]
[0046] At room temperature, crizotinib (900.6mg, 2mmol), methyl 5-bromovalerate (468.0mg, 2.4mmol) and potassium carbonate (830.0mg, 6mmol) were added to 30ml of DMF, stirred at room temperature for 24h , TLC (thin layer chromatography) detection (volume ratio of dichloromethane and methanol 10:1) raw material reaction is complete. Add 30ml of water to the reaction solution, stir until clear liquid, then extract with ethyl acetate (100ml) in 2 equal portions, wash the extract with saturated brine (100ml) in 2 equal portions, dry over anhydrous sodium sulfate, filter, The filtrate was concentrated under reduced pressure and purified by c...
Embodiment 2
[0059] In the structural formula of the c-Met / HDAC dual-target inhibitor in this example, the linker is n=5, synthesized according to the method of Example 1, replacing methyl 5-bromopentanoate with methyl 6-bromohexanoate, and routinely adjusted the test parameters to obtain the target product (I-2). The 1HNMR data is as follows:
[0060] 1H NMR (400MHz, DMSO-d6): ppm: 10.44(s, 1H, N-OH), 7.96(s, 1H), 7.75(d, J=1.68Hz, 1H), 7.56-7.59(dd, J= 4.76Hz, 5.04Hz, 1H), 7.52(s, 1H), 7.44(t, J=8.68Hz, 8.68Hz, 1H), 6.89(d, J=1.4Hz, 1H), 6.06-6.11(q, J =6.48Hz,6.72Hz,6.48Hz,1H),5.67(s,2H),4.06-4.13(m,1H),2.95(d,J=10.96Hz,2H),2.28-2.31(t,J=6.72 Hz, 7.56Hz, 2H), 1.89-2.08(m, 8H), 1.80(d, J=6.72Hz, 3H), 1.22-1.54(m, 6H).
Embodiment 3
[0062] In the structural formula of the c-Met / HDAC dual-target inhibitor in this example, the linker is n=6, synthesized according to the method of Example 1, replacing methyl 5-bromopentanoate with methyl 7-bromoheptanoate, and routinely adjusting the test parameters to obtain the target product (I-3), the 1HNMR data is as follows:
[0063] 1HNMR(400MHz,DMSO-d6):ppm:10.37(s,1H,),8.70(s,1H),7.96(s,1H),7.75(d,J=1.68Hz,1H),7.57(dd,J =4.76Hz, 5.04Hz, 1H), 7.52(s, 1H), 7.44(t, J=8.68Hz, 1H), 6.90(d, J=1.4Hz, 1H), 6.06-6.11(q, J=6.44 Hz,6.72Hz,6.44Hz,1H),5.66(s,2H),4.05-4.11(m,1H),2.94(d,J=11.48Hz,2H),2.29(t,J=7Hz,2H), 1.89-2.06 (m, 8H), 1.80 (d, J=6.72Hz, 3H), 1.23-1.50 (m, 8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com