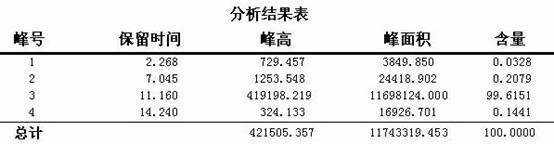
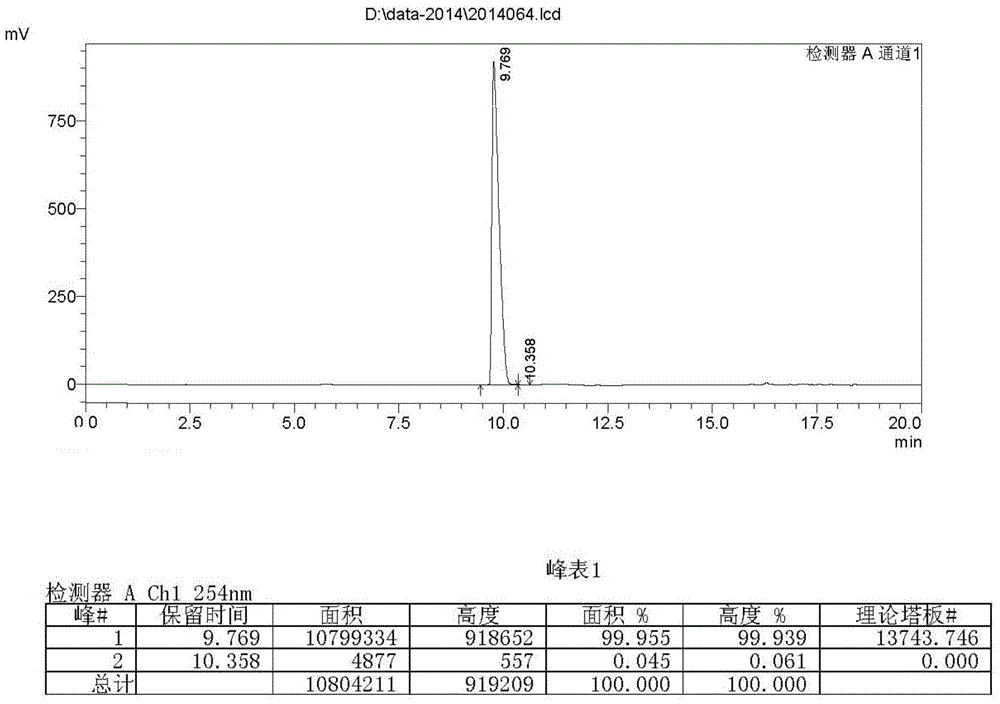
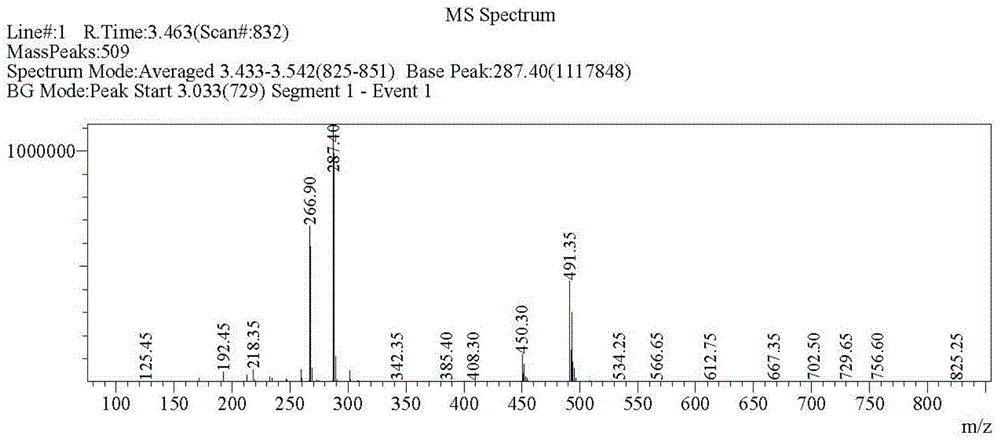
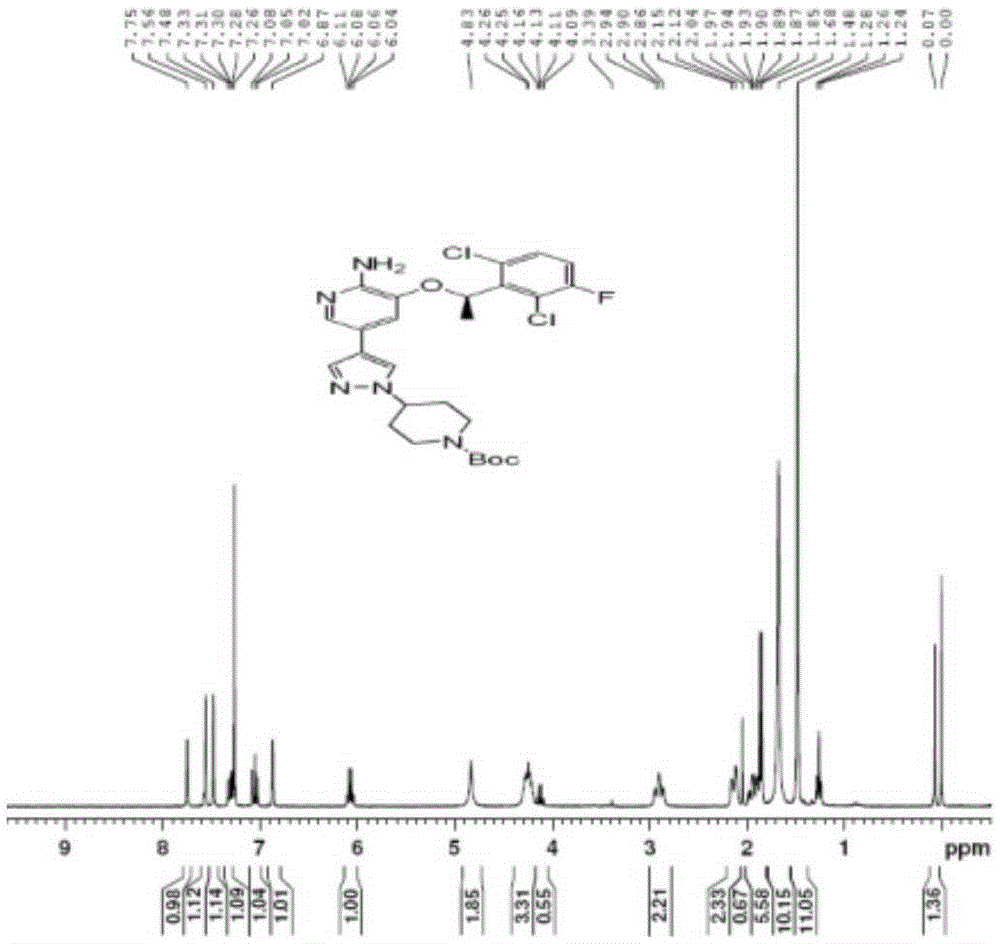
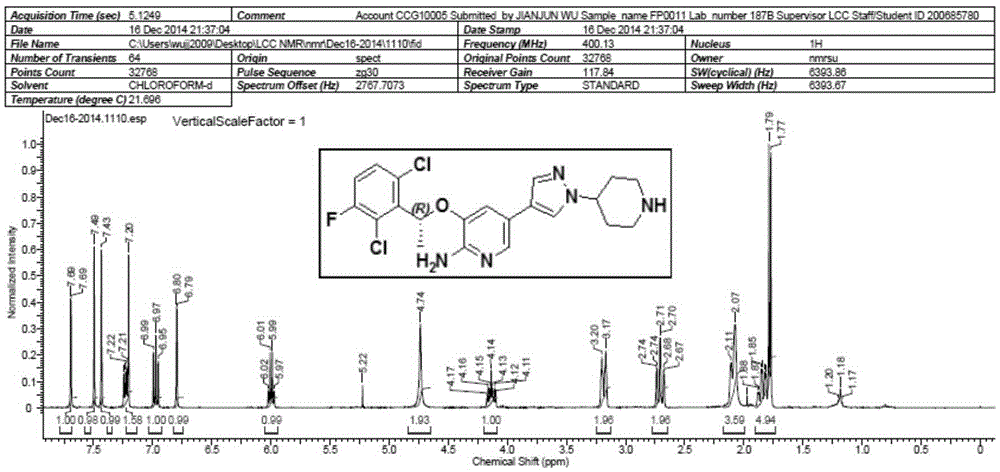
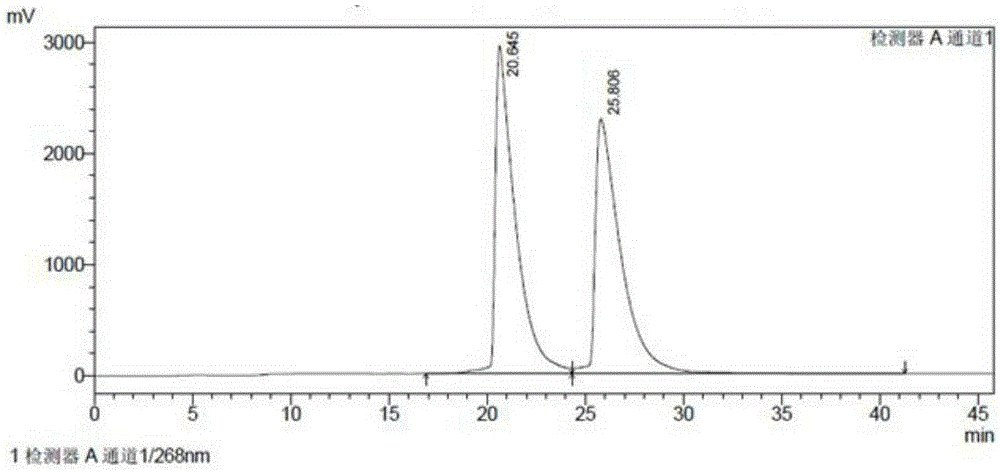
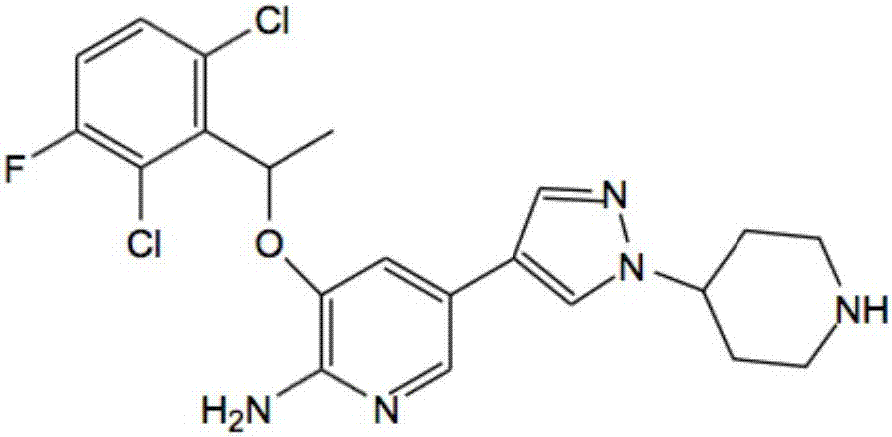
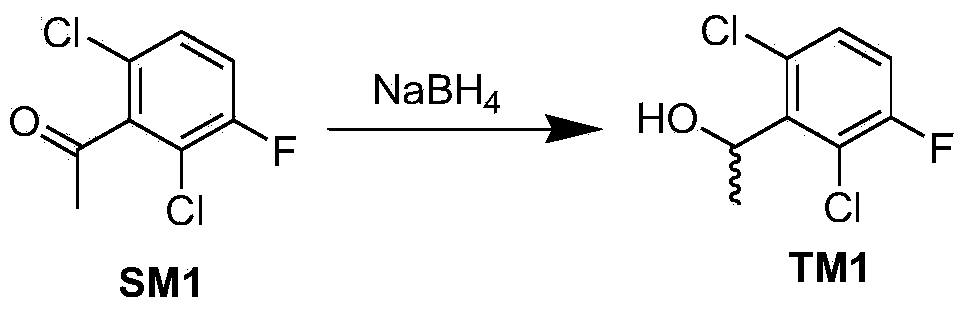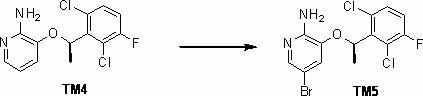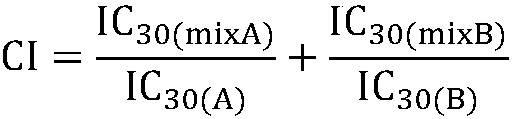Patents
Literature
92 results about "Crizotinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
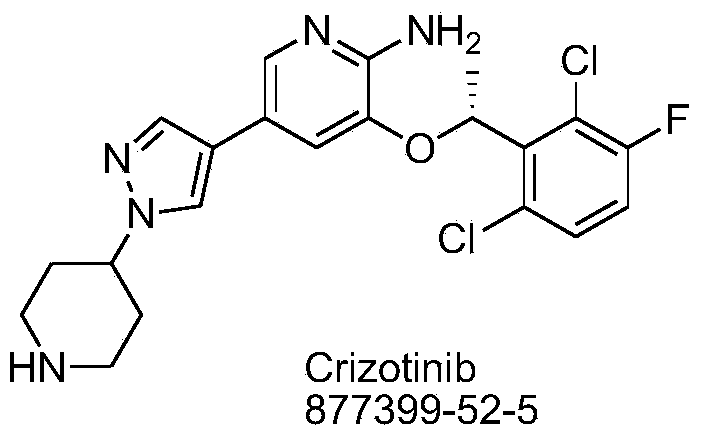
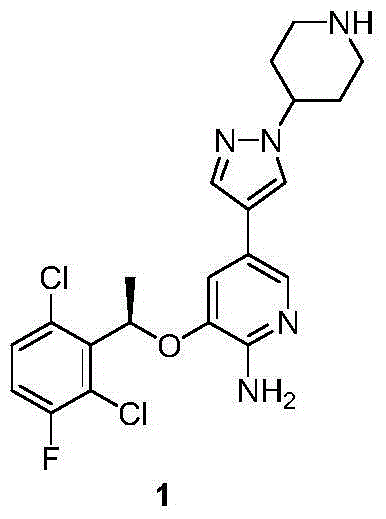
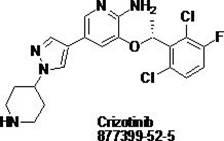
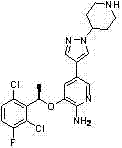
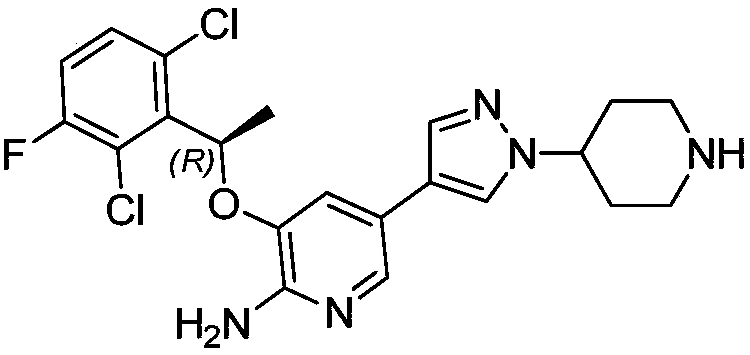
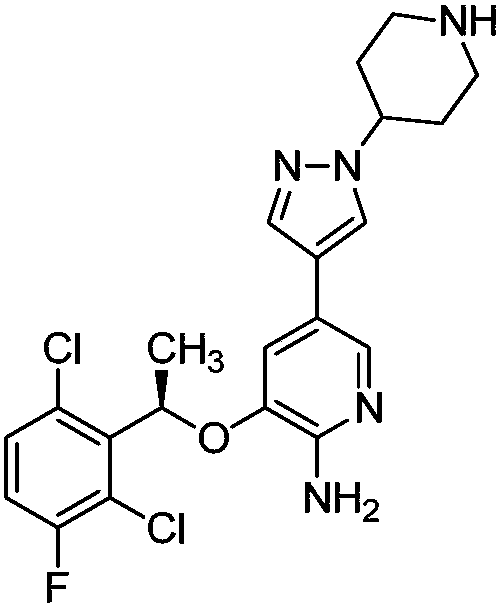
Crizotinib is used to treat certain types of lung cancer.
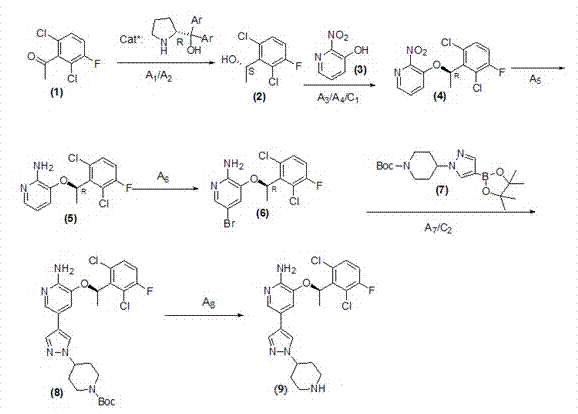
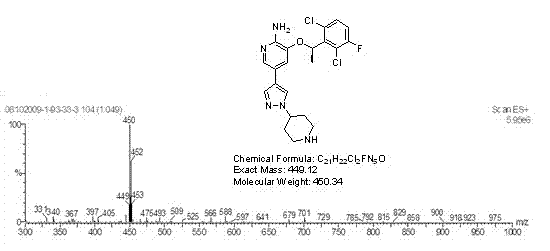
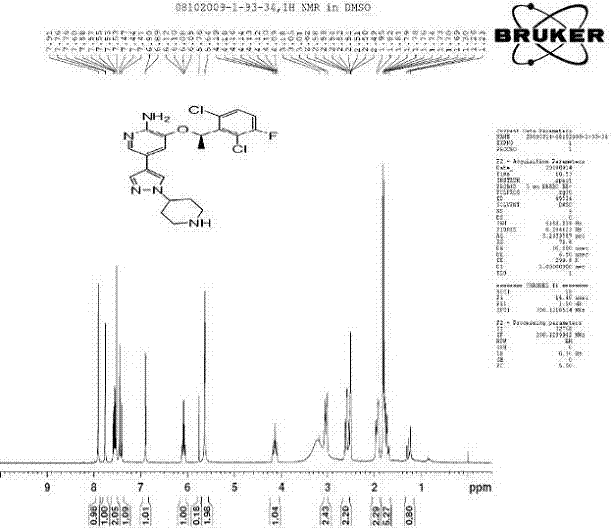
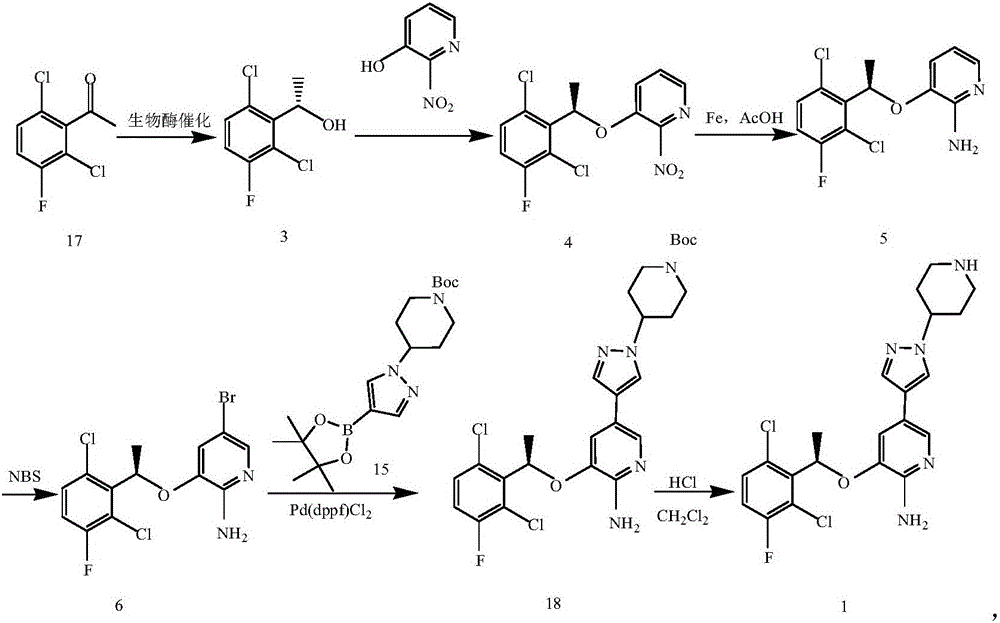
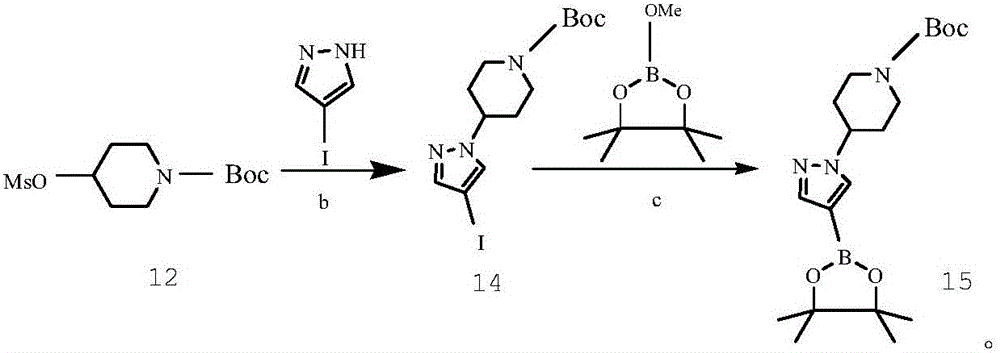
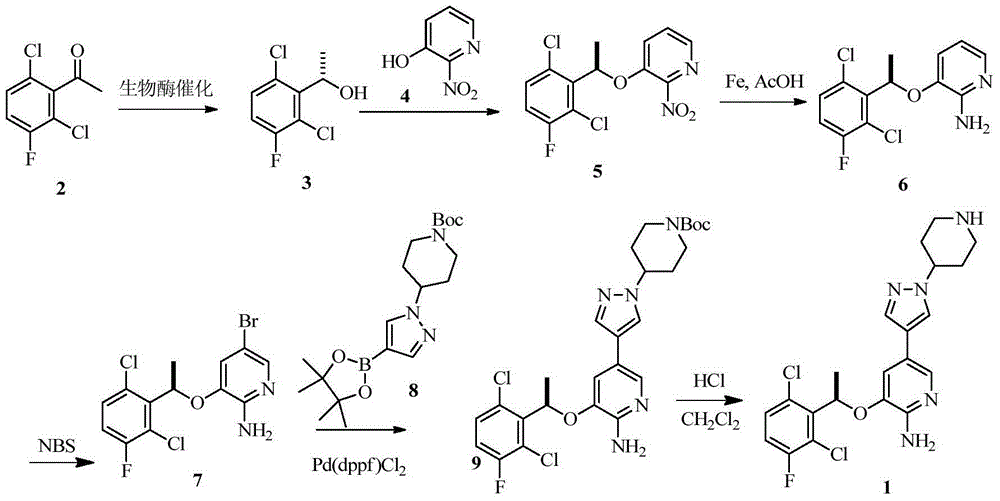
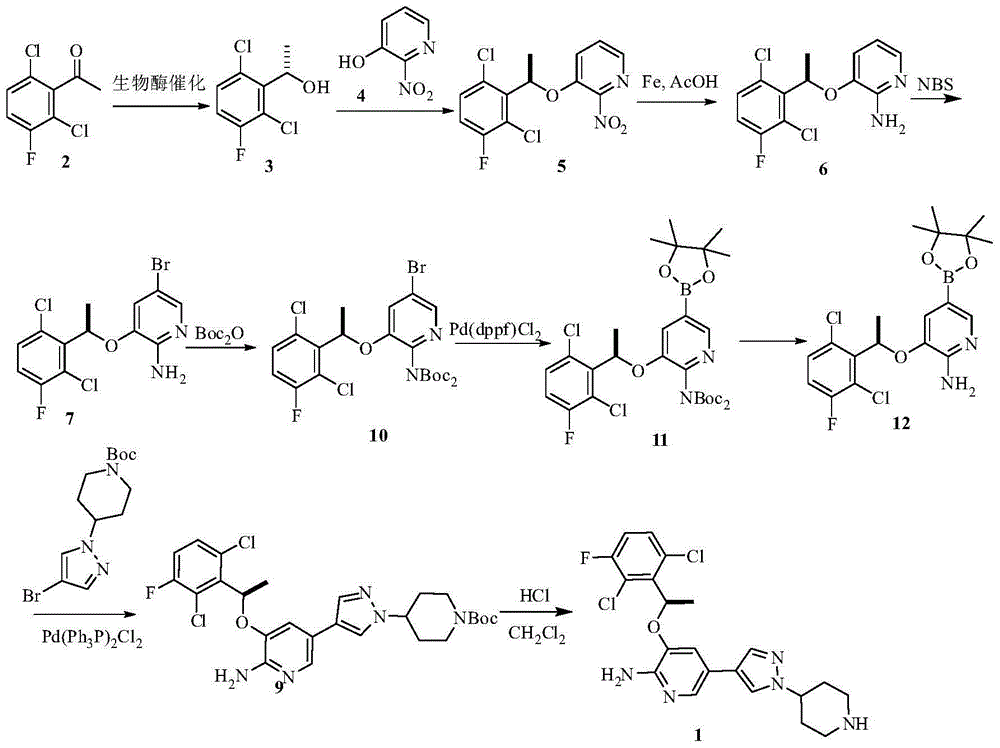
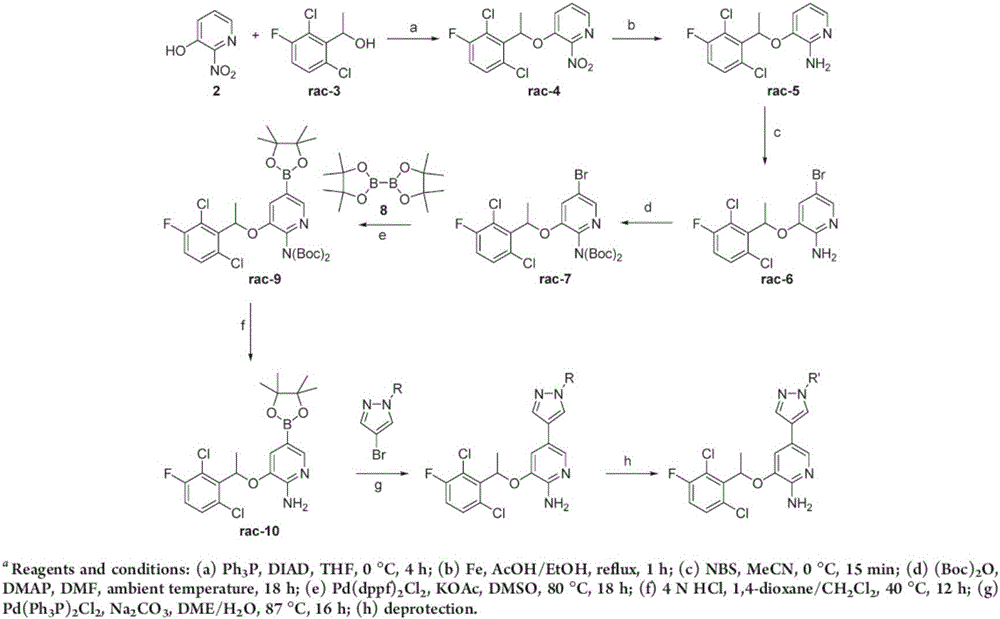
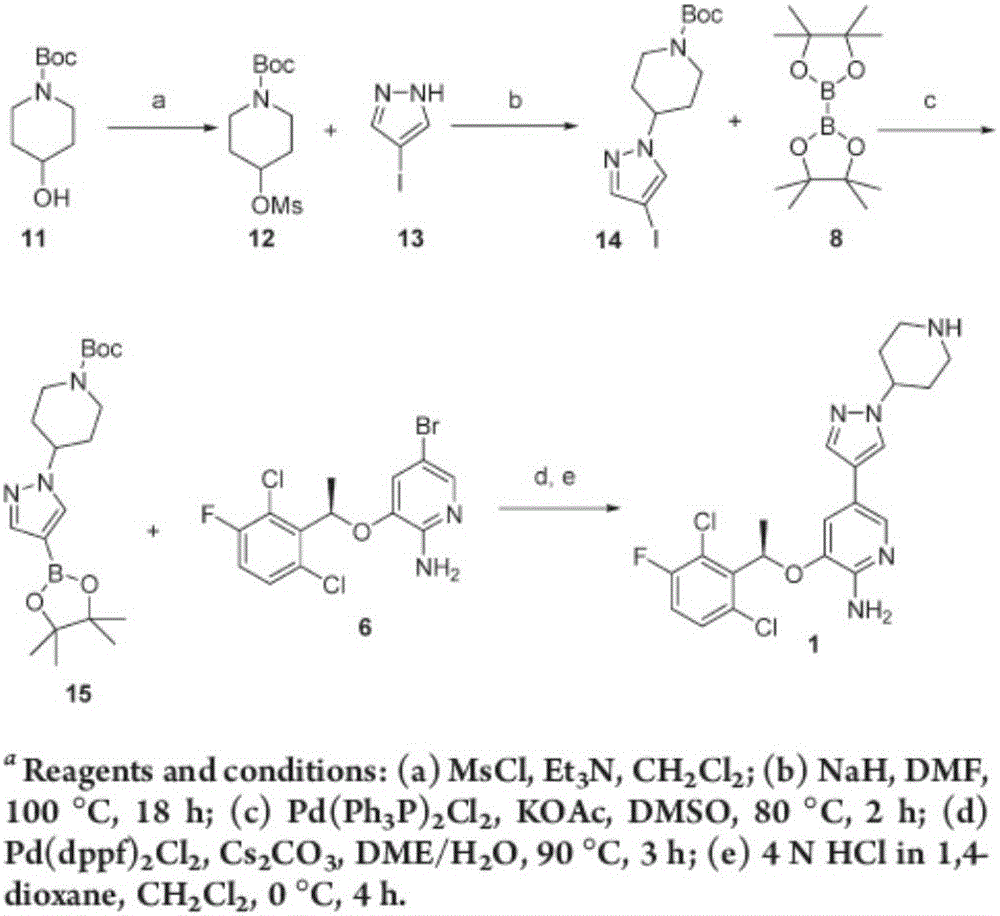
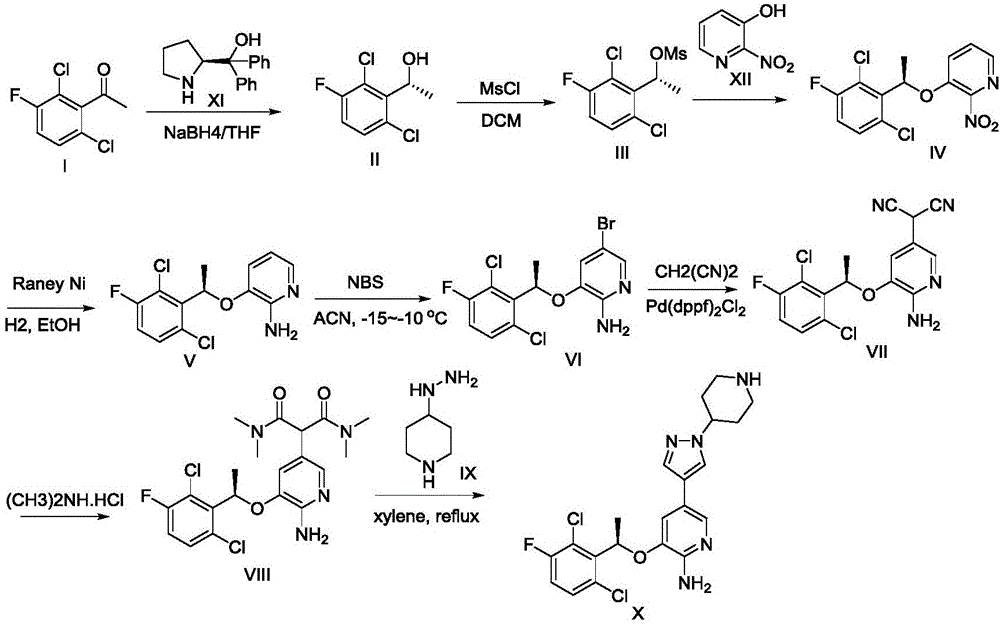
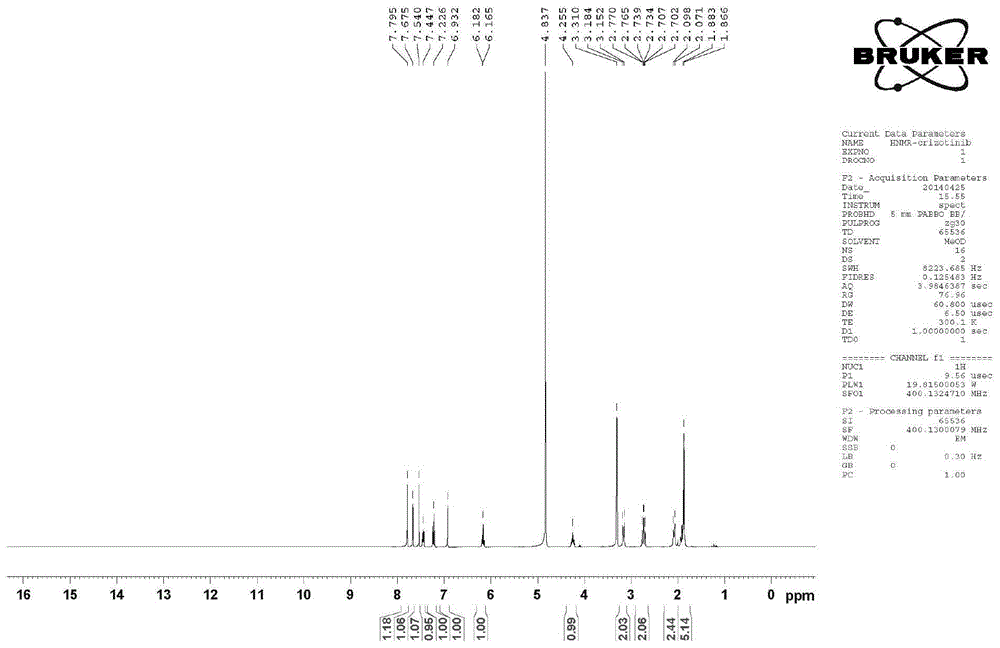
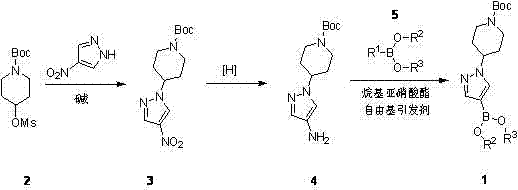
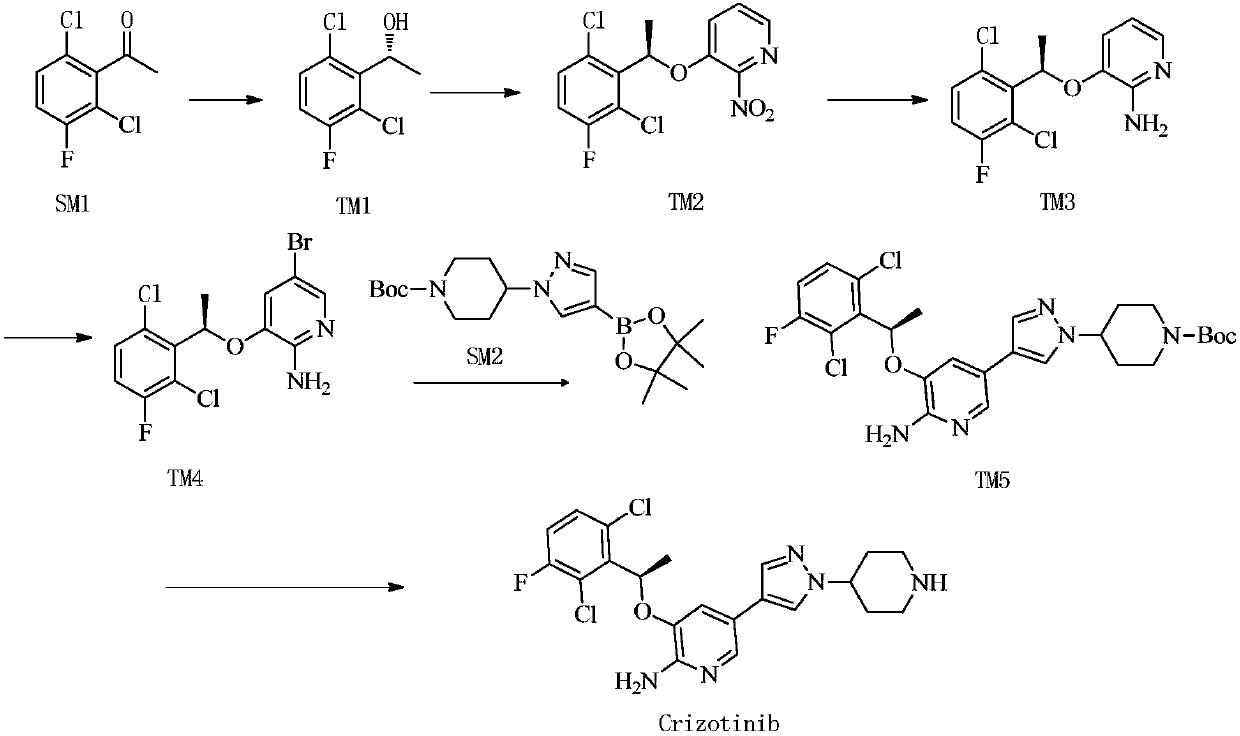
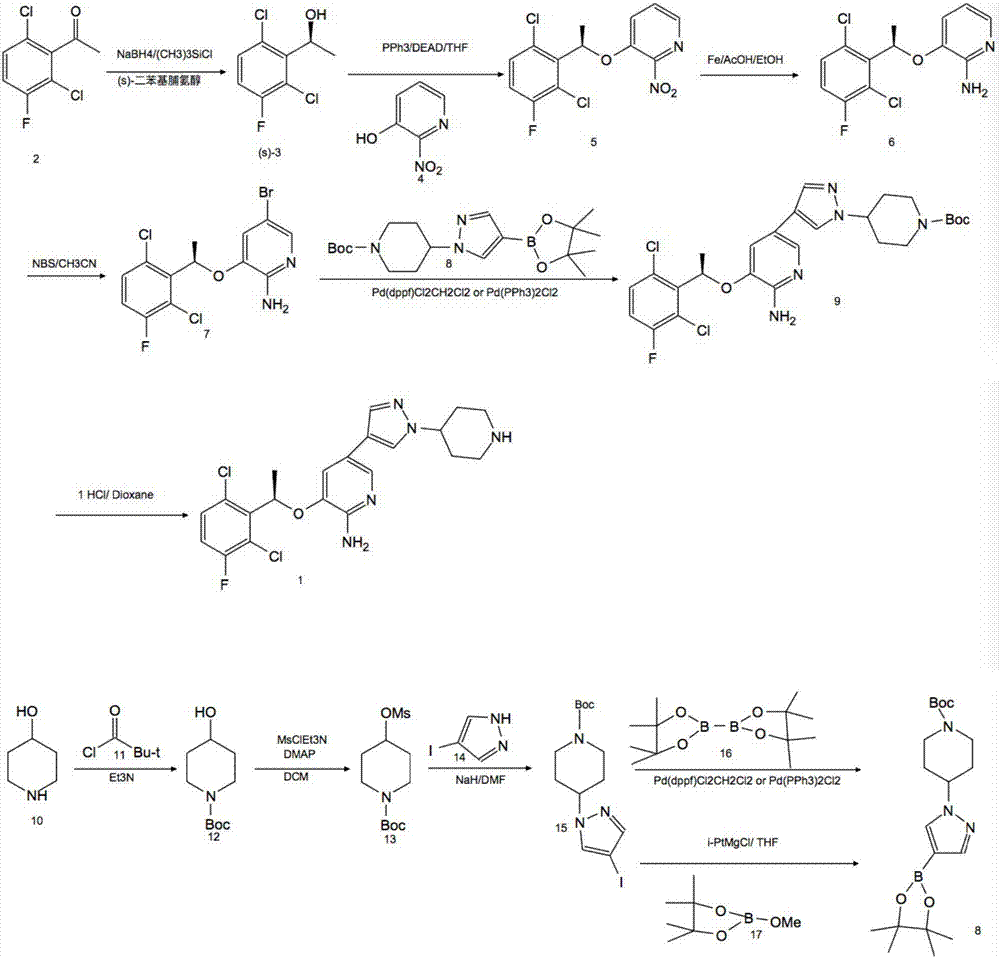
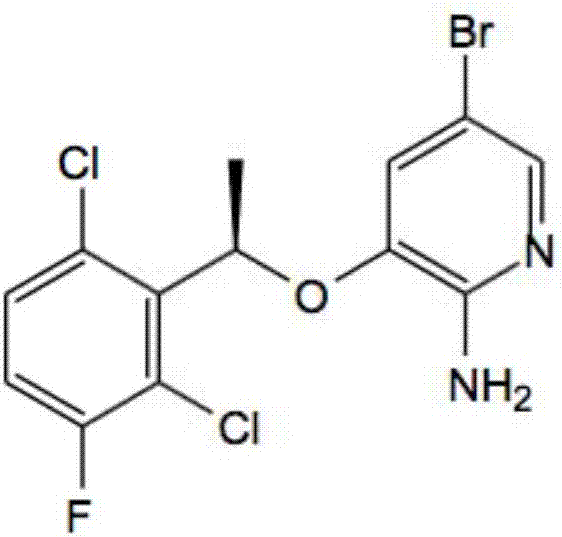
Preparing method of crizotinib
ActiveCN102584795AShort reaction cycleWide variety of sourcesAsymmetric synthesesNitro compoundBenzene
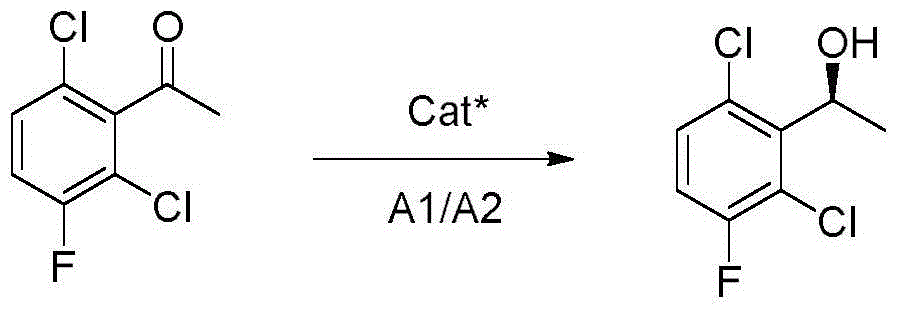
The invention provides a preparing method of crizotinib. The preparing comprises the following steps of: 1) preparing (S)-construction phenethyl alcohol by taking acetophenone the structural formula of which is shown in a formula (1) as a raw material; 2) preparing a nitrocompound; 3) preparing an aromatic amine compound; 4) preparing a bromo-compound; 5) preparing an N-Boc compound; and 6) preparing the crizotinib. Compared with the prior art, the preparing method of the crizotinib has the advantages that 1, the conventional biological enzymatic method and chemical resolution method are replaced by an organic micromolecule catalysis method, thus the whole line is short in reaction cycle, high in yield, simple in operation, wide in raw material source and low in price; and 2) the preparing method of the crizotinib is high in total yield, the obtained product has high optical purity, the required reaction condition and reaction process are easy to control, and a new choice is provided for preparation and production of the medicament crizotinib.
Owner:扬州市三药制药有限公司
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Method of treatment of philadelphia chromosome positive leukaemia
InactiveUS20120244116A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
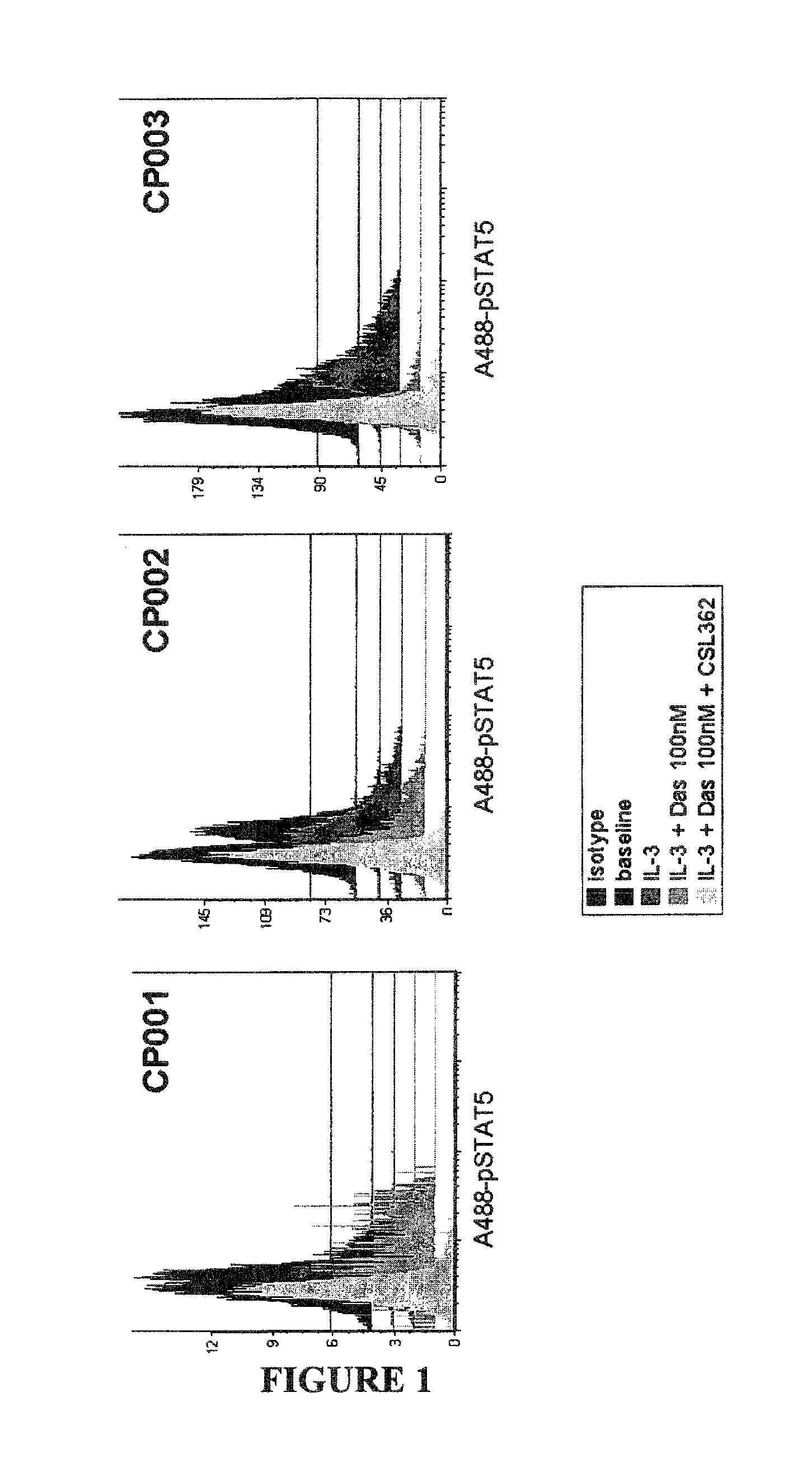
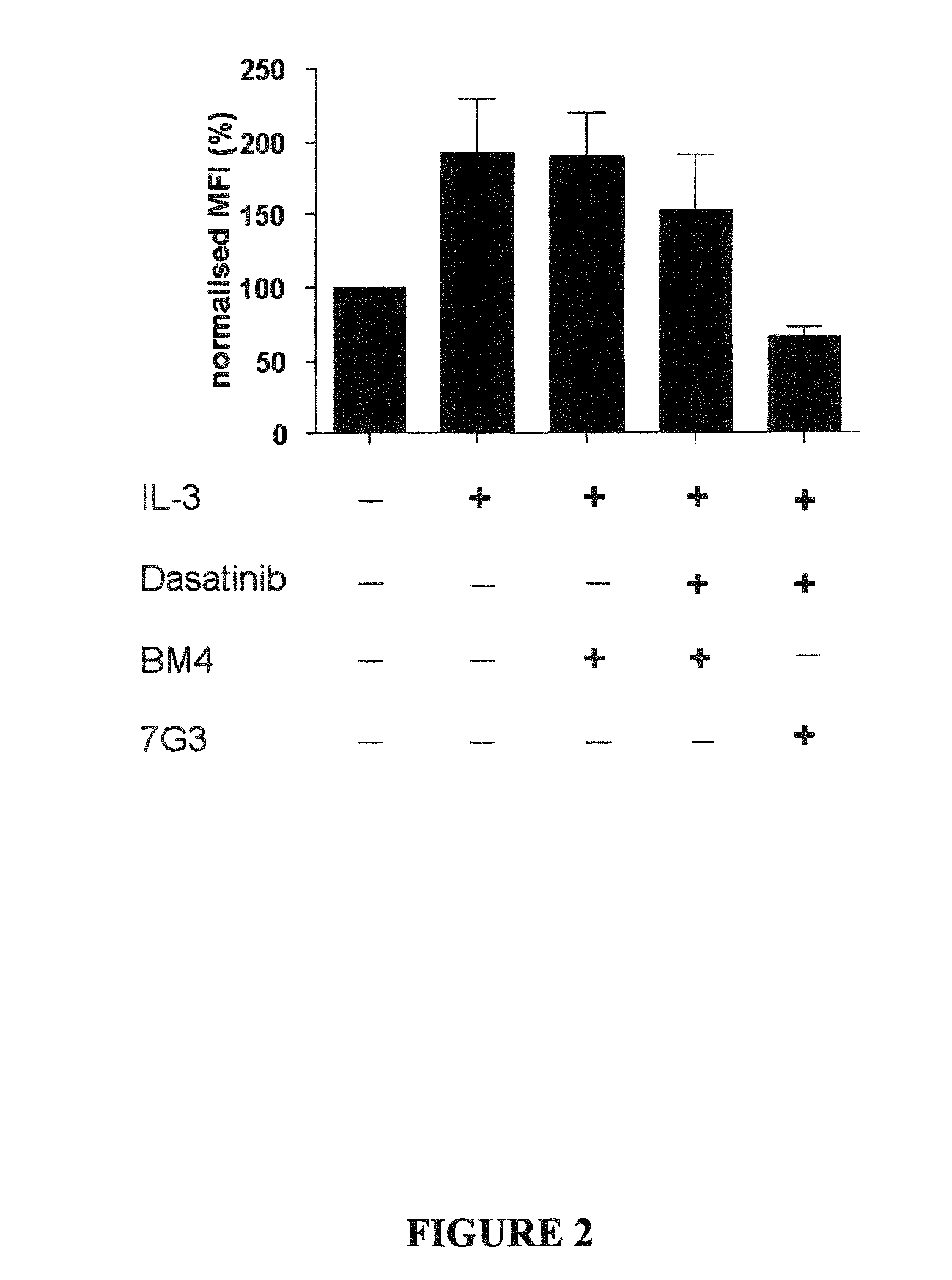
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Methods for inhibiting cell proliferation in ALK-driven cancers
The invention features a method for treating patients who have an ALK-driven cancer, which is, or has become, refractory to one or more of crizotinib, CH5424802 and ASP3026, or which bears an ALK mutation identified herein, by administering a compound of formula (I) to the patient. The invention also features methods, kits, and compositions for characterizing ALK-driven cancers to determine whether they express an ALK mutant.
Owner:TAKEDA PHARMA CO LTD
Synthetic process method for novel antineoplastic molecular targeted drug of crizotinib
ActiveCN103664896AReduce processing stepsEmission reductionPreparation by isomerisationOrganic compound preparationP-Toluenesulfonic acidHydrolysis
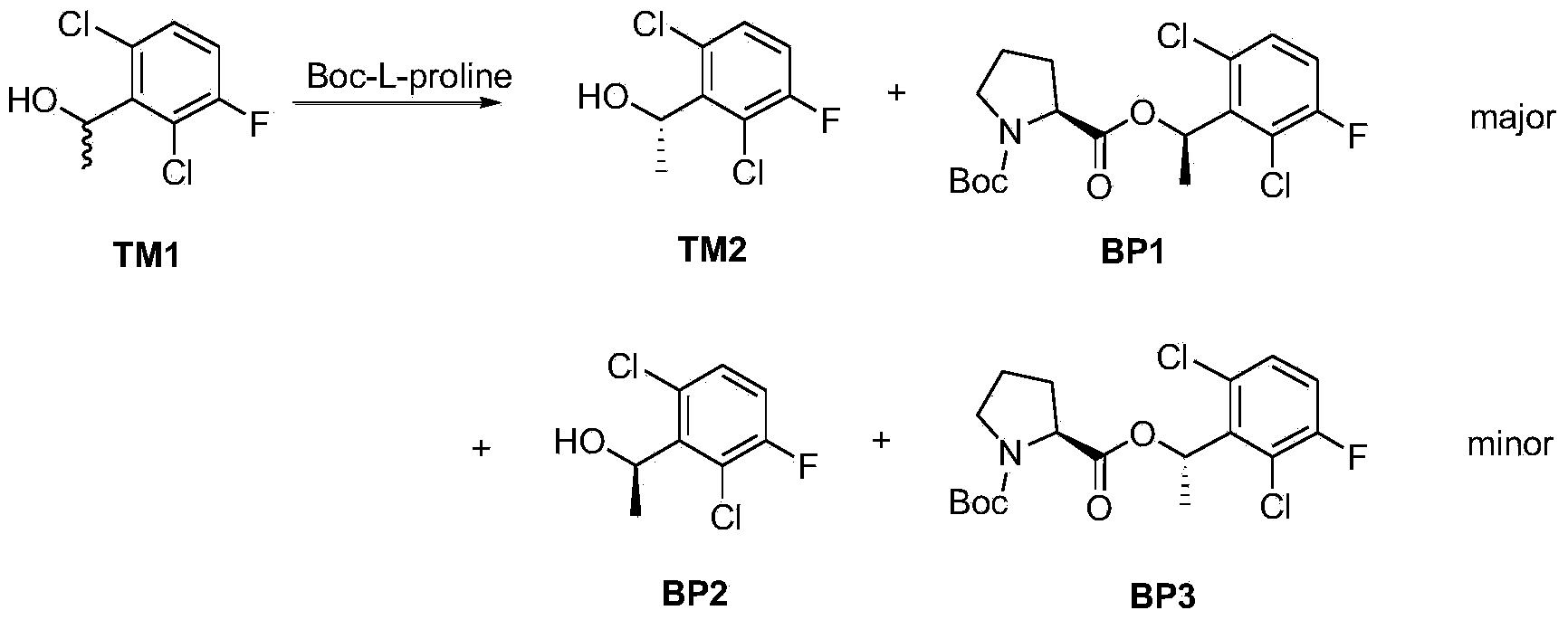
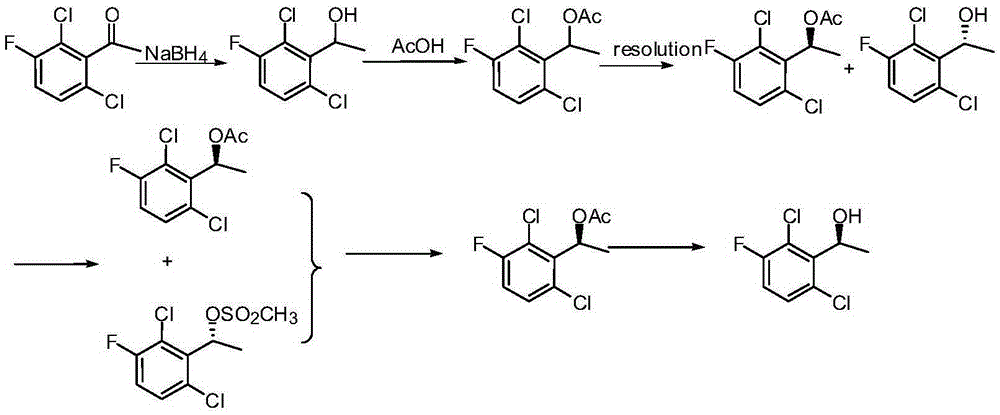
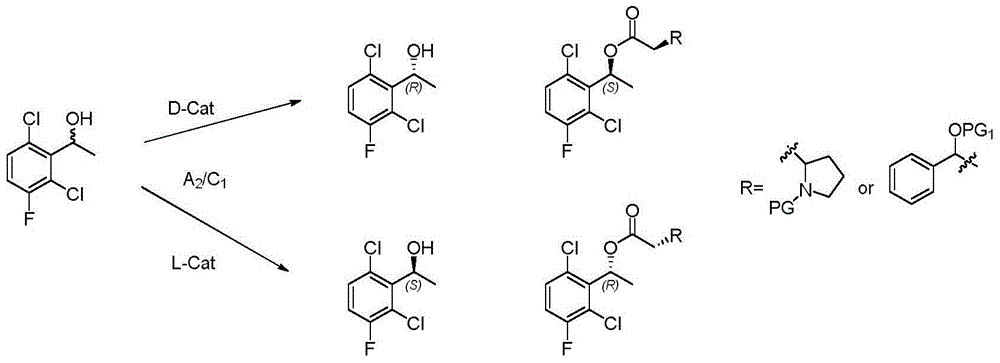
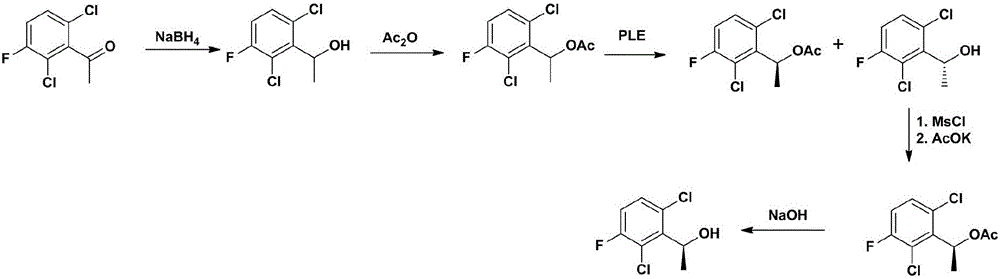
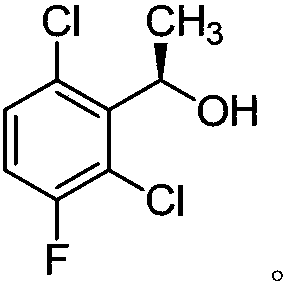
The invention provides a synthetic process method for a novel antineoplastic molecular targeted drug of crizotinib, and relates to the resolution process optimization of chiral isomers of a crizotinib precursor and the recycling of by-products. The method adopts a catalyzing resolution method that Boc-L-proline, namely, N-(tert-butoxycarbonyl)-L-proline) is combined with a catalyst of p-toluenesulfonic acid and a condensating agent of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to split 1-(2,6-dichloro-3-fluorophenyl)ethanol racemate into S-type alcohol and R-type alcohol, and a resolution by-product mixture is subjected to hydrolysis and configuration transition to obtain the (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol; the total yield is improved to 76% from 30%, the time is shortened, pollution is reduced, and application to industrialized production is easy.
Owner:济南良福精合医药科技有限公司
Crizotinib prodrug, as well as preparation and application thereof
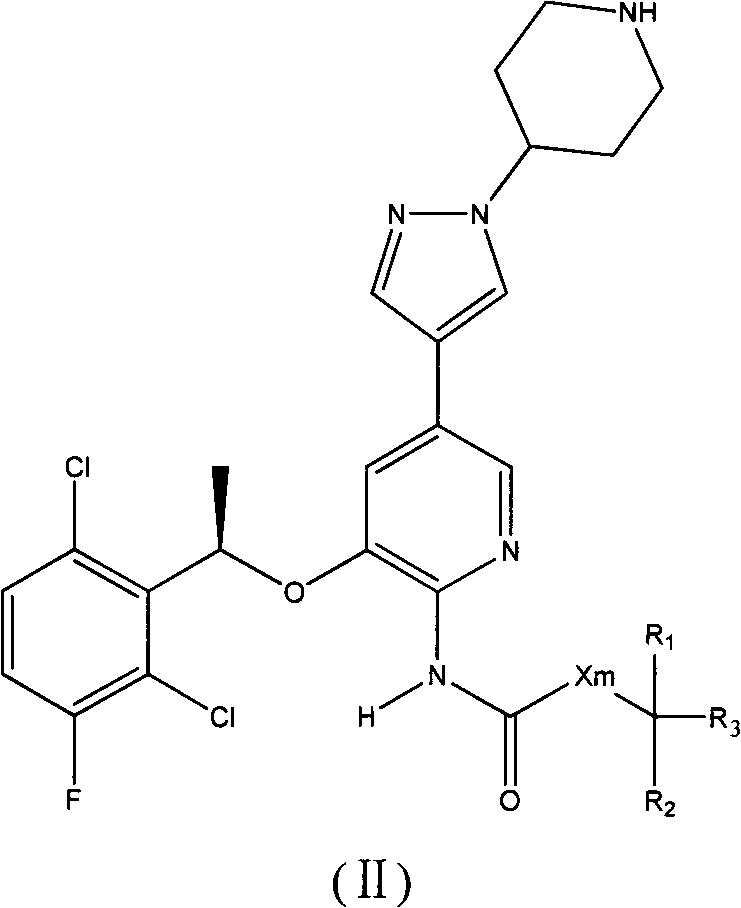
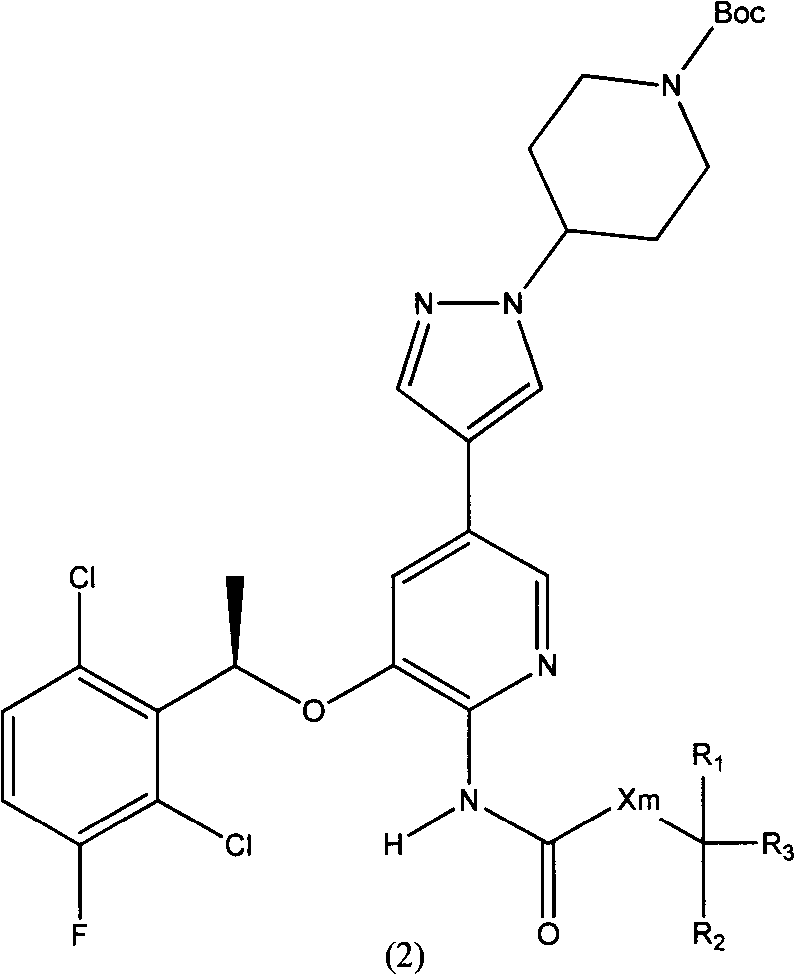
ActiveCN103373986AImprove bioavailabilityReduced inhibitory activityOrganic active ingredientsOrganic chemistryArylHalogen
The invention relates to a crizotinib prodrug, as well as a preparation and an application thereof. The invention provides a compound showed as a general formula (II) or a stereoisomer thereof, or a pharmaceutically acceptable salt, or a solvent compound or a hydrate of the compound, wherein X is O or CH2; m is 0 or 1; R1 and R2 are selected from the same or different groups, and are respectively independently hydrogen, halogen, nitryl, cyan, hydroxyl, amino saturated or unsaturated chain hydrocarbyl of C1-C6, and saturated or unsaturated cyclic hydrocarbyl of C1-C6; R1 and R2 also can exist in the same ring and form ternary-hexahydric ring together; the ternary-hexahydric ring can be substituted or un-substituted cyclic hydrocarbon, aromatic ring or hetero-aromatic ring; the substituent group on the ring can be selected from halogen, nitryl, cyan, hydroxyl, amino, C1-C6 alkyl and C1-C6 alkoxy; R3 is selected from hydrogen, substituted or un-substituted C1-C12 saturated or unsaturated alkyl, substituted or un-substituted phenyl or hetero-aryl, substituted or un-substituted alkyl acyloxy, substituted or un-substituted phosphorus acyloxy and substituted or un-substituted aryl acyloxy or hetero-aryl acyloxy; and the substituent groups are selected from halogen, nitryl, cyan, hydroxyl, amino, phenyl or hetero-aryl, C`-C6 alkyl, C1-C6 acyloxy, C1-C6 vinyl and C1-C6 alkynyl.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Synthetic method of crizotinib intermediate
ActiveCN105906656AImprove efficiencyHigh yieldGroup 3/13 element organic compoundsFermentationLeaving groupBoric acid
The invention relates to the technical field of small-molecule chemical drug, and particularly relates to a preparation method of a crizotinib intermediate. The preparation method comprises: (1) synthesizing a compound 3 from a compound 1 and a compound 2 by flow chemistry; (2) synthesizing the crizotinib intermediate I from the compound 3 obtained in the step (1) and a boric acid ester compound 4 by flow chemistry. The preparation method of the crizotinib intermediate, which is provided by the invention, is high in yield, can greatly reduce energy consumption and cost in the preparation process of crizotinib, is environmental-friendly, is high in safety and high in automation degree, and is suitable for industrial amplification production. A reaction route is as follows: (with reference to the specification), wherein Y represents a leaving group, Z represents an amino protection group, and X is selected from F, Cl, Br and I.
Owner:ASYMCHEM LAB TIANJIN +5
Synthesis method of crizotinib
InactiveCN104693184AHigh purityReduce dosageOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides a synthesis method of crizotinib. The method comprises the following steps: performing chemical exchange reaction for a compound (10) to obtain an intermediate compound (11); adding a compound (6) to react to obtain a compound (12); performing deprotection reaction for the compound (12) to obtain crizotinib. The method is simple in steps, short in line, simple in after-processing, low in cost, and particularly suitable for industrial production; the reaction line is shown in the specification, wherein X is Br or I; Nu is MgX or ZnX; R1 and R2 are independently selected from Boc or H.
Owner:ARROMAX PHARMATECH
Synthesis process for compound crizotinib
The invention provides a new synthesis method for crizotinib. An atomic economic reaction is adopted to reduce environmental pollution. A high-optical purity raw material is obtained by chiral prolinol induced chiral reduction; a chiral centre is constructed through an SN2 substitution reaction; post-processing and purification difficulties caused by Mitsunobu reaction are overcome. Malononitrile derivative is constructed by adopting a coupling reaction of malononitrile and bromo-pyridinium derivative; N,N-dicarboamide derivatives are obtained by performing aminolysis on N,N-dimethylamine hydrochloride; in the N,N-dicarboamide derivatives, N,N-dimethylamine serving as an easy-to-leave group and hydrazine perform a ring closing reaction to construct a pyrazolone ring, so that an expected final product, namely crizotinib, is obtained. According to the method, though continuous steps are used, the reaction of each step is high, the optical purity is high, and the total yield is also high. In addition, raw materials used in the synthesis method are low in cost and easily obtained; the using amount of a catalyst is small; total cost is easy to control. An operating process is simple and convenient and easy to control, and is suitable for industrial production.
Owner:甘肃皓骏药业有限公司
Synthesis method of crizotinib serving as antitumor molecular targeting medicament
ActiveCN102532106AReduce processing stepsEmission reductionOrganic chemistryAntineoplastic agentsPtru catalystEthyl group
The invention discloses a synthesis method of crizotinib serving as an antitumor molecular targeting medicament, which belongs to the field of pharmacy and relates to a novel splitting process of a chiral isomer of a crizotinib precursor and a synthesis method of an intermediate. (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol is prepared by splitting a 1-(2,6- dichloro-3-fluorophenyl)ethanol racemic body into S-alcohol and R-alcohol with a catalytic splitting method for combining Boc-L-proline(N-tert-butoxycarbonyl-L-proline), paratoluenesulfonic acid serving as a catalyst and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride, separating and purifying; the yield is 60 percent; and the excessive fraction ee) of the chiral enantiomer is 99 percent. According to the method, time is shortened, cost is reduced, the generation of waste acids is avoided, environmental pollution is lowered, column chromatography isolation is not required, and industrial production is easier to implement.
Owner:JINAN TRIO PHARMATECH
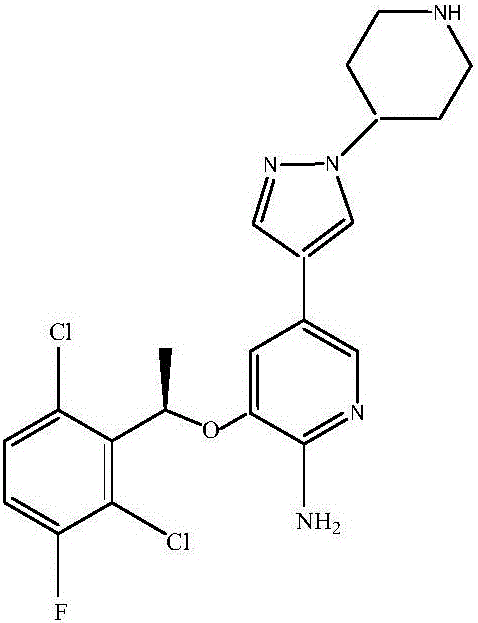
Preparation method of ALK inhibitor crizotinib and analogue or salt thereof
InactiveCN105272966AHigh yieldTake advantage ofOrganic chemistryBulk chemical productionChemical reactionPhenethyl alcohol
The invention discloses a preparation method of an ALK inhibitor crizotinib and analogue or salt thereof. The method comprise the following steps: 1, 1-(4'-N-Boc)-1'-piperidine-3(3''-fluoro-2''-nitro)-5''-pyridylpyrazole is prepared; 2, 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-N-Boc-piperidinyl)-1H-pyrazol-4-yl]-2-nitropyridine is prepared; 3, N-Boc crizotinib is prepared; and 4, crizotinib and analogue or salt thereof is prepared. The method has the following advantages: the synthesis steps are reduced to 4 chemical reaction steps; a total yield is improved to approximately 35%, while the yield of an existing method is only approximately 10%; phenethyl alcohol with (R)- configuration, such as (R)-2,6-dichloro-3-fluorophenylethanol, is directly used as a raw material, such that the raw material can be fully utilized. The method provided by the invention is suitable for industrialized productions.
Owner:NANJING LEIKEXING BIOTECH CO LTD
Preparation method of crizotinib intermediate (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol
InactiveCN105294401AGood catalyticReduce lossOrganic compound preparationHydroxy compound preparationPhenethyl alcoholDiphosphines
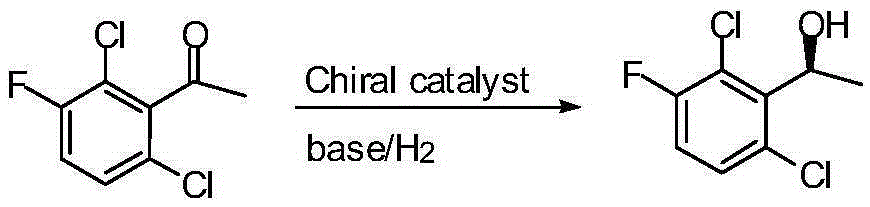
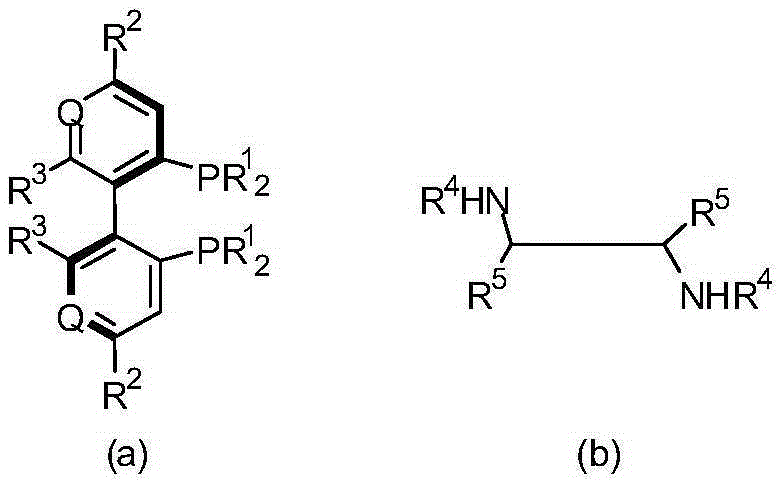
The invention belongs to the technical field of organic chemistry, also belongs to the technical field of medicinal chemistry, and in particular to a preparation method of a crizotinib intermediate (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol. The method uses 1-(2,6-dichloro-3-fluorophenyl) acetophenone as the raw material to obtain a crizotinib chiral intermediate S-1-(2,6- dichloro-3-fluoro) phenethyl alcohol under the effect of a chiral catalyst, alkali and hydrogen. The chiral catalyst is [MX2((S)-a)((R,R)-b)], which is composed of chiral diphosphine ligand compound a, a chiral nitrogen ligand compound b and a metal salt catalyst MX2(P-cymene) in coordination combination. The synthetic method provided by the invention can prepare crizotinib intermediate with high enantioselectivity; the intermediate has ee% reaching 98%; and the mole dosage of the catalyst is only 1 / 150000-1 / 50000 of a reaction substrate 1-(2,6-dichloro-3-fluorophenyl) acetophenone, and the substrate can be completely transformed.
Owner:WUHAN SINO SANTA CHEM TECH CO LTD
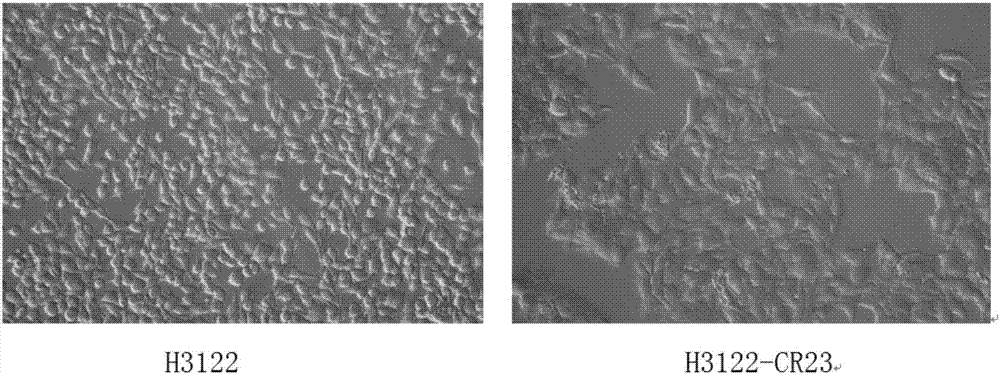
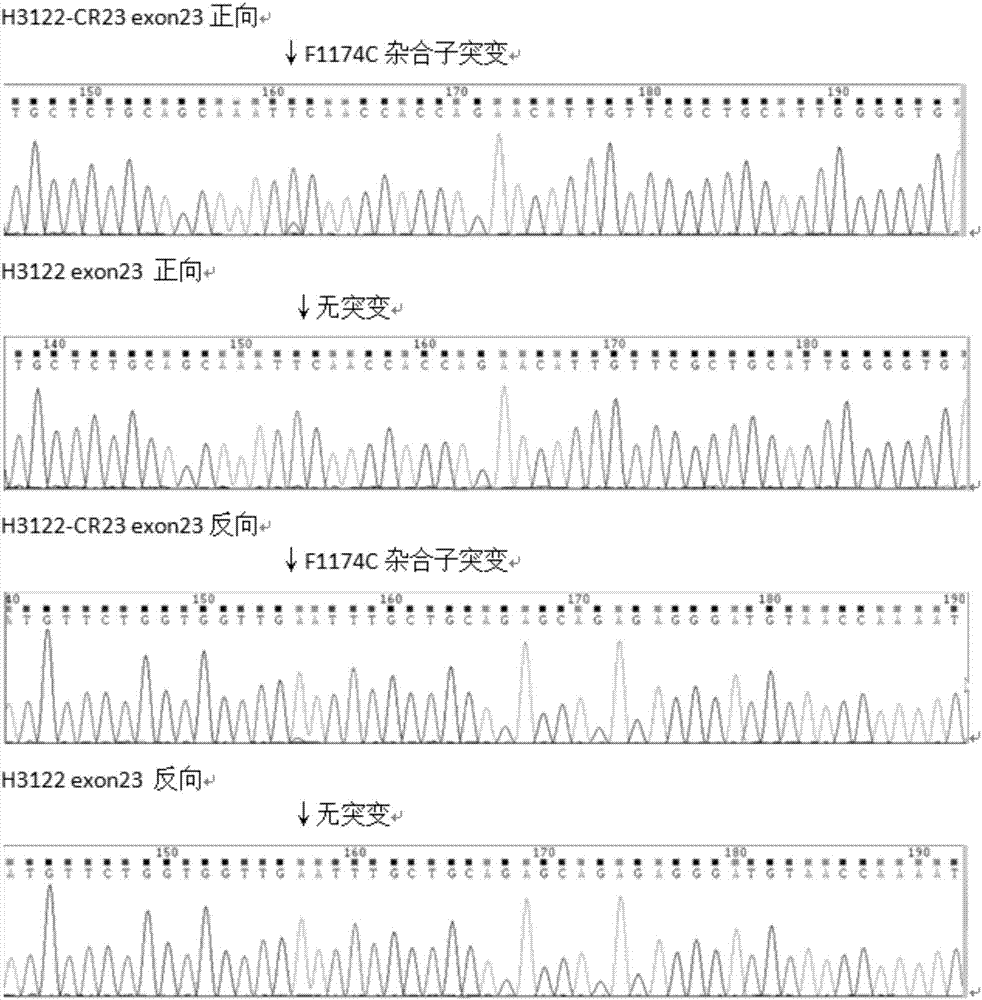
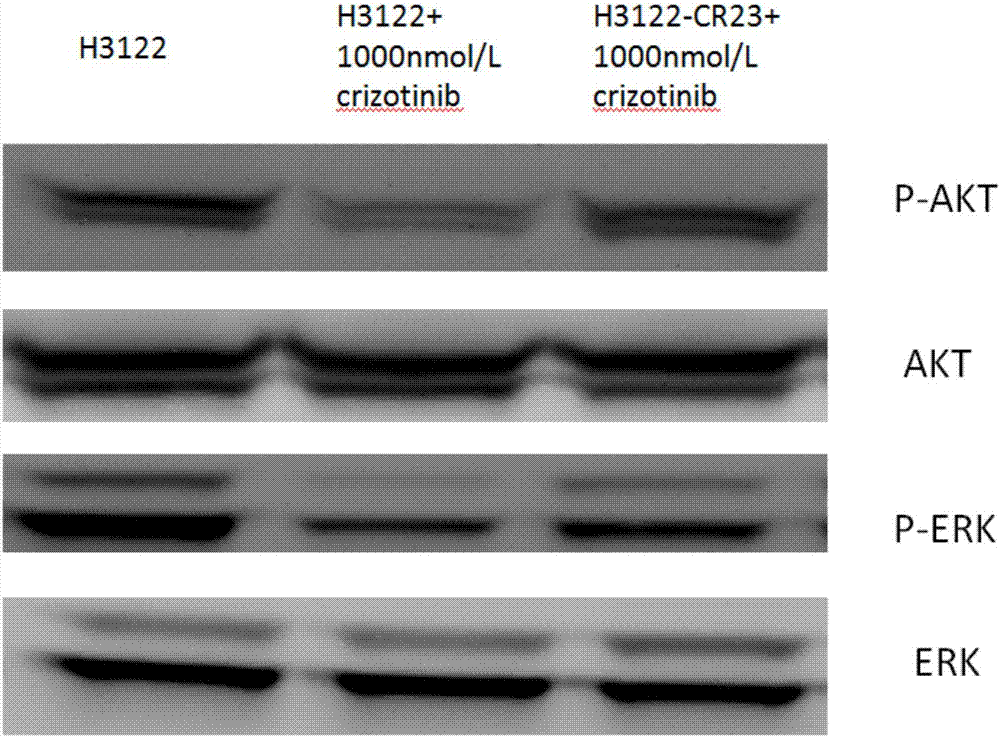
Anti-crizotinib human non-small cell lung cancer cell strain H3122-CR23 and application thereof
ActiveCN107267458AIncrease production capacityMicrobiological testing/measurementMammal material medical ingredientsSocial benefitsSmall cell lung carcinoma cell
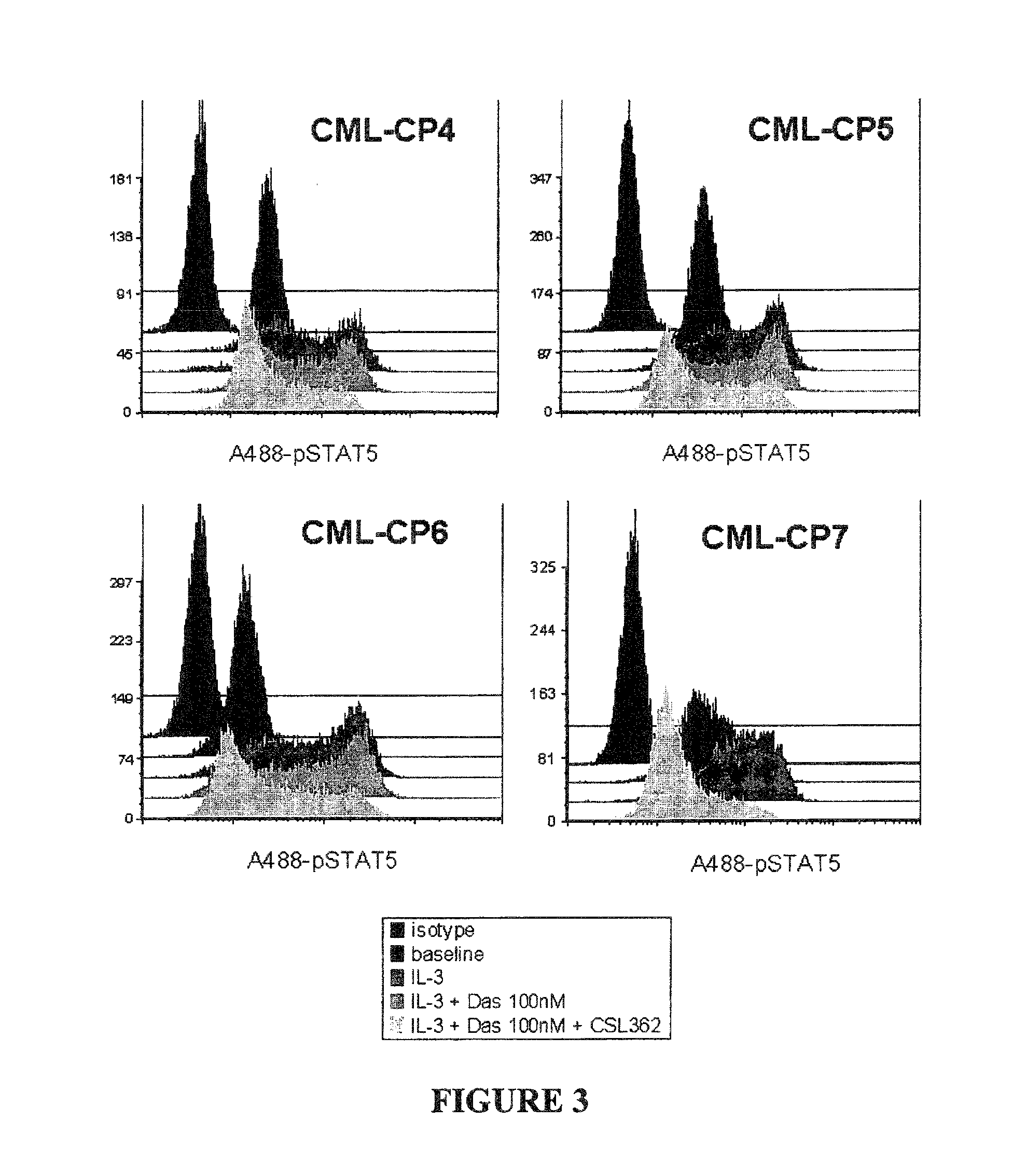
The invention discloses a novel anti-crizotinib human non-small cell lung cancer cell strain which is named as H3122-CR23 and has an accession number of CCTCC No. C201780. The cell strain has F1174C mutation in an ALK kinase area. The cell strain H3122-CR23 is applicable to research on the morphological and biological characteristics of human anti-crizotinib non-small cell lung cancer cells, research on the drug resistance mechanisms of tumors, development of drugs for drug resistance reversal of tumors, analysis of the susceptibility of antitumor drugs, screening and assessment of antitumor drugs, research on more efficient tumor treatment methods, etc., has high application value in scientific research and production, and is expected to produce good scientific research, economic and social benefits.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
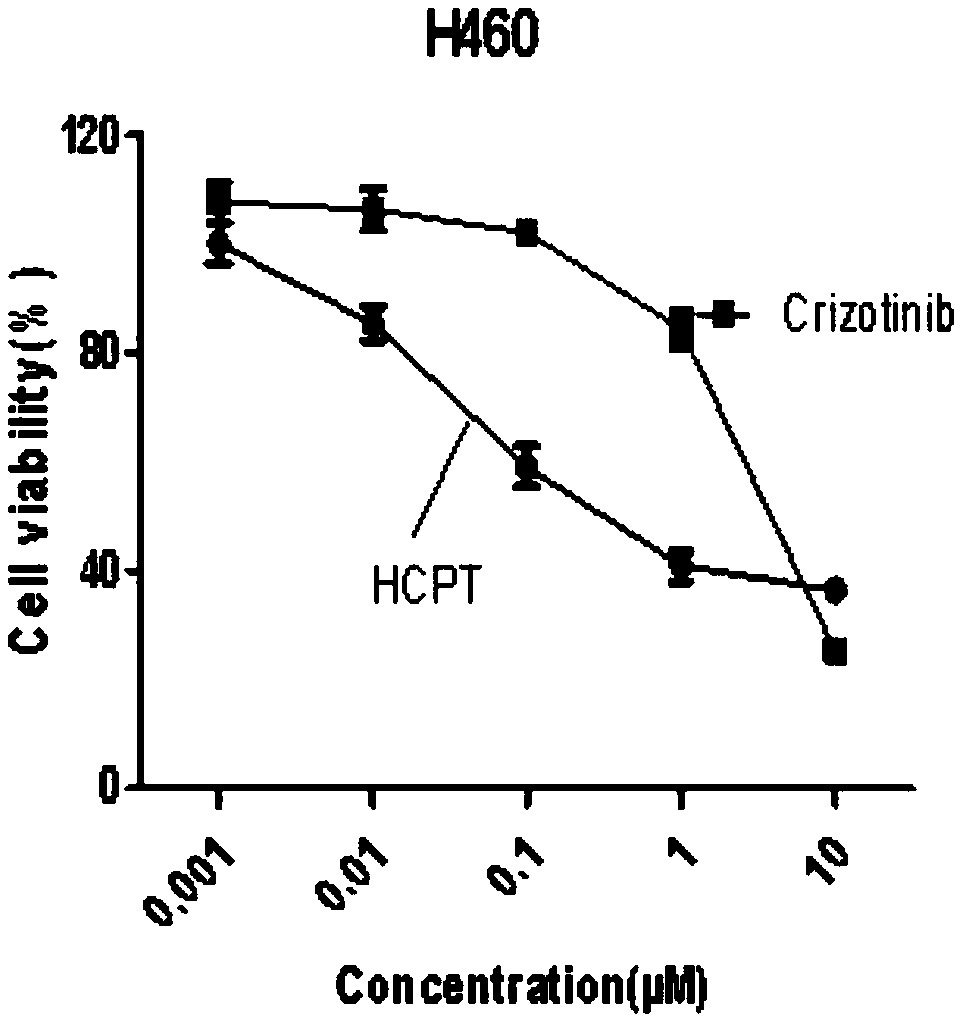
Cancer compound 10-hydroxycamptothecine and crizotinib for treating lung cancer and application
InactiveCN109091480AGood curative effectLittle side effectsOrganic active ingredientsAntineoplastic agentsSide effectWilms' tumor
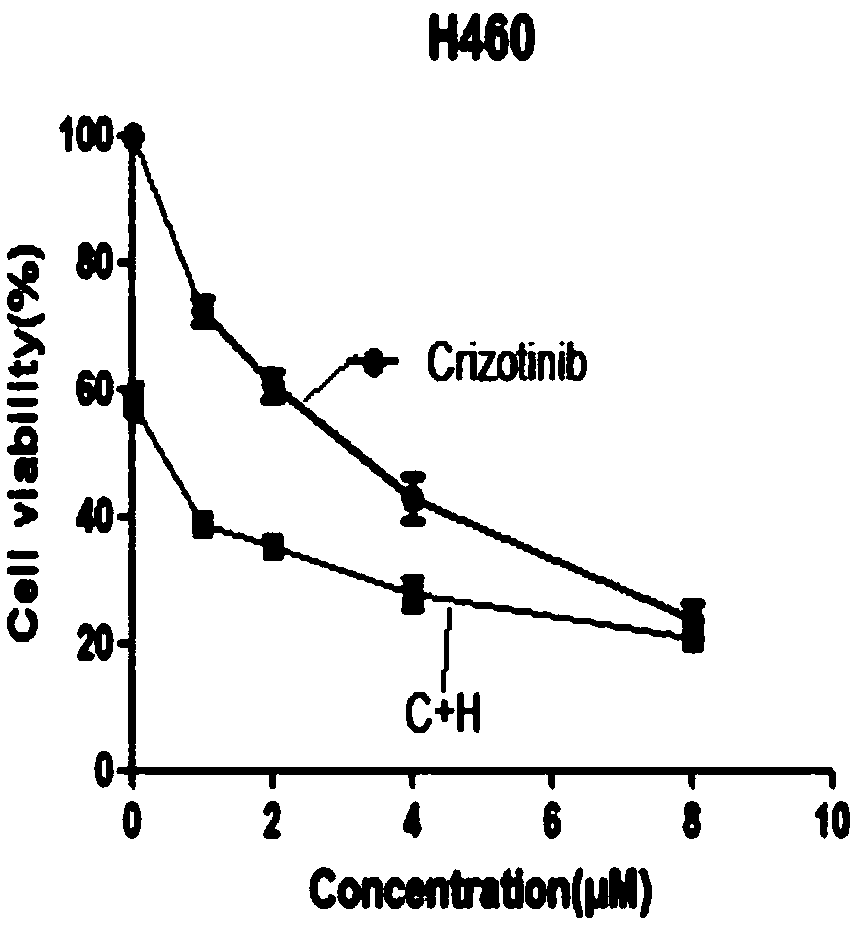
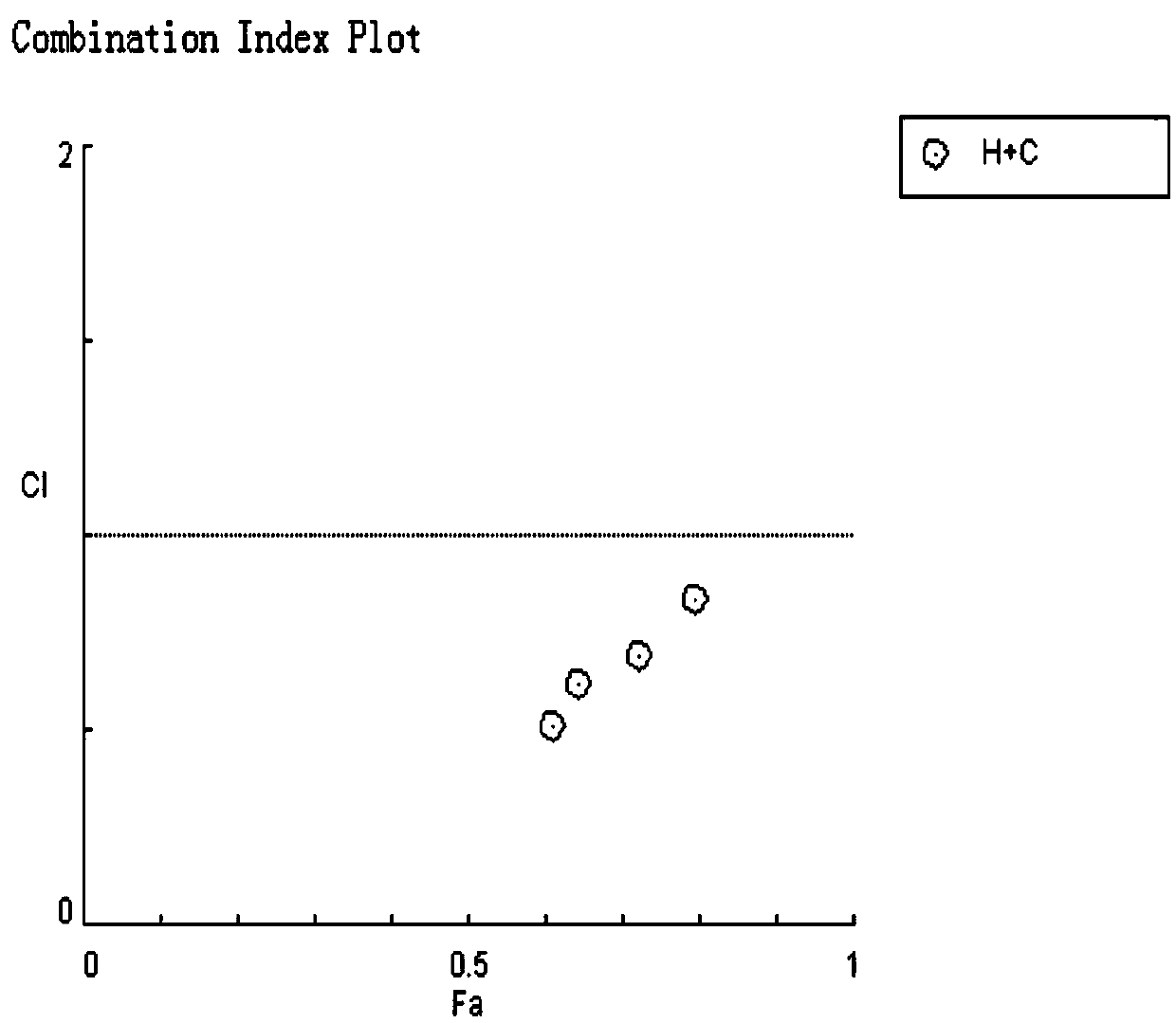
The invention relates to a cancer compound 10-hydroxycamptothecine and crizotinib for treating lung cancer and application. Active components of the medicinal composition include a chemotherapy medicine 10-hydroxycamptothecine and a targeting medicine crizotinib. The invention also relates to application of 10-hydroxycamptothecine and crizotinib in the field of cancer treatment drugs. According tothe cancer compound 10-hydroxycamptothecine and crizotinib for treating lung cancer, the anti-tumor medicine 10-hydroxycamptothecine and the targeting medicine crizotinib are used in match; the in vitro experiment verifies that the chemotherapy medicine 10-hydroxycamptothecine and the targeting medicine crizotinib are obviously synergistically interacted in killing malignant cells. The new application of the drug combination helps reducing the toxic and side effects of chemotherapy medicines; the drug resistance of the targeting medicine can also be reduced; the tumor inhibiting effect is improved; and scientific foundation is supplied to develop new drugs.
Owner:TIANJIN UNIV OF SCI & TECH
Synthetic method for crizotinib intermediate
InactiveCN104402679ALow costQuality is easy to controlOrganic compound preparationHydroxy compound preparationOrganic solventChemical compound
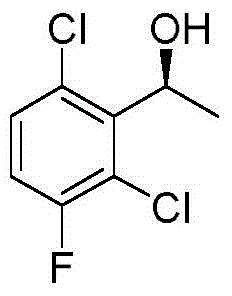
The invention provides a synthetic method for a crizotinib intermediate. The method comprises the following steps: (1), obtaining a crude product through reaction of a compound (I) namely 2,6-dichloro-3-fluoroacetophenone and (-) DIP-Cl in an organic solvent at the temperature of 0-25 DEG C; (2), re-crystallizing the crude product obtained in the step (1) by using normal hexane to obtain a crizotinib intermediate compound (II). A key intermediate is synthesized at one step by using an asymmetric catalysis method, and is controllable in quality, simple and convenient to operate, high in yield, high in optical purity and applicable to industrial production. A reaction structure formula of the method is shown as follows.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Crizotinib intermediate, preparation method and crizotinib preparation method
InactiveCN106317024AGood reaction selectivityImprove controllabilityGroup 3/13 element organic compoundsCrizotinibStereochemistry
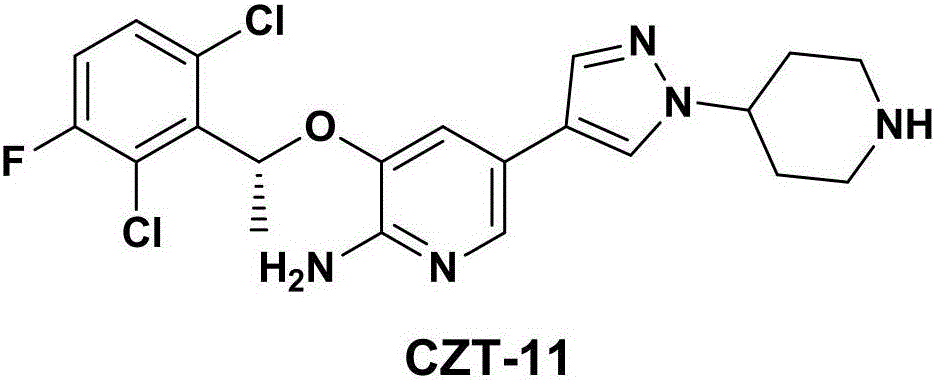
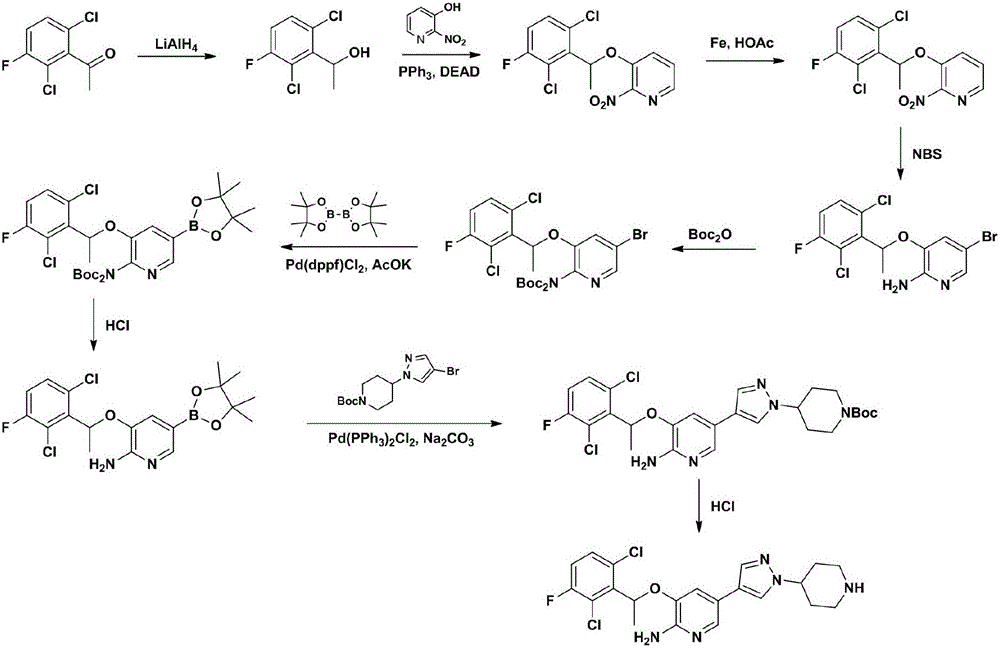
The invention relates to a crizotinib intermediate, a preparation method and a crizotinib preparation method, in particular to an intermediate of crizotinib which has the structure of a formula (CZT-5) as shown in the description and the structure of a formula (CZT-9) as shown in the description, a preparation method of the intermediate and a preparation method of a crizotinib with the structure of a formula (CZT-11). The method provided by the invention has the characteristics of short route, high yield, easiness in acquisition of raw materials, high reaction selectivity and the like, chiral resolution is not required in a synthetic process, the utilization rate of the raw materials is increased, the path costs are low, and therefore, the method can meet requirements of large-scale industrial production.
Crizotinib capsule and preparation method thereof
InactiveCN104971054AContinuous and stable productionImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsTherapeutic effectMagnesium stearate
The invention provides crizotinib capsules and a preparation method thereof, wherein the specifications of the crizotinib capsules can be 200 mg and 250 mg, wherein the auxiliary materials in each capsule include 30-100 mg of microcrystalline cellulose, 50-200 mg of anhydrous calcium hydrophosphate, 20-50 mg of carboxymethyl starch sodium, 5-25 mg of silica gel micro powder and 1-10 mg of magnesium stearate. The preparation method includes following steps: one-step granulation in a fluidized bed, mixing and capsule filling, wherein an 80% ethanol solution is employed as a wetting agent. The preparation method solves the problems of poor flowability, high different of content quantity, low dissolution rate and poor stability of granules of the crizotinib capsules, improves the bioavailability and curative effects of the capsules and is better in curative effects.
Owner:刘小斌 +1
Preparation method for crizotinib
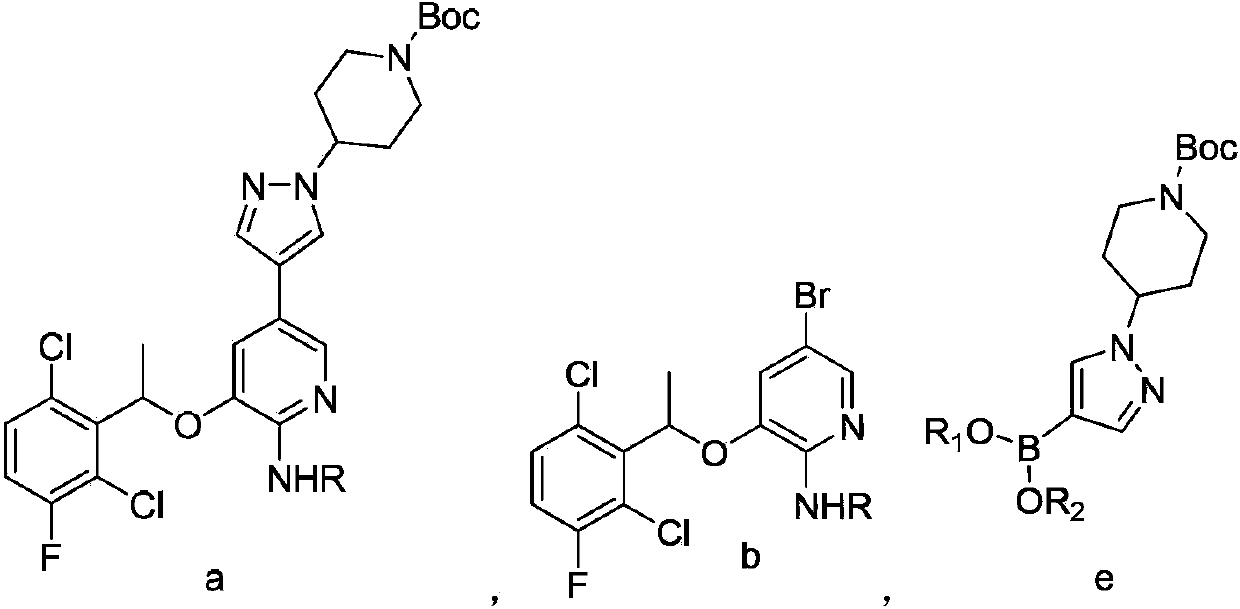
The present invention relates to the field of pharmaceutical synthesis, in particular to a crizotinib preparation method. The method is a preparation method of a compound having a structure of formula (a): conducting Suzuki coupling reaction between a compound having a structure of formula (b) and a compound having a structure of formula (e) to obtain the compound having the structure of formula (a); further conducting a deprotection reaction on the compound having the structure of formula (a) to obtain (±) crizotinib.
Owner:RAYBOW (HANGZHOU) PHARM CO LTD
Method for synthesizing Crizotinib intermediate
InactiveCN102898449AEasy to synthesizeReduce manufacturing costGroup 3/13 element organic compoundsPtru catalystBenzoyl peroxide
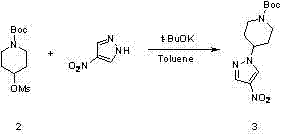
The invention belongs to the technical field of medicine synthesis, and in particular relates to a method for synthesizing a Crizotinib intermediate. The method comprises steps of: a) reacting a raw material 4-mesylate piperidine-1-formic acid tert-butyl ester (2) with 4-nitro pyrazole to prepare a compound 3; b) reducing nitro by using hydrazine hydrate to obtain an amino compound 4; and c) diazotizing the compound 4 by using tert-butyl nitrite, and reacting the compound 4 with a boric acid ester compound 5 in the presence of a free radical initiator benzoyl peroxide, so as to prepare the Crizotinib intermediate (1). Compared with an existing synthesis method, the method provided by the invention has the following advantages: a diazotization method is used to synthesize the boric acid ester product; and compared with an existing Miyaura boronation method catalyzed by Pd, the method avoids the usage of expensive palladium catalyst and ligand, and has the merits of mild reaction condition, high yield, simple operation, cheap and easily available raw materials and short reaction period, and is quite easy for industrialized mass production.
Owner:TONGJI UNIV
Method of treatment of philadelphia chromosome positive leukemia
InactiveUS20150093355A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Crizotinib for use in the treatment of cancer
The present invention relates to the use of ROS kinase inhibitors for treating abnormal cell growth in mammals. In particular, the invention provides methods of treating mammals suffering from cancer mediated by at least one genetically altered ROS. In particular, the invention provides methods of treating mammals suffering from cancer mediated by at least one genetically altered ROS by administration of crizotinib.
Owner:PFIZER INC
Method for resolving racemic crizotinib
ActiveCN112547019AHighly selective adsorptionAchieve splitOther chemical processesOrganic chemistry methodsNanocrystalBuffer solution
The invention relates to a method for resolving racemic crizotinib, wherein the preparation method of an adsorbent comprises the steps: dissolving EDC, NHSS and (L)-Cit in an MES buffer solution, adding MIL-53-NH2 nanocrystals into the MES buffer solution, stirring, centrifuging, washing with deionized water, and carrying out vacuum drying. The adsorbent is applied to resolution of racemic crizotinib, and the method comprises the steps: firstly, soaking the adsorbent into a racemic crizotinib ethanol solution, stirring, carrying out centrifugal treatment, and collecting a supernatant and the adsorbent; adding the adsorbent into the collected supernatant again for centrifugal treatment, and then respectively collecting the supernatant and the adsorbent; and repeating the steps until the excess value of the crizotinib enantiomer in the supernatant is greater than the ee value at the lowest eutectic point, and then carrying out preferential crystallization to obtain (S)-crizotinib with the ee value of greater than 99% and (R)-crizotinib with the ee value of greater than 99%. The method disclosed by the invention is simple and feasible, the method for resolving racemic crizotinib by the adsorbent can obtain two pure enantiomers at the same time, and the separation effect is remarkable.
Owner:SHANGHAI UNIV OF ENG SCI
Synthetic method of bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate and application thereof
ActiveCN106831720ASimple methodProcess is easy to controlOrganic chemistryTesting medicinal preparationsGrignard reactionChemistry
The invention discloses a synthetic method of bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate and an application thereof. The bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate is prepared through a methyl sulfonylation reaction, a hydrocarbylation reaction, a Grignard reaction and a Suzuki coupling reaction by taking cheap and easily available N-Boc-4-hydroxyl piperidine as a raw material. The synthetic method is simple, the process is easy to control, and the yield of the prepared bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate is 86.7%; bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate can be used as a standard substance to detect and monitor synthesis of crizotinib. The synthetic method disclosed by the invention is suitable for synthesizing bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate and the synthesized substance is used for detecting and monitoring synthesis of crizotinib.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Composition containing protein kinase inhibitor and metformin
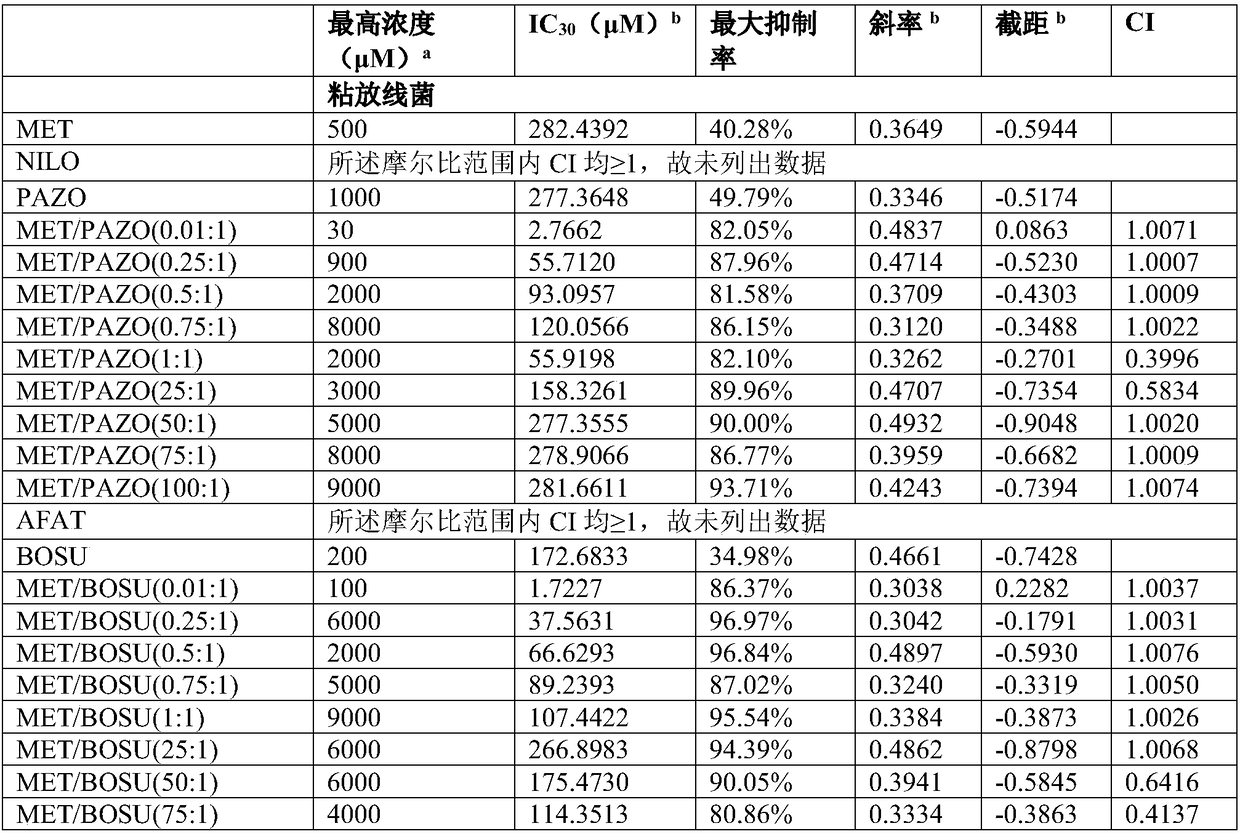
The invention firstly provides a composition containing a protein kinase inhibitor and metformin or pharmaceutically acceptable salts thereof. The composition is characterized in that the protein kinase inhibitor is one selected from nilotinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib and regorafenib or pharmaceutically acceptable salts or solvates thereof or solvates of the pharmaceutically acceptable salts thereof, and the molar ratio of the metformin to the protein kinase inhibitor is (0.01-100):1. In-vitro bacteriostatic tests find that the composition containing the metforminand the protein kinase inhibitor can achieve a synergistic bacteriostatic effect on various bacteria such as staphylococcus aureus in the molar ratio of (0.01-100):1 (at an inhibition rate of 30%, thecombined medication index CI is smaller than 1).
Owner:黄泳华
Palladium removal method for crizotinib intermediate
PendingCN111349083AMeet the requirements of industrial productionLow in palladiumOrganic chemistryOrganic solventPyrazolylchalcone
The invention discloses a method for removing palladium from a crizotinib intermediate. The method comprises the following steps: adding a heavy metal ion remover into a palladium-containing reactionsolution; after the remover is separated, a solid is separated out from an organic phase, and the crizotinib intermediate (R)-4-(4-(6-amino-5-(1-(2, 6-dichloro-3-fluorophenyl) ethoxy) pyridine-3-yl)-1H-pyrazol-1-yl) tert-butyl ester piperidine-1- carboxylate tert-butyl ester with the palladium content as low as 10 ppm is obtained. The method is simple to operate, saves an organic solvent and is suitable for industrial production.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Novel antitumor composition, preparation and application
InactiveCN110522753ADelay drug resistanceGood synergyOrganic active ingredientsAntineoplastic agentsInhibitory effectCrizotinib
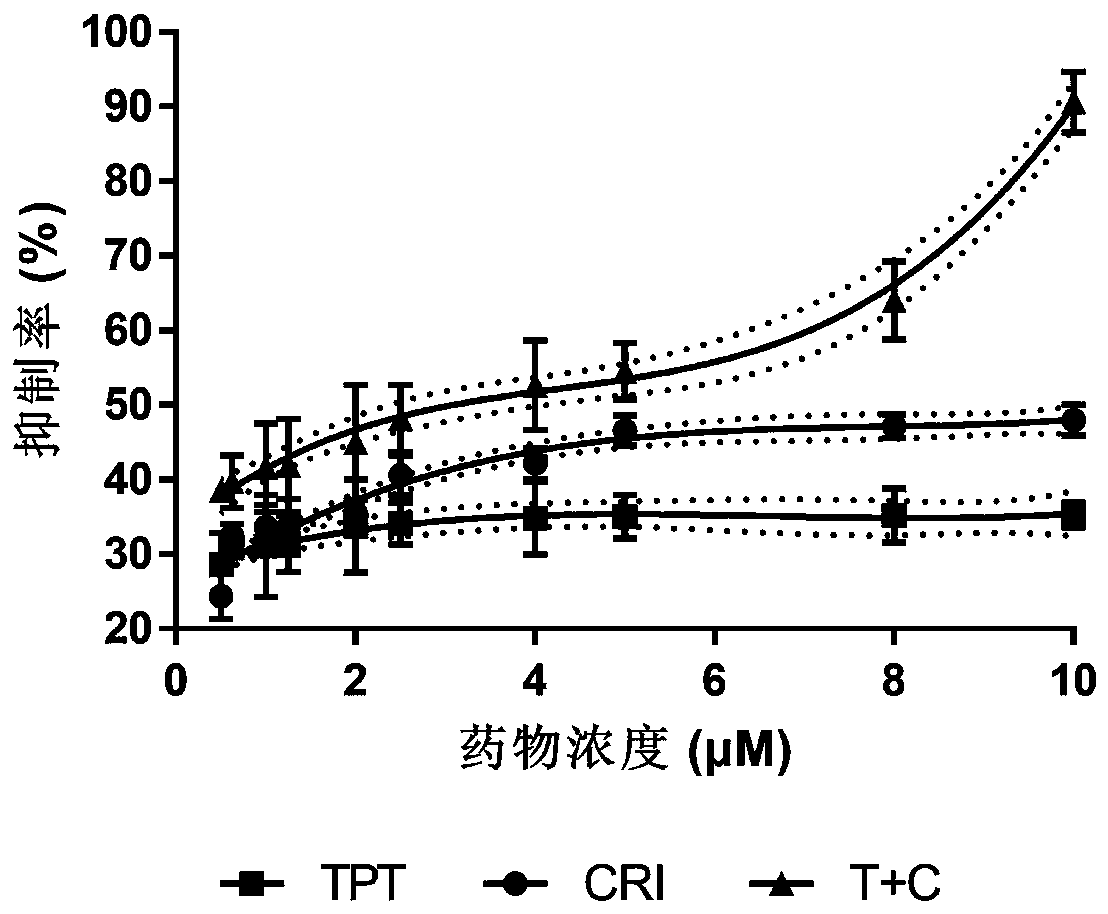
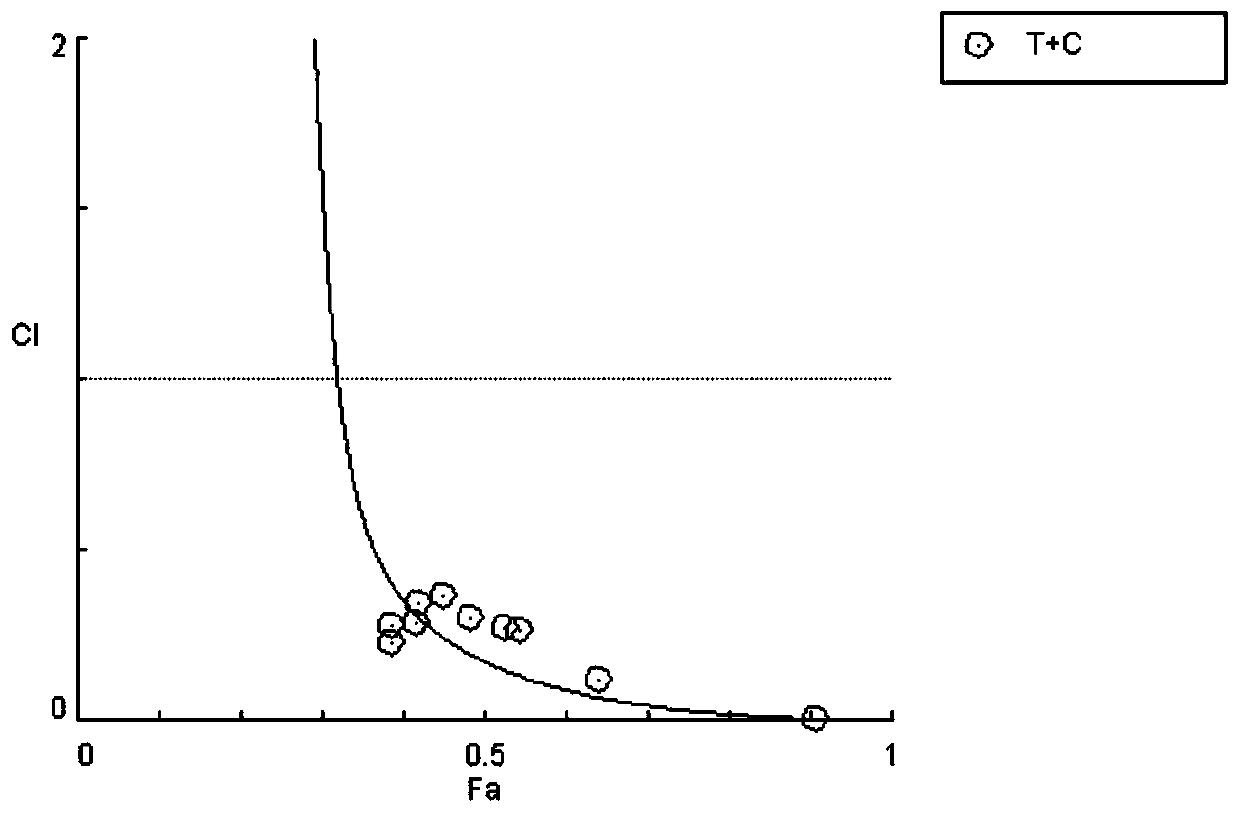
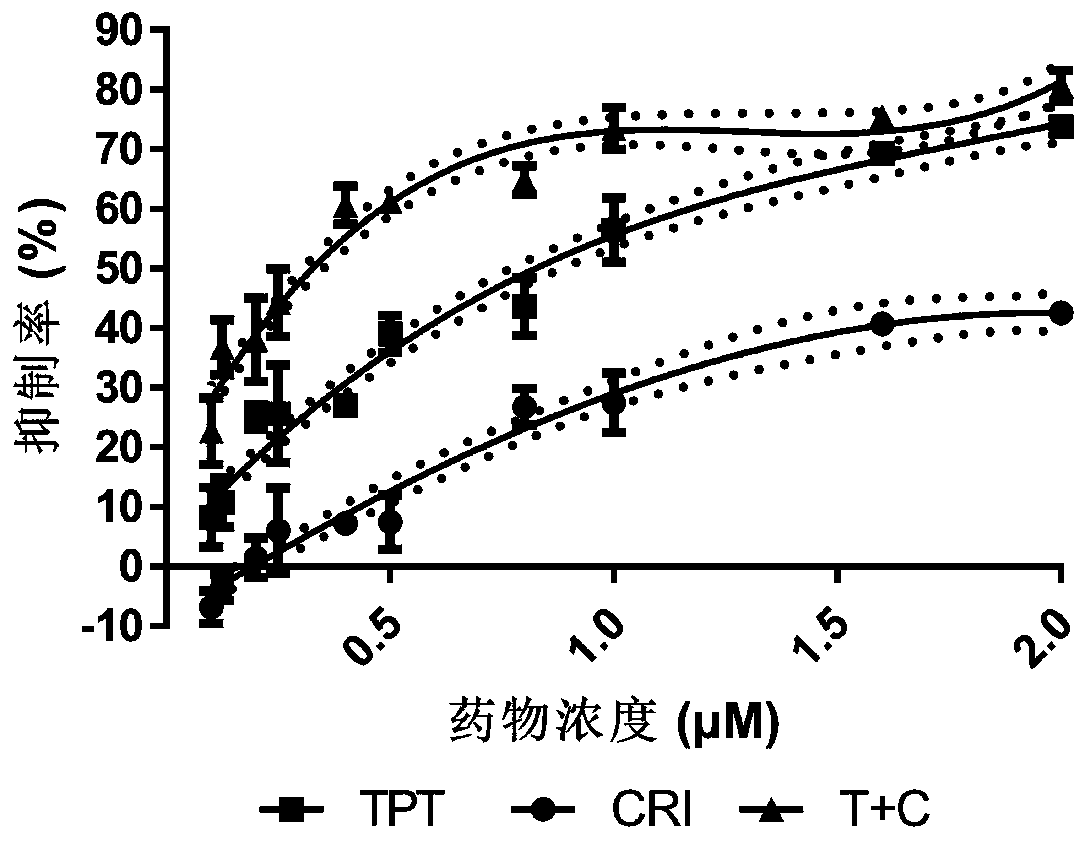
The invention relates to a novel antitumor composition. The composition is the composition of topotecan and crizotinib. According to the combination medicines, the topotecan and the crizotinib are combined for use, and the composition being low in effective dose has notable synergy effects for treatment of non-small cell lung cancer, particularly has synergy effects in the respect of treating non-small cell lung cancer. In vivo experimentation on animals also primarily confirms that the combined medicines are notable in inhibition effects on cell proliferation, the tumor inhibition effects areincreased, a new thought is provided for treatment of patients when generating reverse tolerance of the non-small cell lung cancer, and scientific basis is provided for research and development of new medicines.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Synthesis process of crizotinib intermediate
InactiveCN107903147AHigh yieldReduce pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholHydrogen
The invention discloses a synthesis process of a crizotinib intermediate. The synthesis process comprises the following steps: with 2,6-dichloro-3-fluoroacetophenone as the raw material in an alkali and solvent environment and hydrogen as a reducing agent, reacting under the action of a chiral catalyst to obtain (R)-1-(2,6-dichloro-3-fluorophenyl)ethyl alcohol; the high-chiral-purity crizotinib intermediate is obtained at one step as the process adopts the reduction system, the complex chiral resolution process of the existing process is omitted, the period of the process is greatly shortened,the production cost is low, the reaction condition is mild, the process is stable, the conversion rate is high, the environment pollution caused by the reaction is small, and the synthesis process isfavorable for realizing the industrial production.
Owner:ENANTIOTECH CORP
Preparation method of crizotinib
ActiveCN105294657AHigh yieldHigh optical purityOrganic chemistryBulk chemical productionBoric acidCrizotinib
The invention discloses a preparation method of crizotinib. An intermediate (V) is prepared through boric acid condensation, so that the defects of instable borate serving as a raw material, complexity in operation, low yield and large-scale production difficulty in the prior art are overcome. The preparation method is low in cost, simple and convenient to operate, high in yield, good in optical purity and suitable for large-scale production.
Owner:XIHUA UNIV +1
Preparation method of crizotinib intermediate
The invention discloses a synthetic method of anti-tumor molecular targeted drug crizotinib, belongs to the field of pharmaceuticals, and relates to a synthetic method of a crizotinib intermediate. The method comprises two reduction processes: a bromination reaction process comprises the following reaction steps: a reduction process I: carrying out reduction reaction on a (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine compound and sodium dithionate under a mechanical chemical condition to generate (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-aminopyridine; a reduction process II: dissolving the (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine compound into an organic solvent, carrying out catalytic hydrogenation and reduction treatment to generate (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-aminopyridine; the bromination reaction process comprises a step of carrying out reaction between the compound and potassium hydrogen persulfate as well as bromate to obtain the crizotinib intermediate. The process is low in cost, the raw materials are easy to purchase, the operation is simple, convenient and safe, and the yield is high; moreover, the process is suitable for large-scale production.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Crizotinib prodrug polymeric micelle co-loaded with chemotherapeutic drug and preparation method thereof
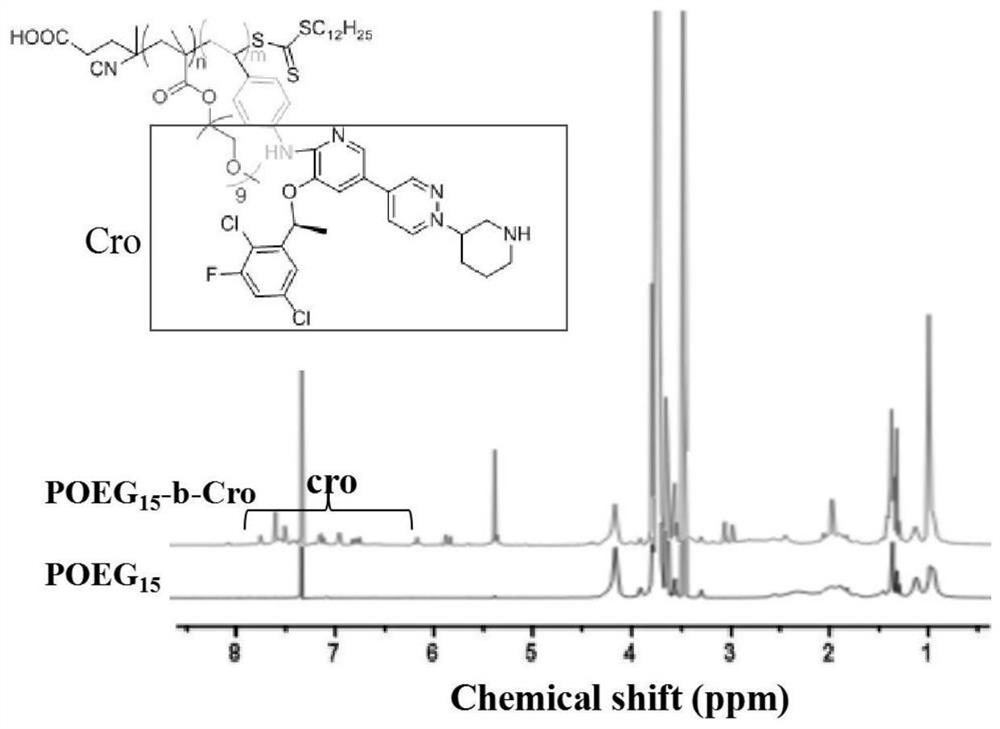
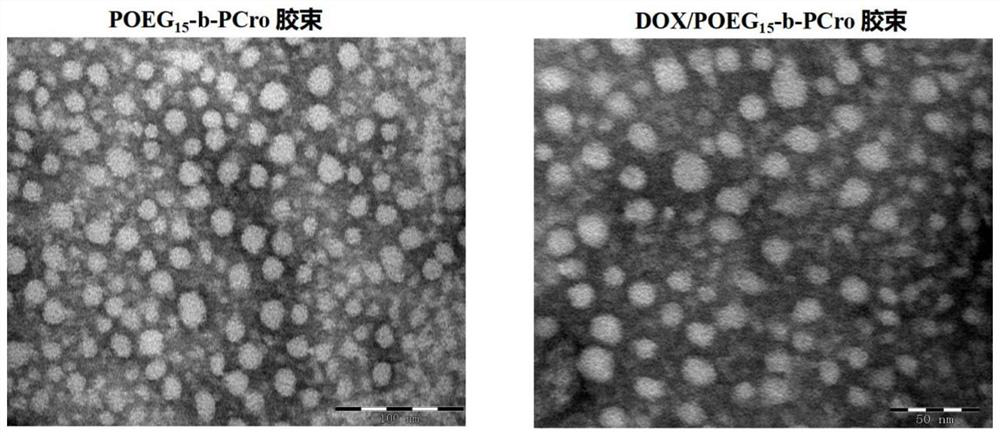
ActiveCN111888357AImprove immunogenic functionTo achieve co-loadingOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical formulationMaterials science
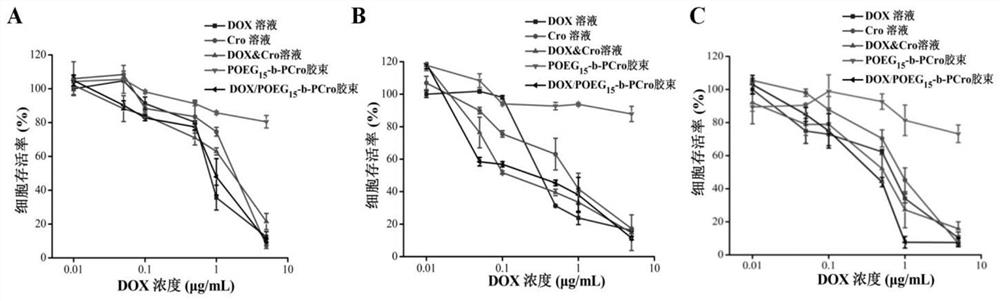
The invention relates to crizotinib prodrug polymeric micelle co-loaded with a chemotherapeutic drug and a preparation method thereof, belongs to the field of a high molecular material and a new dosage form of a medicinal preparation, and particularly relates to crizotinib prodrug polymeric micelle co-loaded with a chemotherapeutic drug, which has an effect of reinforcing tumor immunogenicity, anda preparation method thereof. The prodrug polymeric micelle is characterized by being formed by firstly polymerizing a hydrophilic POEG block and hydrophobic crizotinib through a Reversible Addition-Fragmentation chain Transfer (RAFT) method to obtain an amphiphilic diblock copolymer and then physically loading the chemotherapeutic drug into the diblock copolymer to carry out self-assembling. According to the invention, a co-delivery mode of adopting conventional predrug polymer physical loading or adopting a polymer predrug to carry out self-assembling and physically loading another drug small molecules is broken through, and by a polymerization method using a drug as a monomer, high-efficiency co-loading of the combined drug and in-vivo accurate co-delivery are effectively implemented.
Owner:NINGXIA MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com