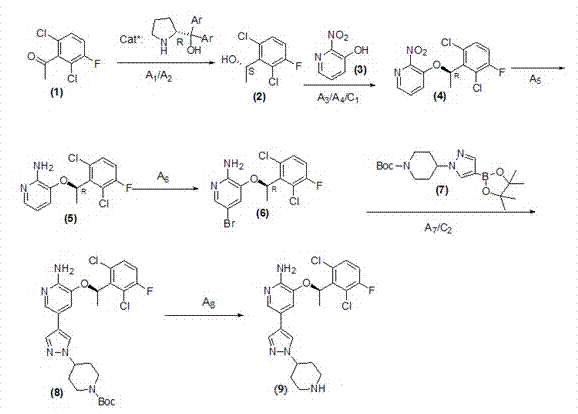
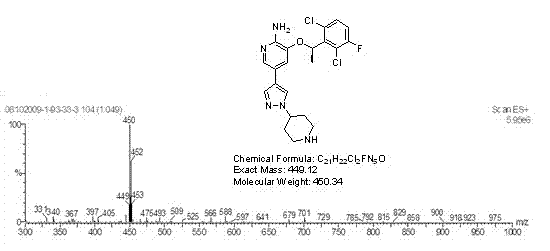
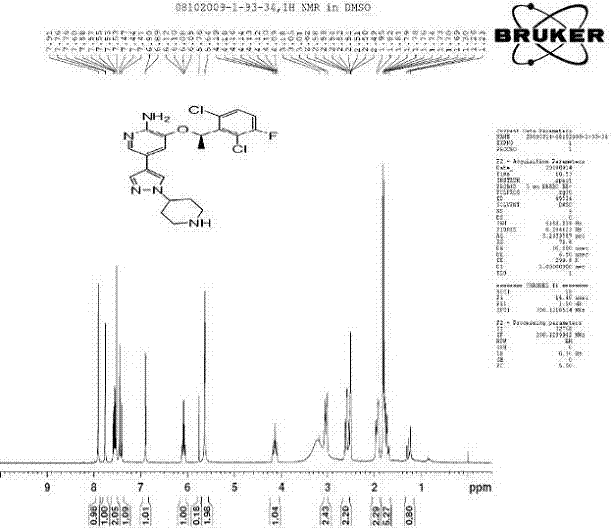
Preparing method of crizotinib
A kind of crizotinib and the technology of the previous step, applied in the field of preparation of crizotinib, can solve the problems such as low yield of chemical splitting method, unfavorable large-scale production, many steps, etc., and achieve a wide source of raw materials, The reaction process is easy to control and the reaction period is short.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Crizotinib
[0044] The method for preparing crizotinib of the present embodiment comprises the following steps:
[0045] 1) Preparation of phenylethyl alcohol in (S)-configuration
[0046] Freshly distilled trimethylchlorosilane (130 g, 1.2 mol) was added to sodium borohydride (45 g, 1.2 mol) in dry THF (5 L). The reaction mixture was heated at 70° C. for 1 hour, cooled to room temperature, and (S)-diphenylprolinol in THF (0.1 mol, 2 L) was added. When no gas was generated, a solution of acetophenone in THF (1 mol, 2 L) was added slowly. After the reaction was complete, 2N aqueous hydrochloric acid (5 L) was added. Extract three times with ether (10 L). The combined organic phases were washed three times with saturated brine. Dry and concentrate. A white solid was obtained with a yield of 98% and an ee value of 96%.
[0047] 2) Preparation of nitro compounds
[0048]Dissolve 94g of triphenylphosphine, 52g of phenethyl alcohol and 38g of...
Embodiment 2
[0057] Example 2 Preparation of Crizotinib
[0058] The preparation method of the present embodiment comprises the following steps:
[0059] 1) Preparation of phenylethyl alcohol in (S)-configuration
[0060] Freshly distilled trimethylchlorosilane (130 g, 1.2 mol) was added to potassium borohydride (65 g, 1.2 mol) in dry THF (5 L). The reaction mixture was heated at 70° C. for 1 hour, cooled to room temperature, and (S)-diphenylprolinol in THF (0.1 mol, 2 L) was added. When no gas was generated, a solution of acetophenone in THF (1 mol, 2 L) was added slowly. After the reaction was complete, 2N aqueous hydrochloric acid (5 L) was added. Extract three times with ether (10 L). The combined organic phases were washed three times with saturated brine. Dry and concentrate. A white solid was obtained with a yield of 96%, ee value: 96%.
[0061] 2) Preparation of nitro compounds
[0062] Dissolve 94g of triphenylphosphine, 52g of phenethyl alcohol and 38g of 3-hydroxy-nitrop...
Embodiment 3
[0071] Example 3 Preparation of Crizotinib
[0072] The preparation method of the present embodiment comprises the following steps:
[0073] 1) Preparation of phenylethyl alcohol in (S)-configuration
[0074] Freshly distilled tert-butyldimethylsilyl chloride (180 g, 1.2 mol) was added to potassium borohydride (65 g, 1.2 mol) in dry THF (5 L). The reaction mixture was heated at 70oC for 1 hour, cooled to room temperature, and (S)-diphenylprolinol in THF (0.1mol, 2L) was added. When no gas was generated, a solution of acetophenone in THF (1 mol, 2 L) was added slowly. After the reaction was complete, 2N aqueous hydrochloric acid (5 L) was added. Extract three times with ether (10 L). The combined organic phases were washed three times with saturated brine. Dry and concentrate. A white solid was obtained with a yield of 97% and an ee value of 96%.
[0075] 2) Preparation of nitro compounds
[0076] Dissolve 94g of triphenylphosphine, 52g of phenethyl alcohol and 38g of 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com