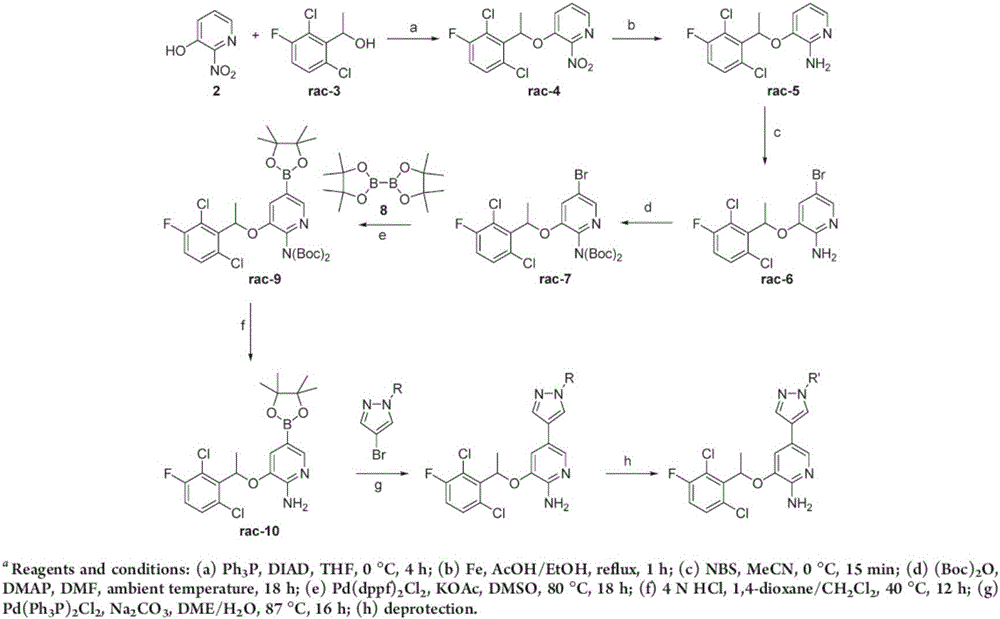
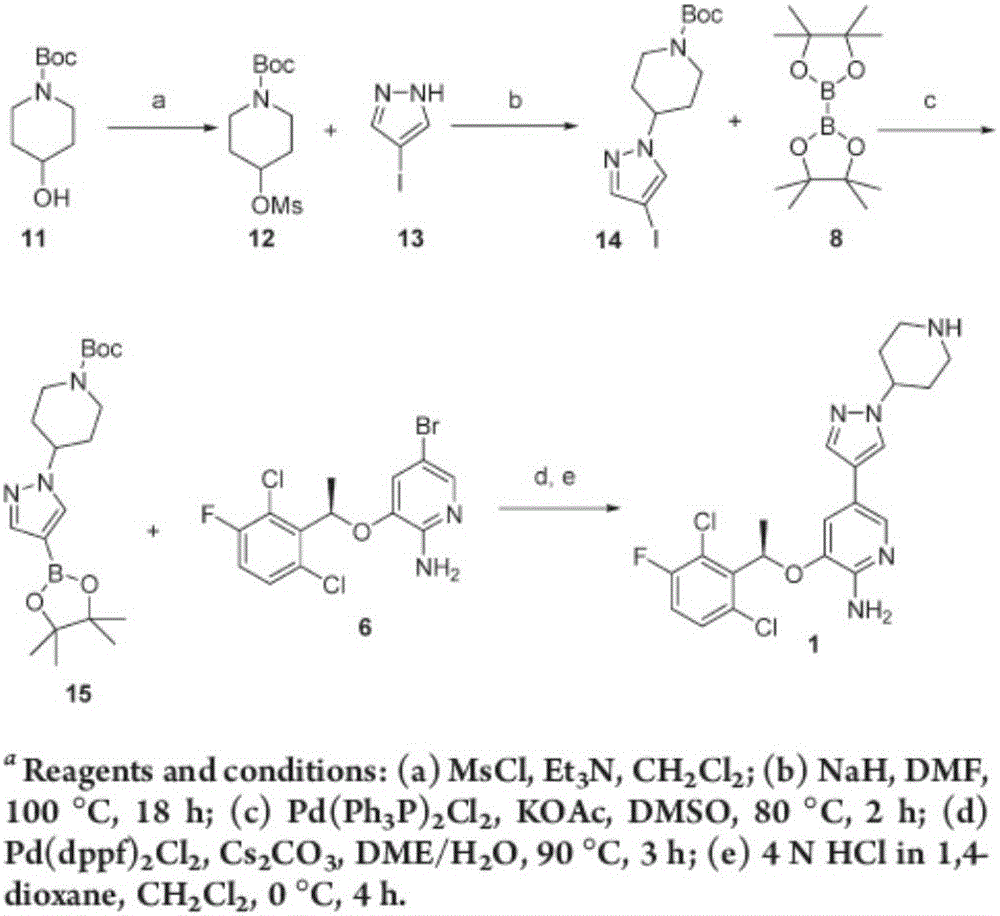
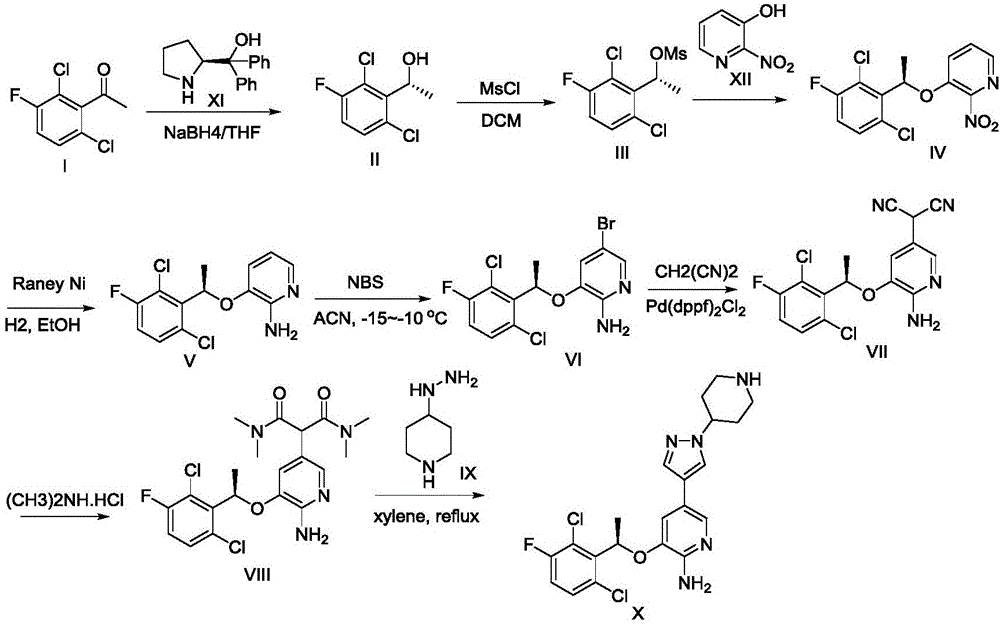
Synthesis process for compound crizotinib
A technology for crizotinib and synthesis process, applied in the field of chemical medicine, can solve problems such as unfavorable industrial production, large usage of precious metals, waste of enantiomers, etc., and achieves easy control of total cost, low usage of catalyst, and total cost. high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) preparation of formula (II) compound
[0040]
[0041] Add formula (I) compound (20.7g, 0.1mol), tetrahydrofuran (200mL) and (S)-diphenylprolinol (2.53g, 0.01mol) in the reaction flask, stir at room temperature for 10min until the solid dissolves completely, use Cool to 1°C in an ice-water bath, add NaBH in batches 4 (4.5 g, 0.12 mol). After the addition was complete, the mixture was raised to room temperature and stirred overnight. TLC showed that the reaction of the raw materials was complete, and the reaction solution was poured into a half-saturated aqueous ammonium chloride solution, stirred for 30 minutes, extracted with ethyl acetate, and the organic phases were combined, and successively washed with 1N HCl, 5% NaHCO 3 Washed with saturated brine, dried over anhydrous sodium sulfate, concentrated acid under reduced pressure to dryness, the yield was quantitative, and the remaining light yellow oil was directly used in the next reaction.
[0042] The com...
Embodiment 2
[0073] (1) preparation of formula (II) compound
[0074]
[0075] Add formula (I) compound (20.7g, 0.1mol), methanol (200mL) and (S)-diphenylprolinol (25.3g, 0.1mol) in the reaction flask, stir at room temperature for 10min until the solid dissolves completely, and use Cool in an ice-water bath to 0-5°C, add NaBH in batches 4 (9 g, 0.24 mol). After the addition was complete, the mixture was raised to room temperature and stirred overnight. TLC showed that the reaction of the raw materials was complete, and the reaction solution was poured into a half-saturated aqueous ammonium chloride solution, stirred for 30 minutes, extracted with ethyl acetate, and the organic phases were combined, and successively washed with 1N HCl, 5% NaHCO 3 Washed with saturated brine, dried over anhydrous sodium sulfate, concentrated acid under reduced pressure to dryness, and the residue was purified by column chromatography to obtain a colorless oil, which was shown to be a racemic product by ...
Embodiment 3
[0077] (1) preparation of formula (II) compound
[0078]
[0079] Add formula (I) compound (20.7g, 0.1mol), 2-MeTHF (200mL) and (S)-diphenylprolinol (12.65g, 0.05mol) to the reaction flask, stir at room temperature for 10min until the solid is completely dissolved , cooled to 0°C with an ice-water bath, and added KBH in batches 4 (13.6 g, 0.36 mol). After the addition was complete, the mixture was raised to room temperature and stirred overnight. TLC showed that the reaction of the raw materials was complete, and the reaction solution was poured into a half-saturated aqueous ammonium chloride solution, stirred for 30 minutes, extracted with ethyl acetate, and the organic phases were combined, and successively washed with 1N HCl, 5% NaHCO 3 Wash with saturated brine, dry over anhydrous sodium sulfate, concentrate acid to dryness under reduced pressure, and purify the residue by column chromatography to obtain a colorless oil, which is 99.5% ee by chiral HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com