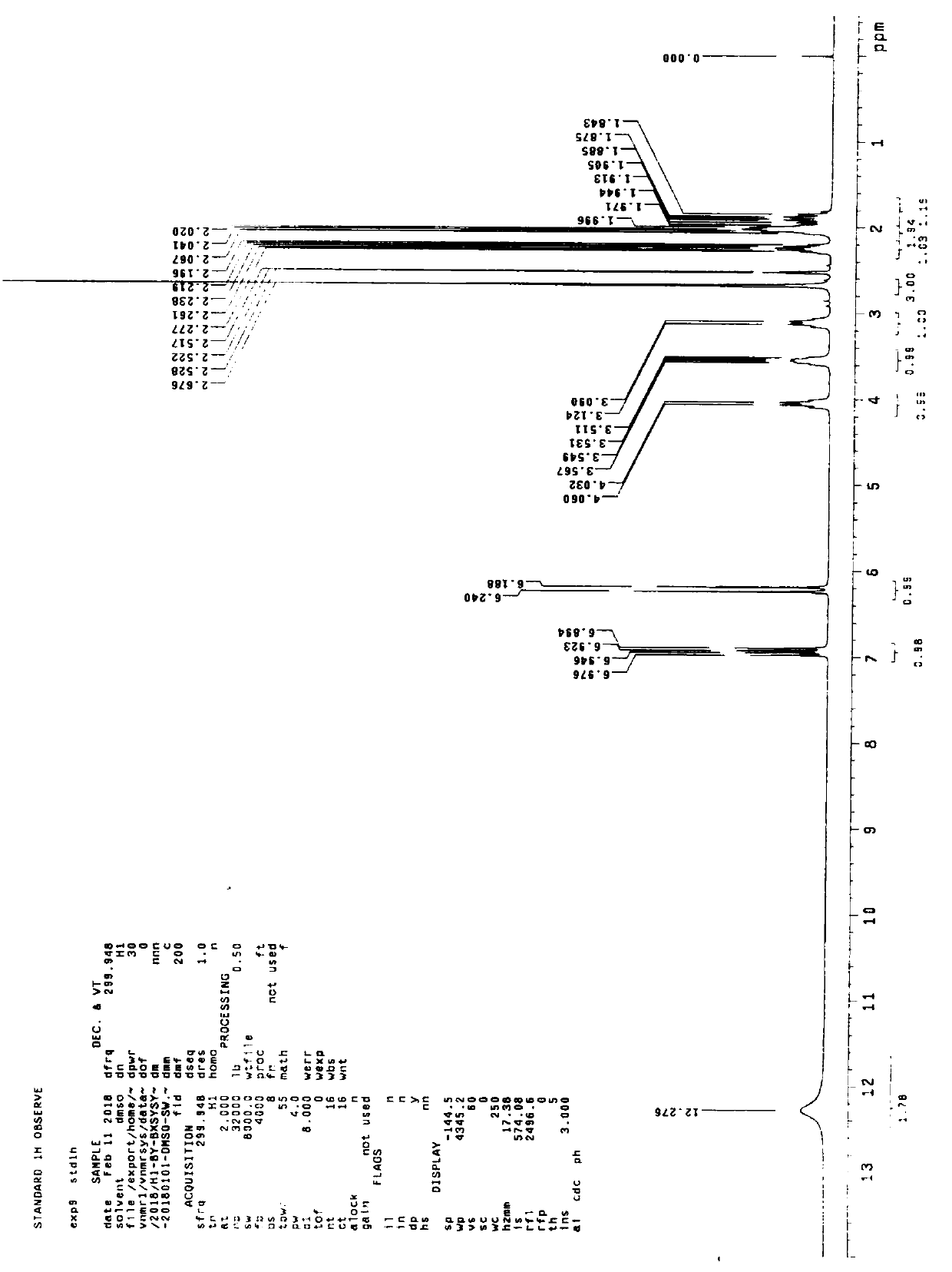
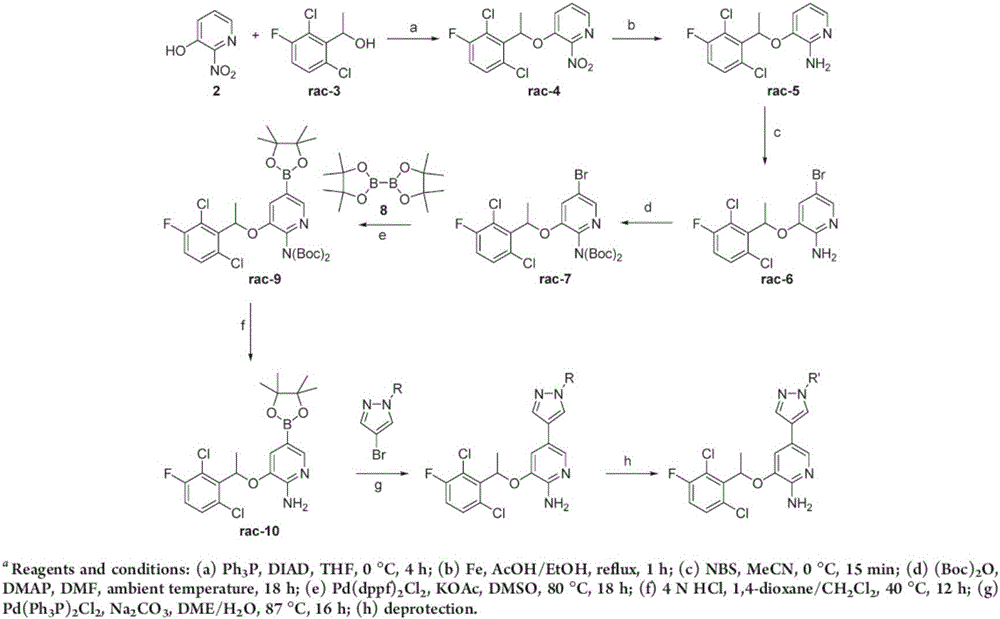
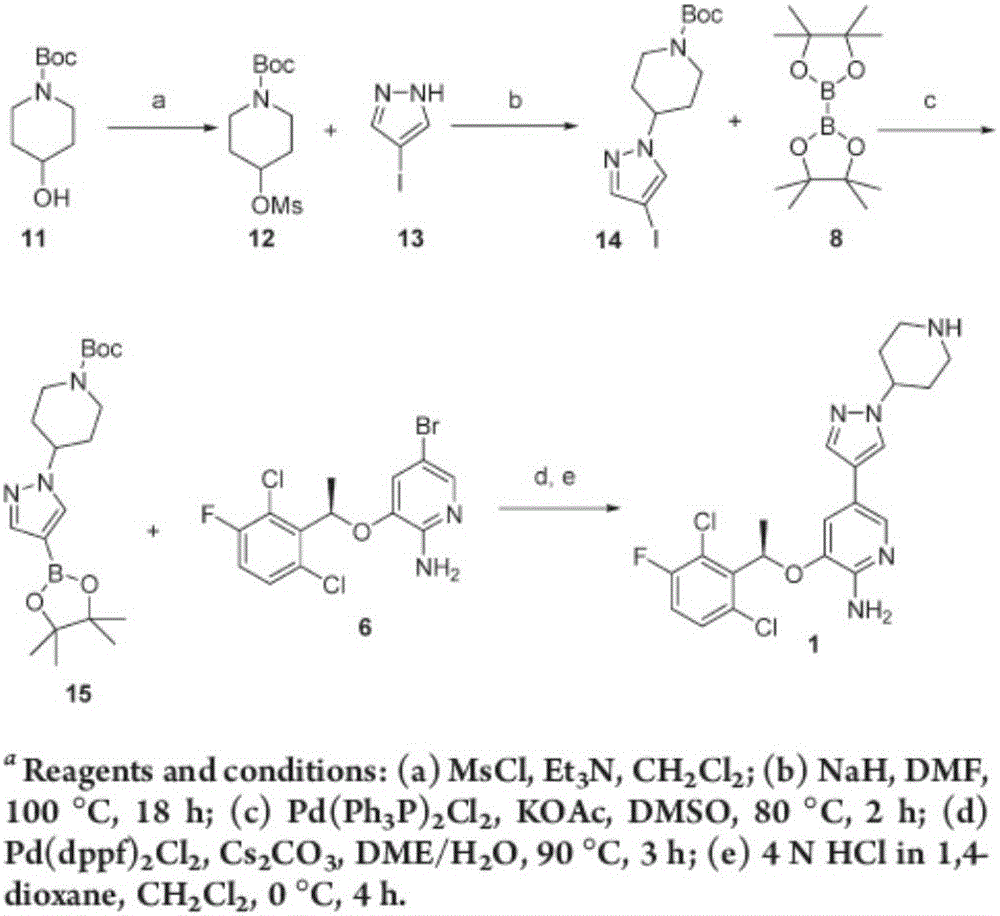
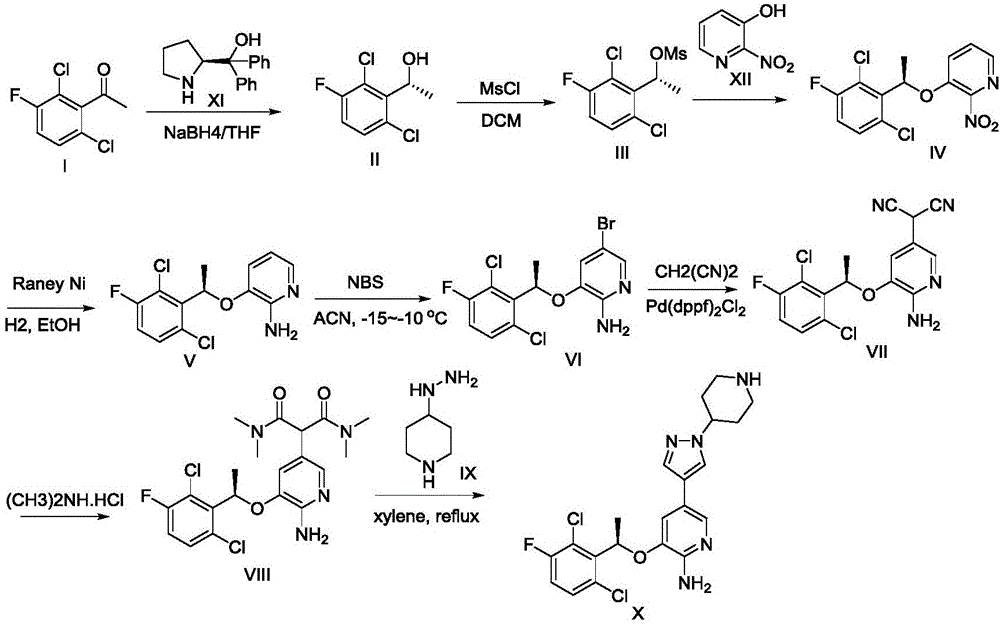
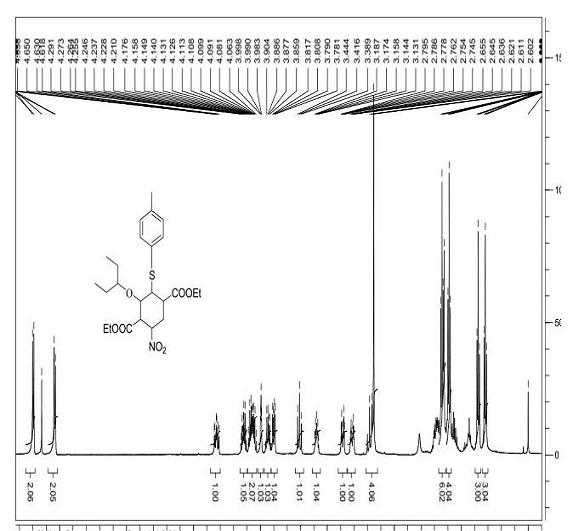
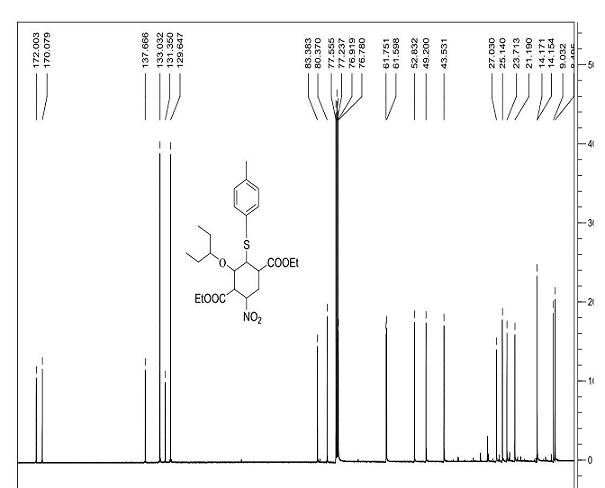
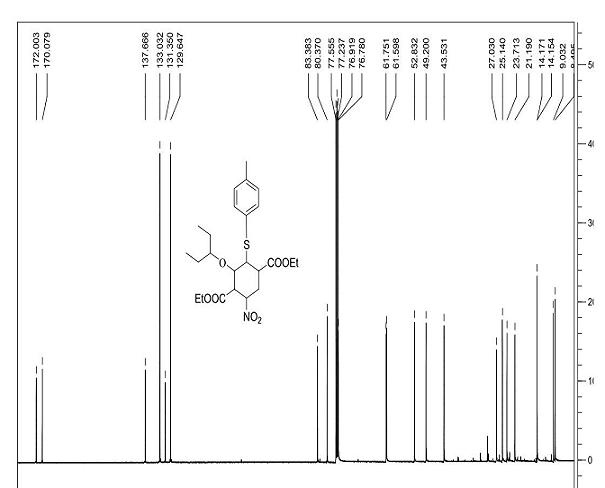
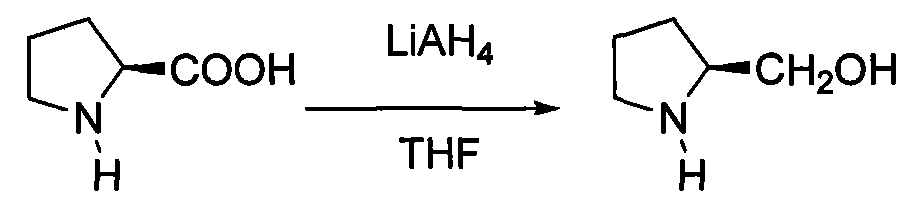
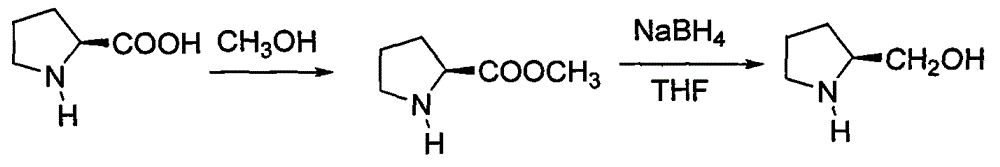
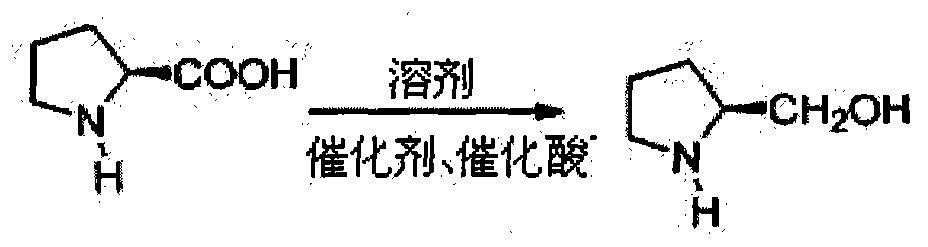
Patents
Literature
68 results about "Prolinol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prolinol is a chiral amino-alcohol that is used as a chiral building block in organic synthesis. It exists as two enantiomers: the D and L forms.
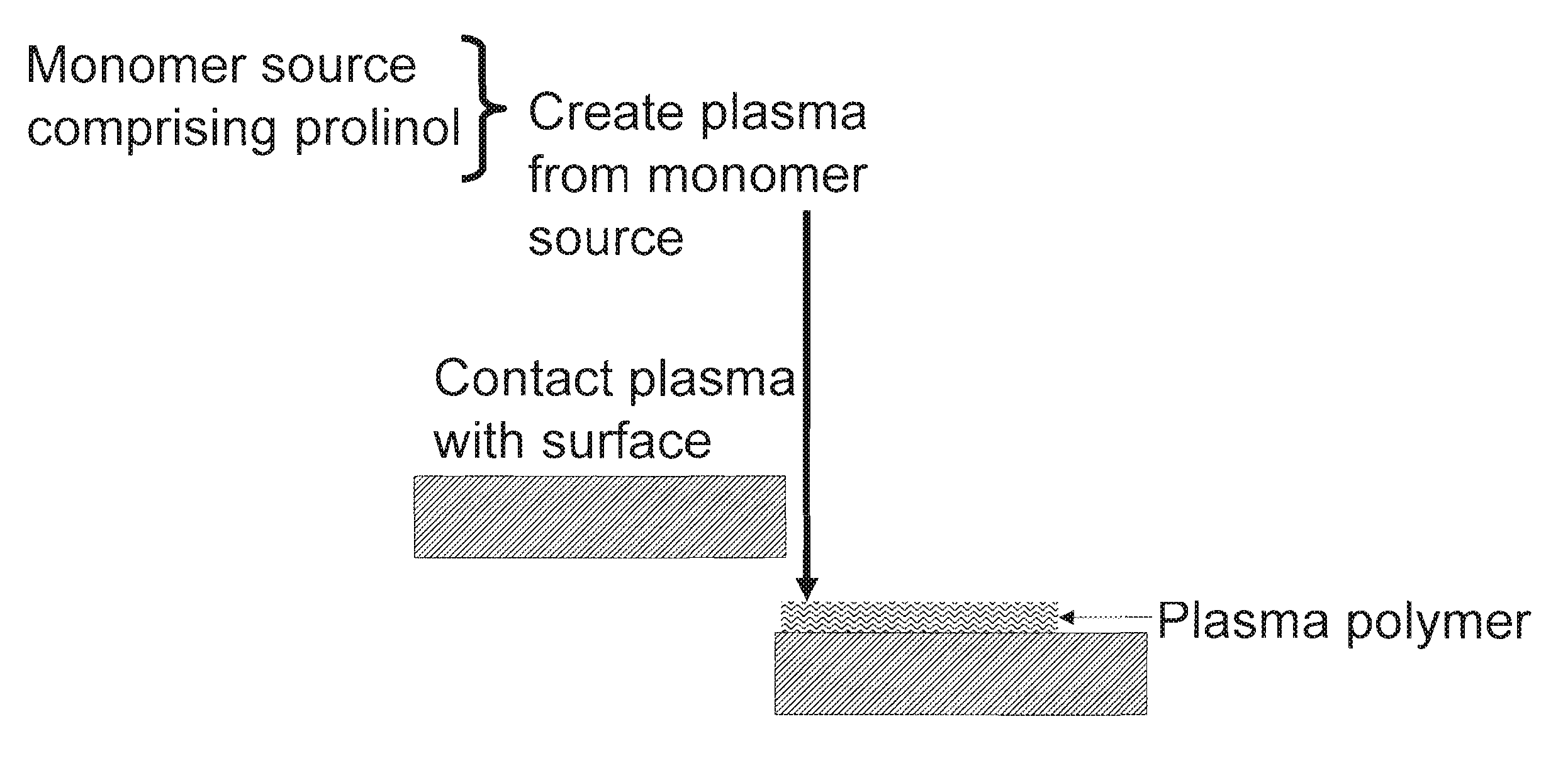
Methods for producing synthetic surfaces that mimic collagen coated surfaces for cell cultue
ActiveUS20100273261A1Low costReduce issueLiquid surface applicatorsCell culture supports/coatingCoated surfaceSimple Organic Compounds
The present invention discloses methods for producing synthetic surfaces that mimic collagen coated surfaces for cell culture comprising: providing a monomer source comprising one or more organic compounds which are capable of polymerization, wherein at least one organic compound is prolinol; creating a plasma of said monomer source; and contacting at least a portion of a surface with the plasma to provide a plasma polymer coated surface. Advantageously, such methods provide an animal-free, synthetic, chemically defined surface that mimics a collagen coated surface for cell culture. Advantageously, such methods not only reduce the cost and / or issues associated with animal-derived collagen but are also amenable to large scale manufacturing.
Owner:CORNING INC
Prolinol derivative induced chiral MOFs material with asymmetric catalysis
ActiveCN101830920AEasy to makeSave raw materialsGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsTert-Butyloxycarbonyl protecting groupProper treatment
The invention discloses a prolinol derivative induced chiral MOFs material with asymmetric catalysis, which belongs to the technical field of a chiral catalytic material. L-BCIP or D-BCIP is used as a chiral source; 5,5'-methylene diiso-phthalic acid, 4,4'-biphenyl acid, 3,3',4,4'-biphenyltetrazole acid or 4,4'-sulfonyl terephthalic acid is used as a connecting ligand; Ln3+ is used as a node; a three-dimensional hole channel structure is constructed by a hydrothermal method; the general formula is as follows: Ln<3+>+L+L-BCIP or D BCIP->Ln-L, wherein Ln<3+> is a rare earth metal ion; L is a connecting ligand; L-BCIP is L-N-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine; and D-BCIP is D-N tert-butoxycarbonyl-2-imidazole-1-pyrrolidine. The material can be used as a heterogeneous catalyst used for an asymmetric silicon cyanation reaction; therefore, the catalyst can be recycled with a yield of 100% and ee value of 99%; and the chiral MOFs material has good application prospect in the aspects of synthesis of pure enantiomer compounds, synthesis of medical intermediates and the like. The material can be recycled after proper treatment.
Owner:DALIAN UNIV OF TECH
Novel synthesizing method of dapoxetine
InactiveCN103304434AHigh yieldHigh stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationHydroxylaminePtru catalyst
The invention discloses a novel synthesizing method of dapoxetine, wherein a series of reactions are carried out on trans-cinnamaldehyde and N-carbobenzoxy hydroxylamine which serve as initiative raw materials under the action of a self-prepared catalyst (s)-alpha-alpha-diisopropyl dimethyl tert-butyl silicon oxygroup prolinol, so that an important intermediate of the dapoxetine, namely an important chiral intermediate (S)-3-amino-3-phenyl propyl alcohol (compound 4), is obtained. The invention further discloses a preparation method of capecitabine, wherein the compound serving as a raw material is methylated and protected by hydroxyl and then reacts with 1-naphthol to form a salt, so that the capecitabine is obtained. The preparation method is easy in raw material obtainment, good in stereoselectivity and high in yield, thereby being suitable for industrial production.
Owner:湖南欧亚药业有限公司
Synthetic method of benzopyran chiral compound
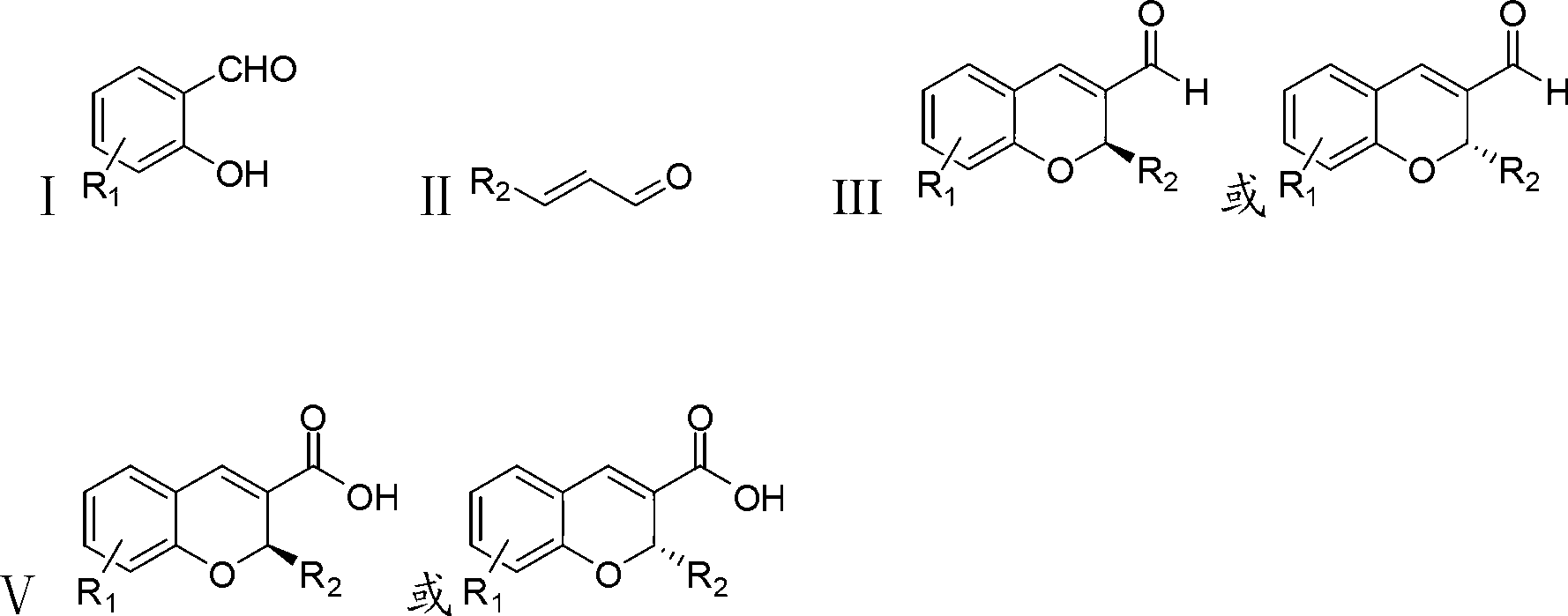
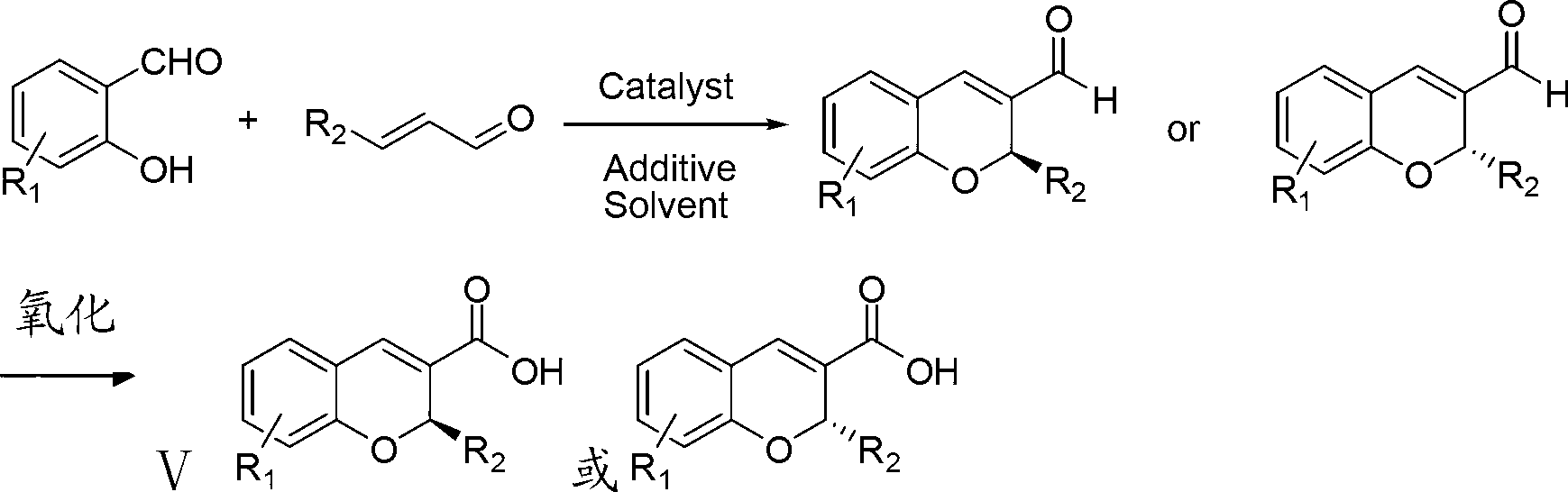
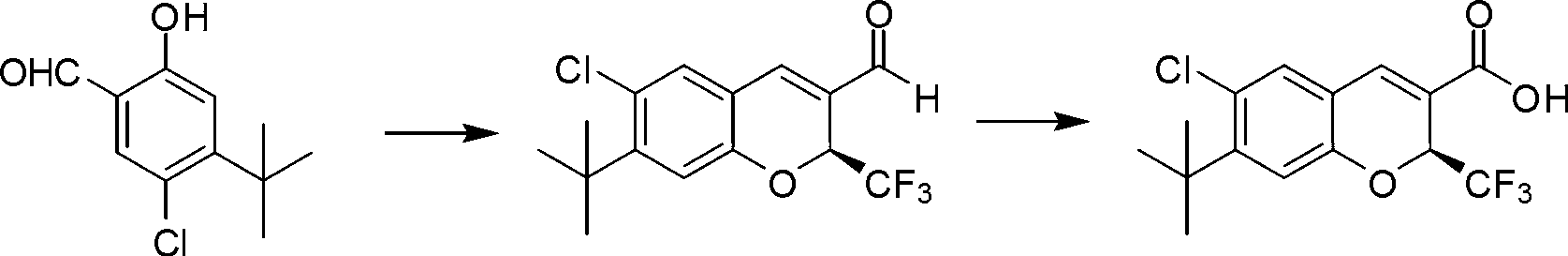
The invention discloses a synthetic method of a benzopyran chiral compound. The method comprises the following steps: a compound with the structural formula I and a compound with the structural formula II carry out the condensation reaction to generate a compound with the structural formula III, and the compound with the structural formula III is oxidized to obtain the benzopyran chiral compound; the chemical additive adopts a benzoic acid or a substituted benzoic acid compound; and the chiral catalyst is a diphenyl prolinol ester compound or dinaphthyl prolinol ester compound. The synthetic method has the advantages that under the mild condition, relatively cheap raw materials are used, the benzopyran chiral compound derivate is synthesized with high yield, and the derivate is high in optical purity, accessible in raw material, and beneficial for large-scale production and application.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
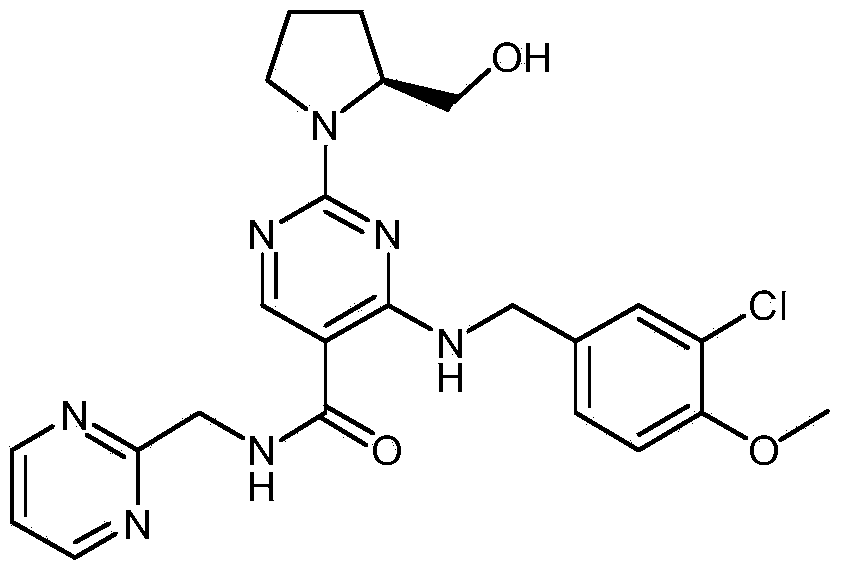
Avanafil
InactiveCN103483323ASimple structureImprove product qualityOrganic chemistryRoom temperature4-methoxybenzylamine
The invention provides an avanafil intermediate A and an avanafil intermediate B, and a synthetic method of the avanafil intermediates A and B and avanafil. The synthetic method of the avanafil comprises the following steps: reacting a compound with the formula (I) with 2-methylaminopyrimidine at a temperature ranging from -10 DEG C to 5 DEG C to obtain the avanafil intermediate A; agitating the avanafil intermediate A with 3-chloro-4-methoxybenzylamine at the temperature ranging from 0 DEG C to 3 DEG C and reacting for 0.2-0.4 hour to obtain the avanafil intermediate B; and agitating the avanafil intermediate B with L-prolinol at the room temperature and reacting for 18-22 hours to obtain the avanafil. The structural formulas of the avanafil intermediates A and B and the compound with the formula (I) are shown in the specification. The avanafil intermediates A and B provided by the invention are simple in structure and good in product quality; the cost of the synthetic method is low; the synthetic route of the whole process is short and reaction steps are few, so that the reaction time is shortened and the yield and the purity of the avanafil intermediates A and B, and the avanafil are also improved.
Owner:SUZHOU UUGENE BIOPHARMA
Compound (e)-3-(1-methylpyrrolidin-2-yl)-acrylic acid hydrochloride and its synthesis method
The invention discloses a compound (E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride and a synthetic method. The compound is structurally as shown in a formula (I). The synthetic method of the compound comprises the following steps: using BOC-L-prolinol (or BOC-D-prolinol) as an initial material, and by oxidization, forming aldehyde; removing a BOC protective agent; then reacting with haloalkane; then by a Wittig reaction, synthesizing (S,E)-3-(1-methylpyrrolidine-2-yl)-ethyl acrylate; after hydrolysis, salifying to obtain (S,E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride [or (R,E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride]. The compound, as a medical intermediate, can be used for preparing quinazoline or quinolines medicine derivatives. The formula is shown in the description.
Owner:SHANDONG BOYUAN PHARM CO LTD
Synthesis process for compound crizotinib
The invention provides a new synthesis method for crizotinib. An atomic economic reaction is adopted to reduce environmental pollution. A high-optical purity raw material is obtained by chiral prolinol induced chiral reduction; a chiral centre is constructed through an SN2 substitution reaction; post-processing and purification difficulties caused by Mitsunobu reaction are overcome. Malononitrile derivative is constructed by adopting a coupling reaction of malononitrile and bromo-pyridinium derivative; N,N-dicarboamide derivatives are obtained by performing aminolysis on N,N-dimethylamine hydrochloride; in the N,N-dicarboamide derivatives, N,N-dimethylamine serving as an easy-to-leave group and hydrazine perform a ring closing reaction to construct a pyrazolone ring, so that an expected final product, namely crizotinib, is obtained. According to the method, though continuous steps are used, the reaction of each step is high, the optical purity is high, and the total yield is also high. In addition, raw materials used in the synthesis method are low in cost and easily obtained; the using amount of a catalyst is small; total cost is easy to control. An operating process is simple and convenient and easy to control, and is suitable for industrial production.
Owner:甘肃皓骏药业有限公司
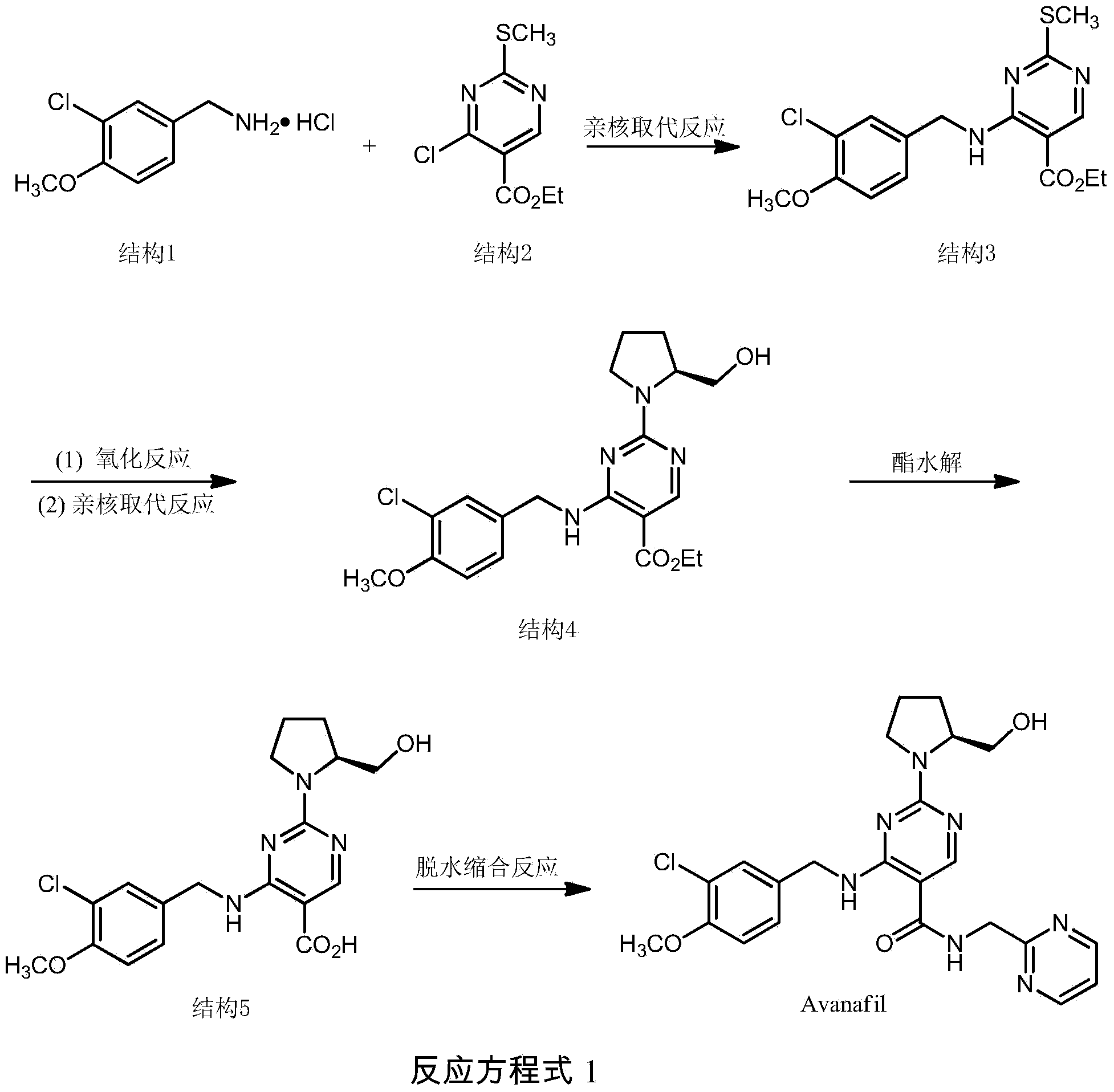
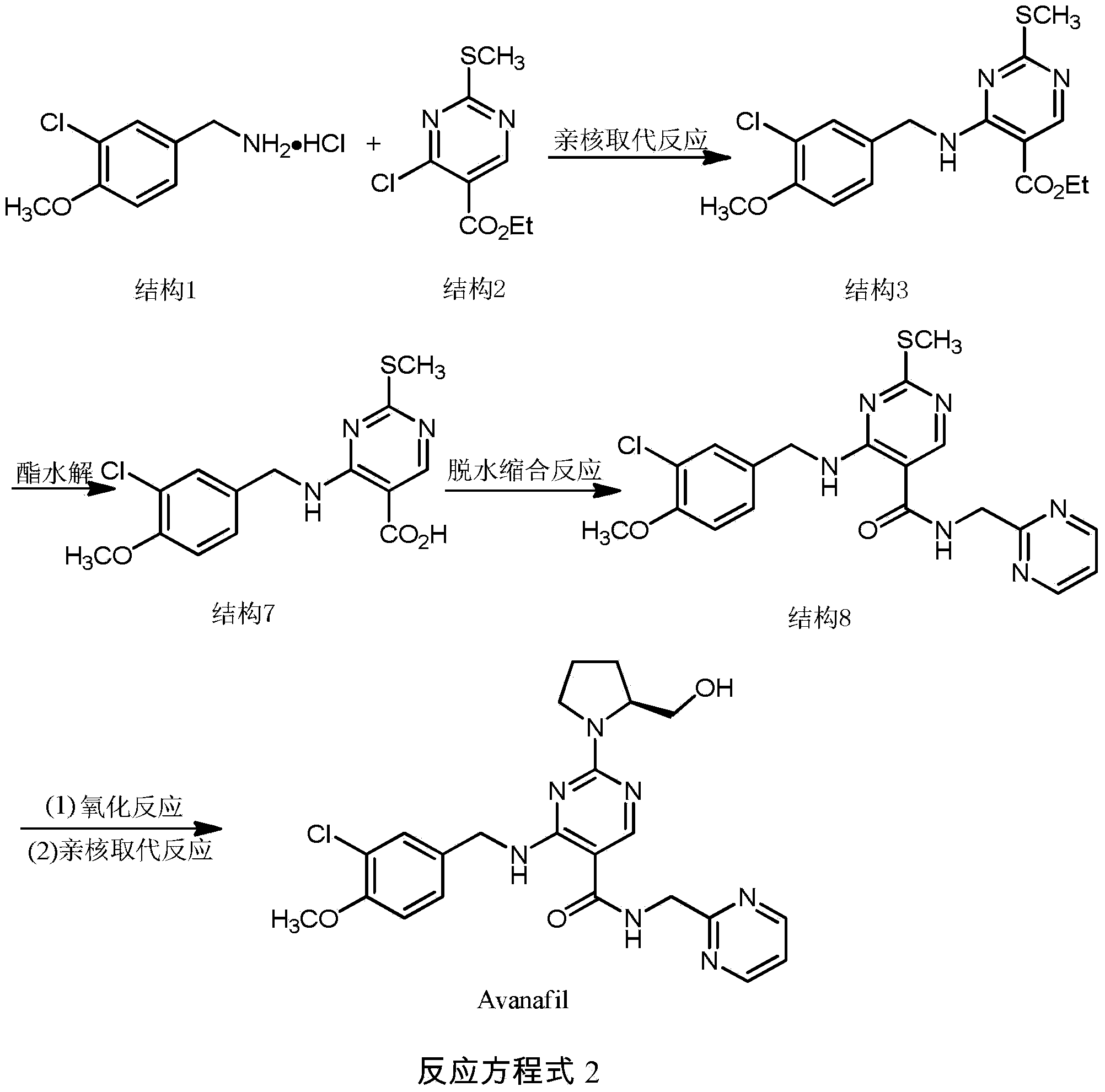
Synthesis method of avanafil
InactiveCN104003981AHigh purityRaw materials are cheap and easy to getOrganic chemistryChemical synthesisSynthesis methods
The invention relates to the field of the organic chemistry synthesis and in particular relates to a synthesis method of avanafil. The method comprises the following steps: carrying out nucleophilic substitution on raw materials which are 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine and 3-chloro-p-methoxy benzyl amine hydrochloride to obtain 4-(3-chloro-4-methoxy benzyl amine hydrochloride)-5-ethoxycarbonyl-2-methylthiopyrimidine, then sequentially carrying out oxidation reaction, nucleophilic reaction with L-prolinol, esterolysis and dehydration synthesis to finally obtain avanafil. The method has the advantages that the used raw materials are cheap and easily available; the cost is low; the operation is simple and convenient; the prepared final product has few impurities and is of a solid state. The final product can be directly re-crystallized to obtain a pure product; the product is high in purity and suitable for industrial production.
Owner:HEBEI KANGTAI PHARMA
Chirality sulfonamide aminol ligand based on prolinol, its preparing method and application
The invention discloses a making method and application of chiral sulfonamide amide-alcohol ligand based on proamide-alcohol, which is characterized by the following: adopting L-proamide-alcohol as raw material; making each ligand framework possess 1-3 chiral center; proceeding selectivity loop-opening reaction for cycloethyliminum; improving receiving rate obviously; adopting chiral sulfonamide amide-alcohol ligand and similarity as catalyst ligand in the asymmetrical synthetic reaction; fitting for applying in the asymmetrical diethyl zinc additioning reaction, alkynyl zinc additioning reaction for carbonyl compound, reducing reaction and aldehyde alcohol condensing reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Optically active 3-substituted indole derivatives as well as synthesis method and application thereof
ActiveCN104557665AHigh enantioselectivityGood inhibitory effectOrganic chemistryAntineoplastic agentsBenzoic acidChemical synthesis
The invention discloses optically active 3-substituted indole derivatives as well as a synthesis method and an application thereof. The optically active 3-substituted indole derivatives are with structures as shown in a formula (Ia) and a formula (Ib). The target products which are the optically active 3-substituted indole derivatives are prepared through one-step reaction at the temperature of 0-40 DEG C, wherein the raw materials include a diazo compound, an indole derivative and an alpha, beta-unsaturated aldehyde, the water absorbent is a 4-angstrom molecular sieve, the catalysts include a metal catalyst, chiral diaryl prolinol silicon ether and substituted benzoic acid, and the solvent is an organic solvent. The synthesis method disclosed by the invention has the advantages of high atom economy, high selectivity, high yield and mild reaction condition and is easy and safe to operate. The pair of optically active 3-substituted indole derivatives has high enantioselectivity and bioactivity and can be used for preparing antitumor medicines.
Owner:广东和博制药有限公司
Synthesis process of (S)-rivastigmine
ActiveCN103304447AFew stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateFormate
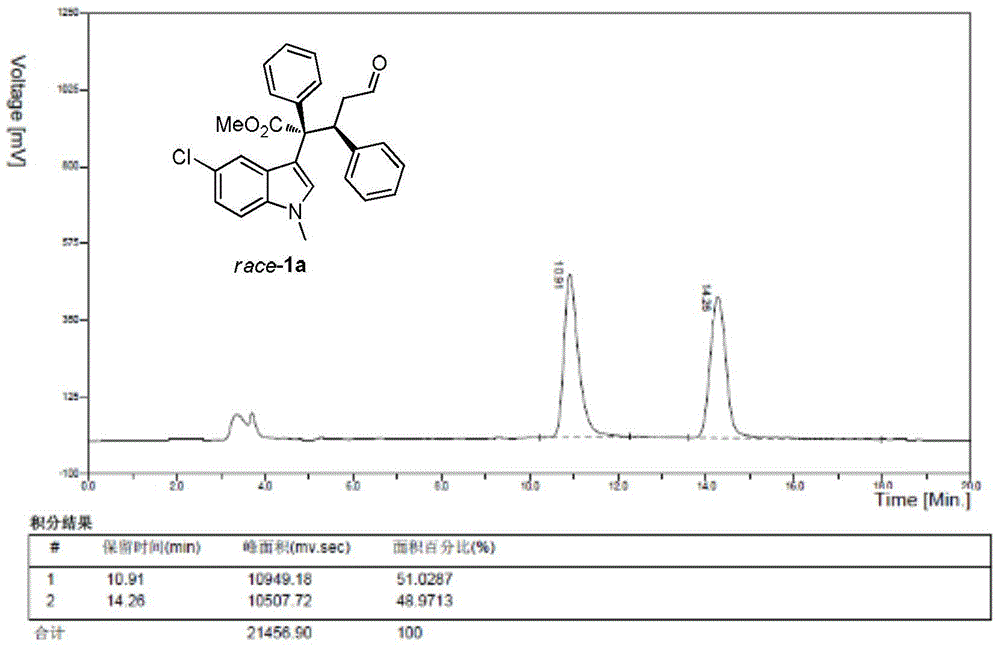
The invention discloses an unsymmetrical synthesis method of (S)-rivastigmine. The method comprises the following steps of: reacting hydroxyacetophenone with methylethylcarbamoyl chloride to generate N-ethyl-N-methyl-carbamate 3-acetylphenyl formate, carrying out chiral reduction on the N-ethyl-N-methyl-carbamate 3-acetylphenyl formate under the catalysis effect of a complex of (S)-(-)-alpha, alpha-diphenyl prolinol and trimethyl borate, carrying out methanol dissociation to generate N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate, and reacting the N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate with methylsufonyl chloride and dimethylamine hydrochloride to obtain the (S)-rivastigmine. According to the method disclosed by the invention, a target product can be prepared from conventional reagents only through a three-step reaction, the reaction yield reaches 40% and is far higher than that by a racemic resolution method and that by other chiral synthesis methods, the application of virulent reagents and highly corrosive reagents are avoided in the process, and the unsymmetrical synthesis method is easy to operate, environment-friendly, low in production cost, and suitable for industrialized production.
Owner:YABAO PHARMA GRP CO LTD
Compound (E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride and synthetic method
The invention discloses a compound (E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride and a synthetic method. The compound is structurally as shown in a formula (I). The synthetic method of the compound comprises the following steps: using BOC-L-prolinol (or BOC-D-prolinol) as an initial material, and by oxidization, forming aldehyde; removing a BOC protective agent; then reacting with haloalkane; then by a Wittig reaction, synthesizing (S,E)-3-(1-methylpyrrolidine-2-yl)-ethyl acrylate; after hydrolysis, salifying to obtain (S,E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride [or (R,E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride]. The compound, as a medical intermediate, can be used for preparing quinazoline or quinolines medicine derivatives. The formula is shown in the description.
Owner:SHANDONG BOYUAN PHARM CO LTD
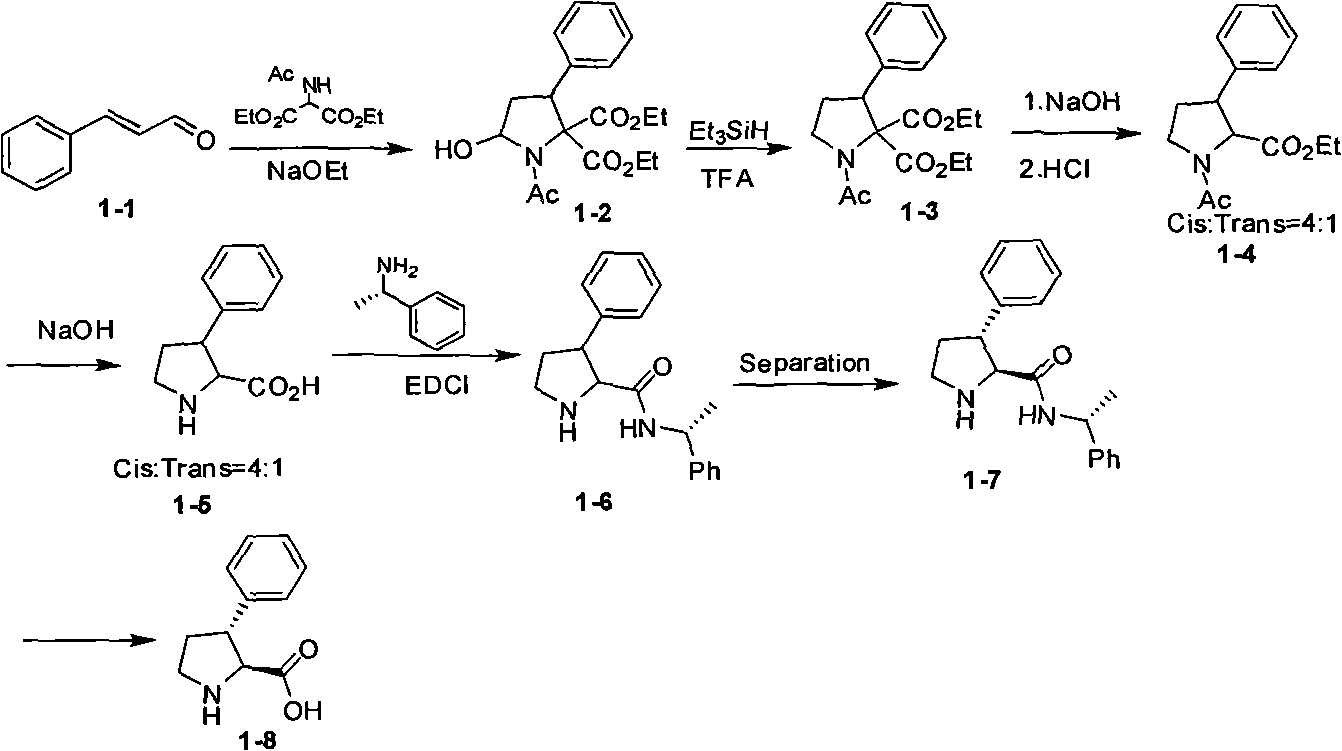
Synthesis method of cis 3-phenyl substituted s-proline derivative
InactiveCN101863815AHigh stereoselectivityMild reaction conditionsAsymmetric synthesesSynthesis methodsPhenyl group
The invention provides an optical isomers pure cis 3-phenyl substituted s-proline derivative synthesis method. A route is as follows: taking the substituted cinnamaldehyde and nitroethyl alcohol as raw materials, taking (S)-diphenyl prolinol trimethyl silicyl oxide as an asymmetric catalyst, and obtaining the optical isomers pure cis 3-phenyl substituted s-proline derivative through asymmetric michael addition reaction, hydrogenation and boc protection reaction, and oxidation and deprotection reaction. The synthesis method has the advantages of high yield, good three-dimensional selectivity, mild reaction conditions, convenient post-treatment, no need of column chromatography purification, low cost and the like, and is a synthesis route with industrial prospect.
Owner:JIAXING EPOCHEM PHARMTECH
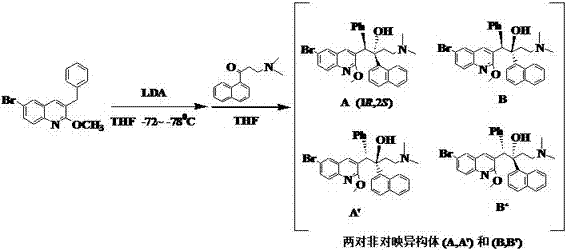
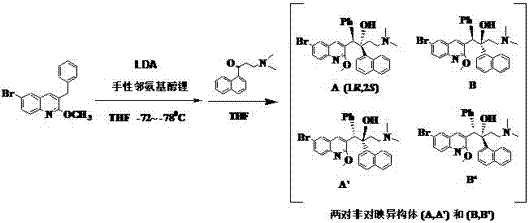
Chiral inducer for synthesizing (1R,2S)-Bedaquiline
The invention relates to a chiral inducer for synthesizing (1R,2S)-Bedaquiline. Di(isopropyl)lithium ammonium takes off benzyl-bit protons from 6-bromo-3-benzyl-2-methoxy quinoline at a low temperature in the presence of the chiral inducer, i.e., lithium N-benzyl-L-prolinol and then is subjected to addition with 3-dimethylamino-1-naphthyl-1-acetone. Chiral o-amino lithium alkoxide remarkably increases the proportion of a target enantiomer, i.e., (1R,2S)-Bedaquiline and can be used for further preparing a drug, i.e., (1R,2S)-Bedaquiline fumarate.
Owner:FUJIAN INST OF MICROBIOLOGY
Mesoporous hollow silicon dioxide nanospheres loaded with prolinol catalyst as well as preparation method and application of mesoporous hollow silicon dioxide nanospheres
ActiveCN112121853AIncrease loadReduce thicknessOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhysical chemistry
The invention discloses mesoporous hollow silicon dioxide nanospheres loaded with a prolinol catalyst as well as a preparation method and application of the mesoporous hollow silicon dioxide nanospheres. Mesoporous silicon dioxide with an ultra-large specific surface area is firstly prepared, a shell layer is very thin, and the maximization of the loading capacity is ensured. The mesoporous hollowsilicon dioxide nanospheres are effectively combined with the prolinol catalyst, and the defect that silicon dioxide is not easy to activate is overcome. The hollow silicon dioxide nanospheres and the prolinol catalyst are effectively combined and successfully applied to Michael / Michael / aldol cascade reaction with strict catalytic requirements, and a good result (ee is close to 100%, and Dr is 98: 2) is obtained. The catalytic effect is decreased progressively only after the catalyst is recycled for 8 times, which indicates that the structure of the silicon dioxide spheres is stable and a method for combining a hydrolysis method and an organic group is very suitable.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Preparation method of avanafil
The invention provides a preparation method of avanafil, and specifically relates to the technical field of pharmaceutical chemistry. The preparation method of avanafil comprises the following steps:sequentially carrying out cyclization and chlorination reactions on methyl thiourea sulfuric acid and diethyl ethoxymethylene malonate which serve as initial raw materials to obtain 4-chloro-2-methylthiopyrimidine-5-carboxylic acid ethyl ester; then substituting and hydrolyzing with 3-chloro-4-methoxybenzylamine; condensing with 2-pyrimidinemethanamine to obtain a key intermediate, namely, 4-[(3-chloro-methoxybenzyl)amino]-2-methylthio-N-(2-pyrimidine methyl)-5-pyrimidine formamide; oxidizing the intermediate and reacting with L-prolinol to generate the avanafil. The preparation method has theadvantages of easy availability of raw materials, easiness and convenience in operation, mild reaction conditions and higher product yield.
Owner:苏州盛达药业有限公司
Synthesis method of 1,3-disubstituted allene with high optical activity
ActiveCN104193568AReduce dosageWide applicabilityCarbamic acid derivatives preparationCarboxylic acid esters preparationSynthesis methodsAlkyne
The invention discloses a synthesis method of 1,3-disubstituted allene with high optical activity, namely a method for preparing 1,3-disubstituted allene with high optical activity in one step of functionalizing terminal alkyne, aldehyde and chiral alpha, alpha-diphenyl-L-prolinol under catalysis of a bivalent copper salt. The method is simple to operate, adopts easily available raw materials and reagents, uses a substrate with wide universality, can be compatible with a plurality of functional groups, such as a plurality of glycosidic units, primary alcohol, secondary alcohol, tertiary alcohol, amides and dimethyl malonate, and does not need further protection; and the obtained axial-chirality allene is moderate to good in yield and excellent in diastereoselectivity or enantioselectivity.
Owner:ZHEJIANG UNIV
Tamiflu intermediate and synthesis method thereof
InactiveCN102180821AHigh yieldReduce manufacturing costSulfide preparationOrganic solventSynthesis methods
The invention relates to a Tamiflu intermediate and a synthesis method thereof, solving the technical problems of effectively reducing the production cost of the Tamiflu intermediate and effectively improving the yield of the Tamiflu intermediate and the content of the Tamiflu intermediate in a product. The method comprises the following steps: 1) catalytic reaction: in an organic solvent, reacting 3-pentyloxy acetaldehyde (1) and 3-nitro ethyl acrylate (2) in a molar ratio of 2: (1-2) in the presence of salt which is prepared by premixing (R)-N,N-dimethlbenzylamine prolinol silicon ether (3) with Bronzed acid in a molar ratio of 1: (1-8), so as to obtain an addition product (4); 2) carrying out reaction on the addition product (4) and 2-(diethoxy, phosphoryl) ethyl acrylate (5) in a molar ratio of 1: (1-2) and carrying out a reaction on cesium carbonate and the 2-(diethoxy, phosphoryl) ethyl acrylate (5) in a molar ratio of 2:1, so as to produce a produce (6); and 3) carrying out michael addition on the product (6) and p-methylthiophenol in a molar ratio of 1: (2-10), so as to obtain cyclohexane derivative, namely Tamiflu intermediate.
Owner:HANGZHOU NORMAL UNIVERSITY
Serial water-soluble hydroxycamptothecine naphthenic amino alcohol derivative and preparation method and use thereof
InactiveCN103113381AInhibit apoptosisInhibit inflammationOrganic chemistryAntineoplastic agentsOxygenTumor cell apoptosis
The invention discloses serial water-soluble hydroxycamptothecine naphthenic amino alcoholderivative. The serial water-soluble hydroxycamptothecine naphthenic amino alcohol derivative is synthesized by leading naphthenic amino alcohol methylene into a hydroxycamptothecine structure for the first time; and the hydroxycamptothecine with strong cell toxicity is decorated into the hydroxycamptothecine naphthenic amino alcohol derivative with medium cell toxicity. Therefore, the selectivity of induction of tumor cell apoptosis is improved; the situation that the hydroxycamptothecine prolinol has special hydrogenation reduction metabolic pathway in the body is found out for the first time; existing camptothecin derivatives do not have special hydrogenation reduction metabolic pathway; and active oxygen in the cell can be controlled, so as to inhibit cell proliferation, selectively induce cancer cell apoptosis, and inhibit cell inflammatory necrosis. Normal cell function is not affected.
Owner:DALIAN UNIV OF TECH
Prolinol rapid desulfurizer and preparation method thereof
ActiveCN111943935APromote degradationRapid oxidative degradationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRaw materialProlinol
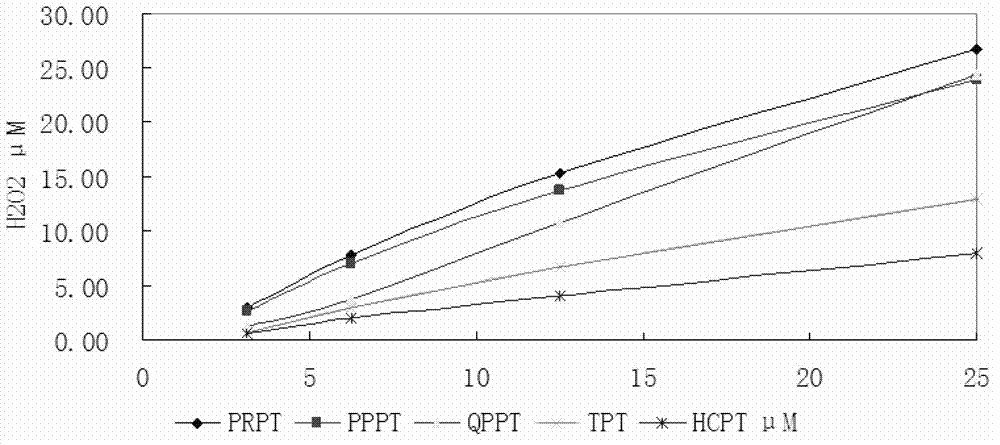
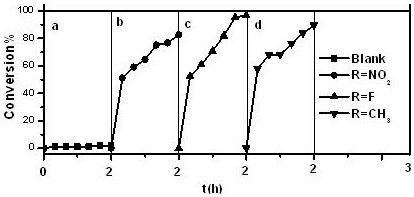
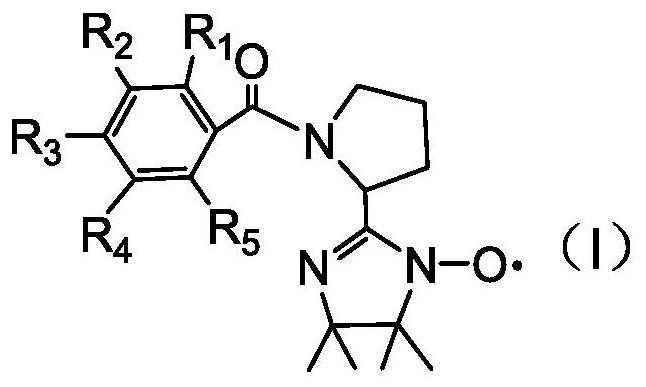
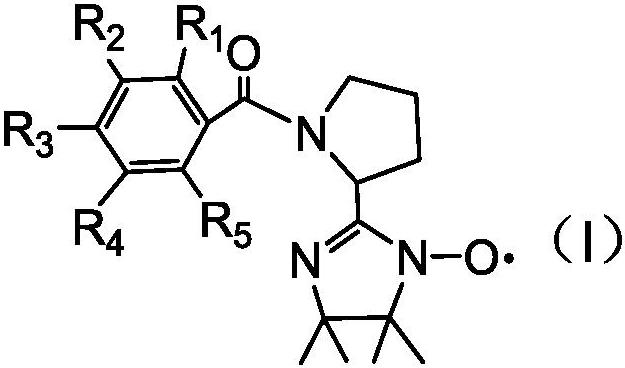
The invention relates to the technical field of environmental protection, in particular to a prolinol rapid desulfurizer and a preparation method thereof. The problems that in the prior art, the visible light utilization rate is very low, point location requirements of various catalytic reactions are difficult to meet at the same time, meanwhile, photo-generated electron hole pairs are easy to compound, and the quantum efficiency is low are solved. The invention provides the prolinol rapid desulfurizer, the general formula of the desulfurizer is shown in the specification, wherein R1 to R5 areH, OCH3, NO2, X (F, Cl, Br), COOH and CF3. The desulfurizer is formed by condensation of R1 to R5 substituted phenylacetaldehyde and 2,3-dimethyl-2,3-dihydroxylamino butane, and R1 to R5 are respectively and independently selected from H, OCH3, NO2, halogen, COOH and CF3. The preparation method is simple, raw materials are easy to obtain, operation is easy, and the method is suitable for large-scale industrial production.
Owner:XIAN TECHNOLOGICAL UNIV
Preparation method of chiral CBS catalyst
ActiveCN105618135AFew reaction stepsEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsAlkanePtru catalyst
The invention provides a preparation method of a chiral CBS catalyst. The preparation method comprises the following steps: by adopting proline as a raw material, protecting nitrogen and carboxylic acid by trimethyl chlorosilane, and reacting with a phenyl grignard reagent to obtain diphenyl prolinol; then reacting with boron trihalide and lithium alkylide, after the reaction is ended, quenching by acetic acid, performing backflow filtering on alkane, and performing cooling crystallization to obtain a chiral product. The process is short in synthetic route, the operation is simple, the raw material is easy to get, the yield and the product purity are high, racemization of a chiral center is avoided in the whole preparation process, and the method is more suitable for large-scale production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
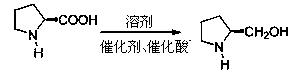
Method for preparing L-prolinol through high-pressure hydrogenization of L-proline
The invention discloses a method for preparing L-prolinol through high-pressure hydrogenization of L-proline. According to the method, L-proline is reduced for 4-12h in a solvent at the temperature of 120-180DEG C under the reaction pressure of 4-10MPa in the presence of a catalyst and catalytic acid, to obtain a target product L-prolinol, wherein the solvent is isopropanol preferably, the catalyst is 5% Ru / C preferably, and the catalytic acid is phosphoric acid preferably. The method for preparing L-prolinol is short in process route, high in yield and simple for aftertreatment.
Owner:江苏恒祥化学股份有限公司
Radiation protection compound as well as synthesis method and application thereof
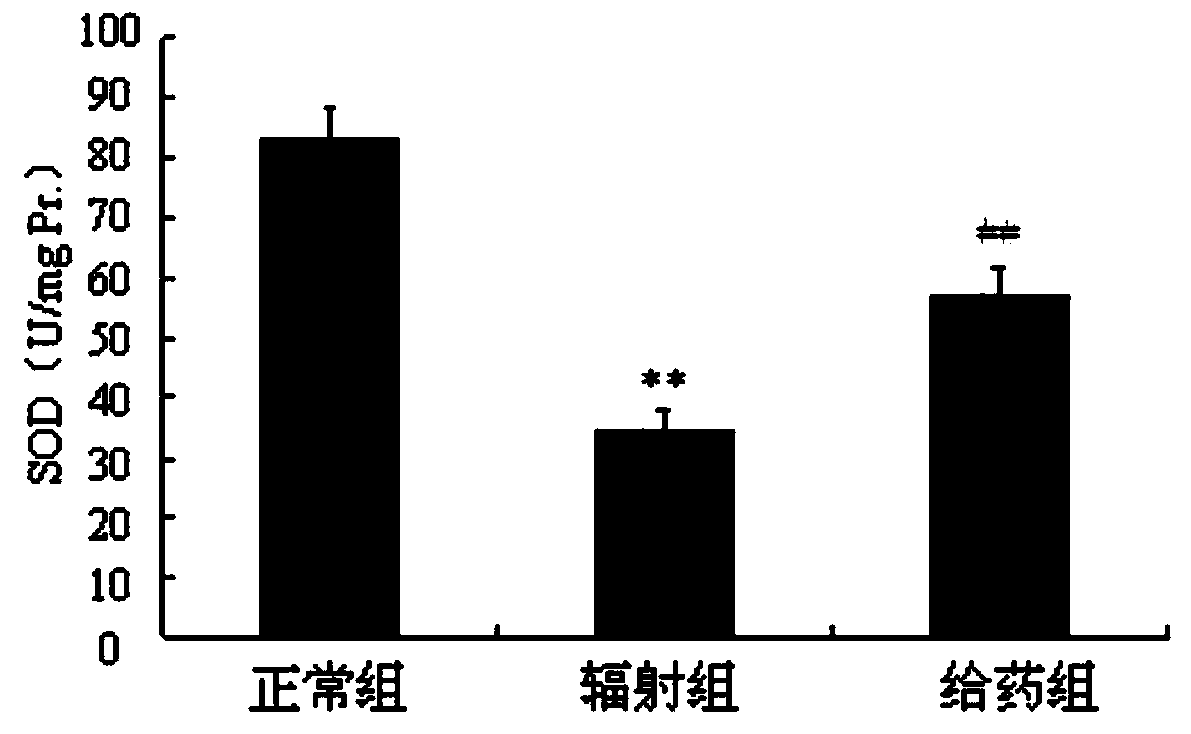
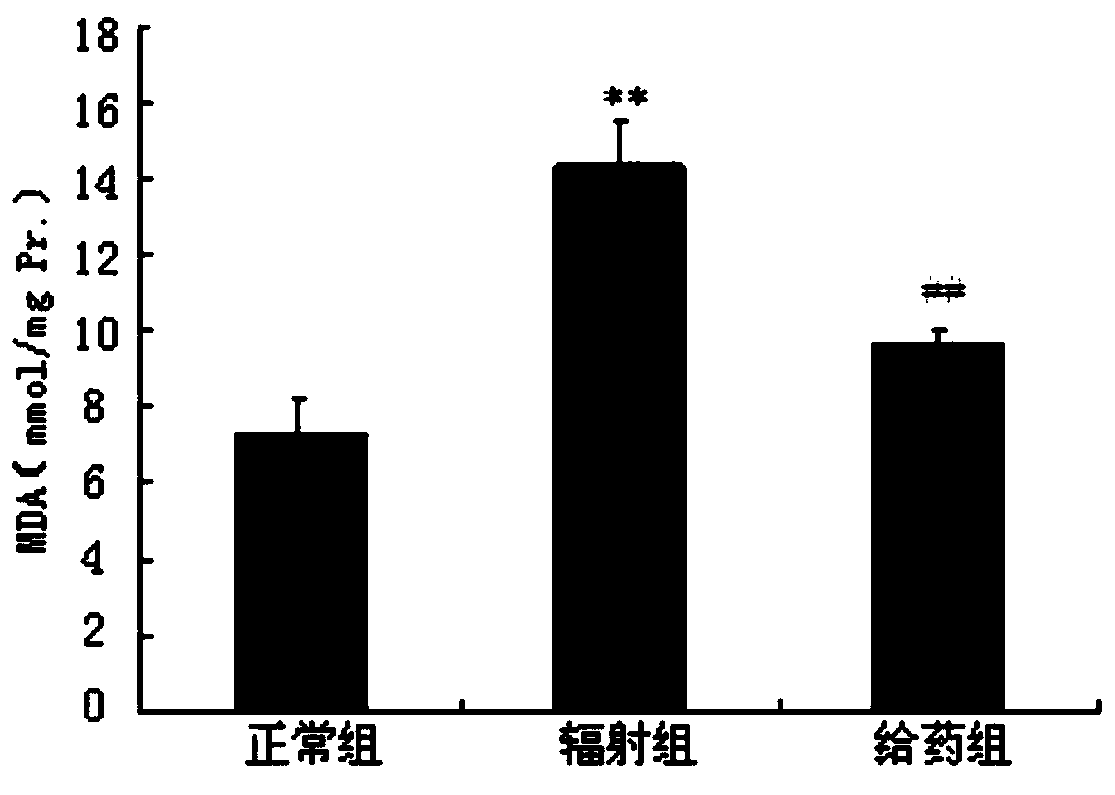
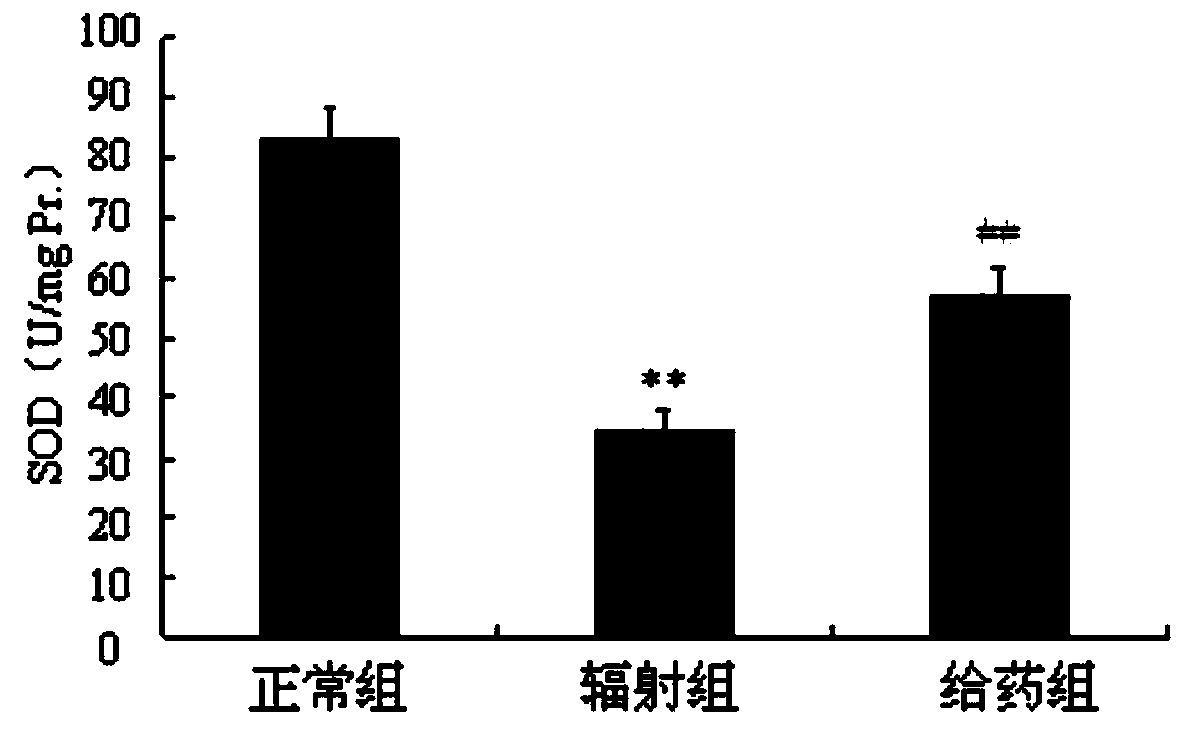
InactiveCN111018913AGood biocompatibilityGood radiation protectionOrganic active ingredientsGroup 5/15 element organic compoundsPharmaceutical SubstancesNitroxide radical
The invention discloses a radiation protection compound with a structural general formula (I) or (II). The synthesis method comprises the steps: enabling triphenylphosphine to react with 6-bromohexanoic acid or 1,6-dibromohexane to obtain a triphenylphosphine cationic compound, sequentially performing acylation, etherification, reduction and oxidation on L-prolinol or D-prolinol, and combining with the triphenylphosphine cationic compound to obtain TPP-L / D-NIT-1 or TPP-L / D-NIT-2; the radiation protection compound is applied to the anti-radiation damage pharmaceutical composition. The radiationprotection compound is a mitochondria targeting nitroxide free radical compound with chirality and has an obvious radiation protection effect at an animal level, and a chiral prolinol structural unitin the radiation protection compound is similar to a proline structure in a biological organism, so that the biocompatibility is better; the synthesis method disclosed by the invention is mild in condition and good in reproducibility; the radiation protection compound provided by the invention has relatively high practical value.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for high pressure hydrogenation preparation of L-prolinol from L-proline
The invention discloses a method for high pressure hydrogenation preparation of L-prolinol from L-proline. The method comprises that L-proline in a solvent is reduced in the presence of a catalyst and a catalysis acid at a temperature of 120-180 DEG C under reaction pressure of 4-10MPa for 4-12h to produce a desired product L-prolinol, wherein preferably, the solvent is isopropanol, the catalyst is Ru / C with content of 5% and the catalysis acid is phosphoric acid. The method for high pressure hydrogenation preparation of L-prolinol has the advantages of short technology route, high yield and simple and easy post-treatment processes.
Owner:吴志明
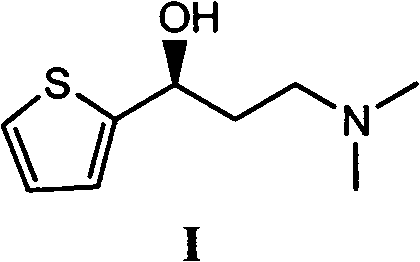
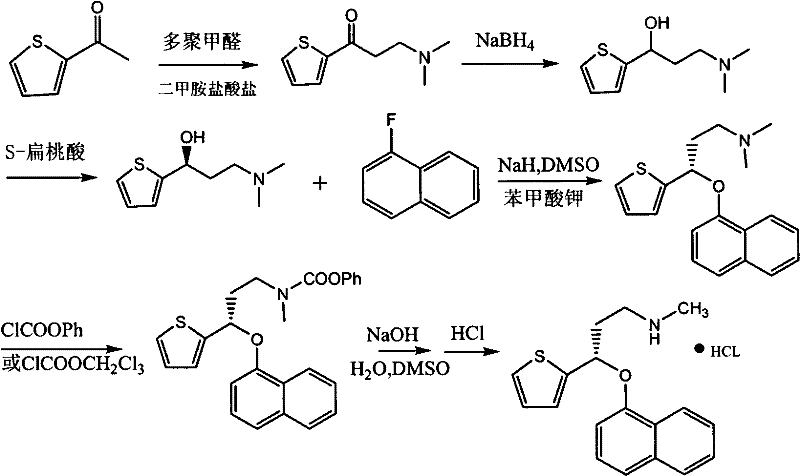
Asymmetric synthesis method of duloxetine intermediate-(S)-N, N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propylamine
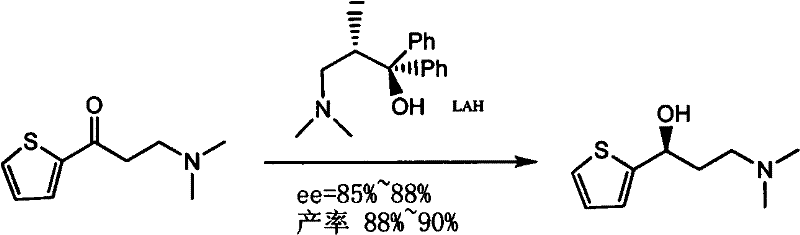
The invention provides an asymmetric synthesis method of duloxetine intermediate-(S)-N, N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propylamine, which mainly comprises the following steps that: the (S)-N, N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propylamine is obtained by reduction in the presence of (R)-(+)-alpha alpha-diaryl prolinol or (R)-(+)-alpha, alpha-diaryl prolinol silyl ether which is used as a catalyst and metal hydride complexes such as sodium borohydride and potassium borohydride which are used as a reducing agent. The method is simple and feasible, has high yield and optical purity, and is suitable for large-scale production.
Owner:EAST CHINA UNIV OF SCI & TECH
R-diphenyl prolinol chiral organic small molecule compound with cyclopropane structure and synthesis method of R-diphenyl prolinol chiral organic small molecule compound
InactiveCN103450073AReasonable workmanshipEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention provides a synthesis method of R-diphenyl prolinol with a cyclopropane structure. The method comprises the following steps of: step 1, performing a Simmons-Smith cyclopropane reaction on (R)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrole ethyl formate; step 2, respectively performing grignard reactions on enantiomers obtained by the step 1; and step 3, respectively performing amino-protection removing actions on reaction products obtained by the step 2, thereby obtaining the R-diphenyl prolinol with the cyclopropane structure. The method is reasonable in process, simple to operate and low in cost, and can be used for obtaining the enantiomer products with optical purity while chiral preparation and separation are not required further. Thus, the method is ideal for synthesis of the R-diphenyl prolinol with the cyclopropane structure.
Owner:JIAXING UNIV
Method for synthesizing chiral purine noncyclic nucleoside through dynamic and kinetic resolution of purine, aldehyde and anhydride
ActiveCN107827890AHigh stereoselectivitySimple structureOrganic-compounds/hydrides/coordination-complexes catalystsOptically-active compound separationSynthesis methodsPurine
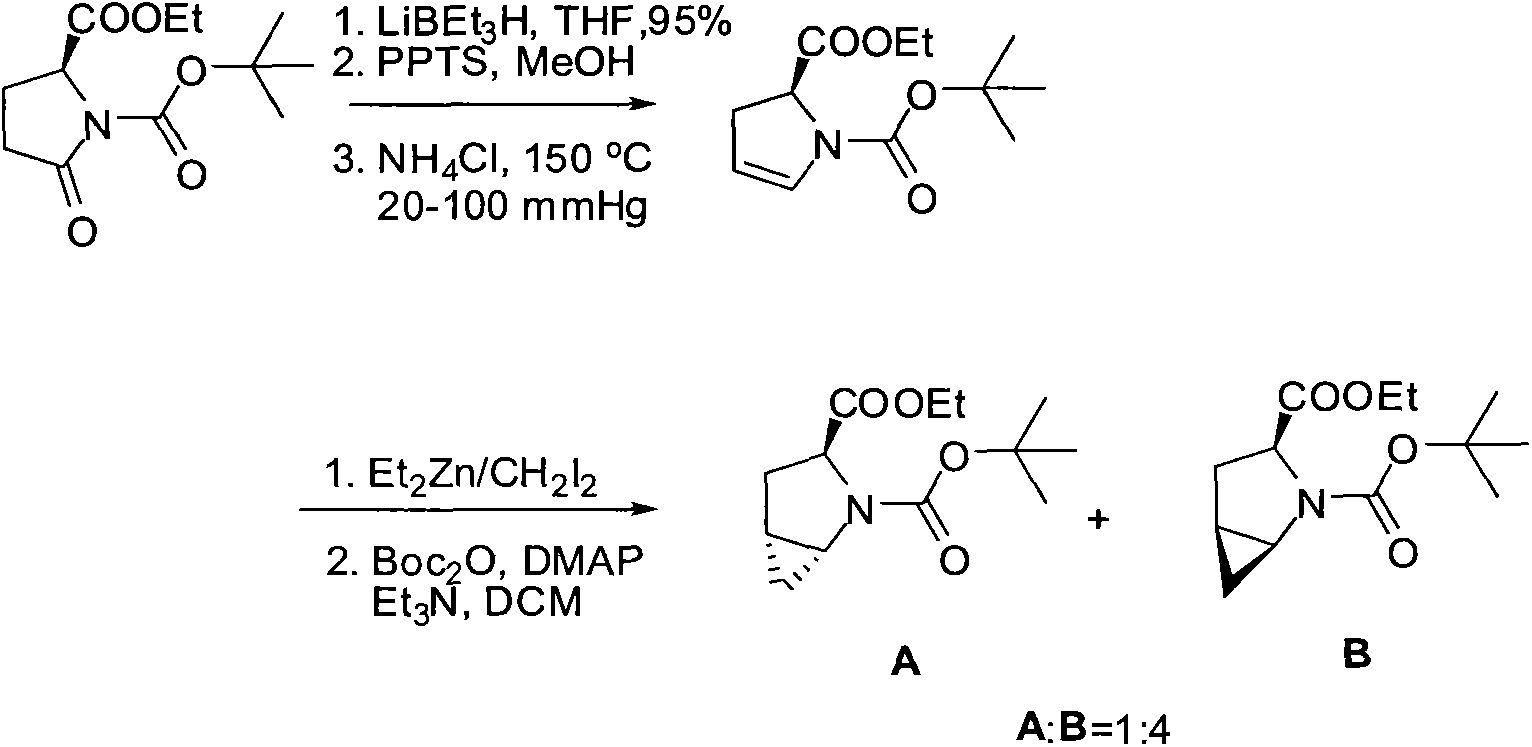
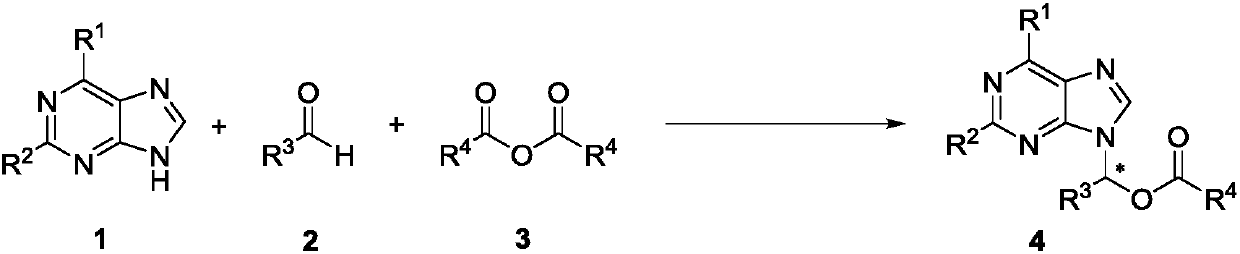
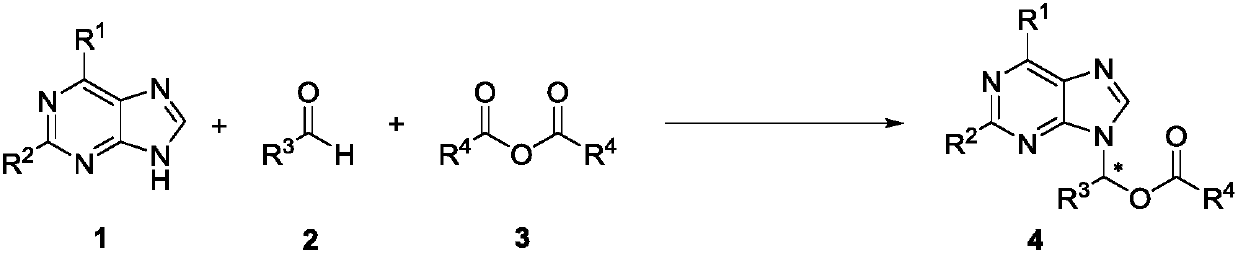
The invention discloses a method for synthesizing chiral purine noncyclic nucleoside through dynamic and kinetic resolution of purine, aldehyde and anhydride and belongs to the field of asymmetric synthesis in organic chemistry. Purine, aldehyde and anhydride are taken as raw materials and subjected to a catalytic reaction under catalysis of a PPY-3-acyl prolinol catalyst, and a chiral purine noncyclic nucleoside analogue is obtained. The simple, cheap and efficient synthesis method is provided for chiral noncyclic purine, raw materials for the reaction are easy to obtain, and the product hasa rich structure and high stereoselectivity.
Owner:HENAN NORMAL UNIV
Use of aryloxy-functionalized prolinol chiral ligand as catalyst
ActiveCN109851540AWide applicabilityHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRare earthLanthanum
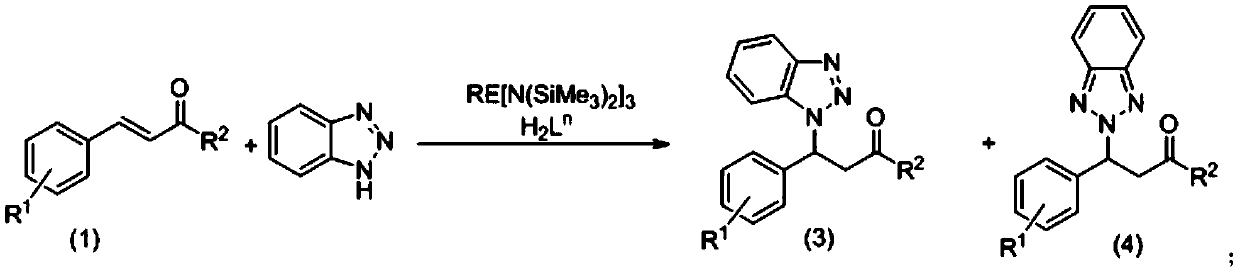
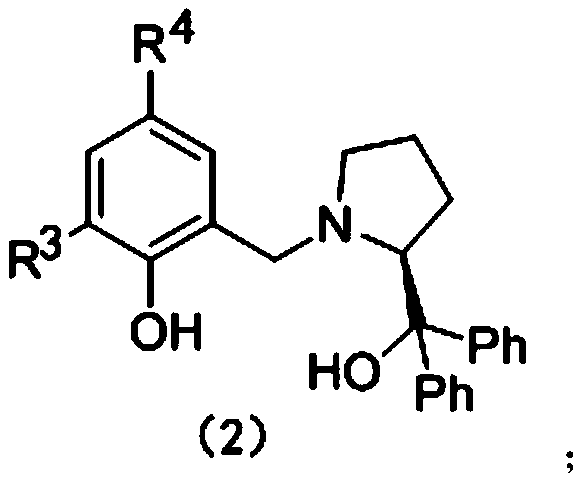
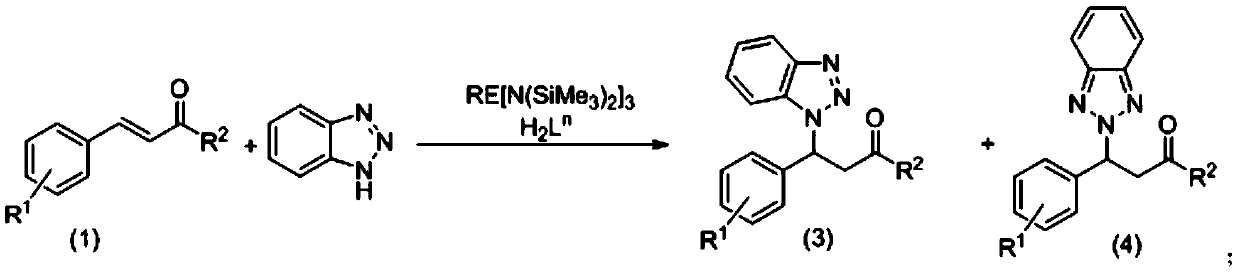
The invention relates to use of an aryloxy-functionalized prolinol chiral ligand for catalyzing the asymmetric addition reaction of chalcone compounds with benzotriazole. In the water-free and oxygen-free conditions containing protective atmosphere, alpha, beta-unsaturated ketones in the formula (1) and benzotriazoles react in the organic solvent at 40-20 DEG C under the catalyst action of the aryloxy-functionalized prolinol chiral ligand and rare earth metal amines, so as to obtain compounds shown in the formula (3) and the formula (4) which are described in the description, wherein R1 is selected from the group consisting of hydrogen, alkyl, halogen, methoxy, nitro or CF3; R2 is selected from the group consisting of phenyl, C1-C4 alkyl substitute phenyl, C1-C4 alkoxy substitute phenyl, halophenyl, furyl or thienyl; H2Ln is that aryloxy-functionalized prolinol chiral ligand; the structural formula of H2Ln is as shown in the formula (2), wherein R3, R4 are independently selected from C1-C4 alkyl, cumyl, hydrogen or halogen; RE[N(SiMe3)2]3 is the rare earth metal amine compound, wherein RE represents a rare earth metal and RE is selected from the group consisting of scandium, lanthanum, neodymium, samarium, yttrium or ytterbium.
Owner:SUZHOU UNIV
Prolinol derivative induced chiral MOFs material with asymmetric catalysis
ActiveCN101830920BLow priceChemically stableGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsTert-Butyloxycarbonyl protecting groupProper treatment
Owner:DALIAN UNIV OF TECH
L-prolinol synthetic method
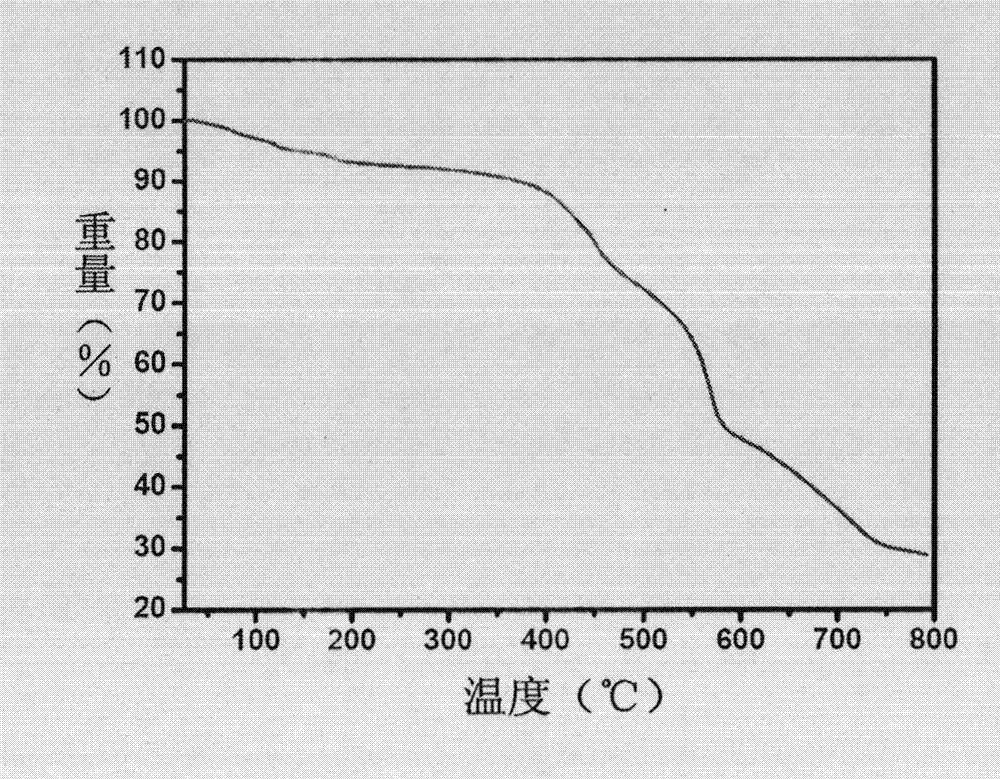
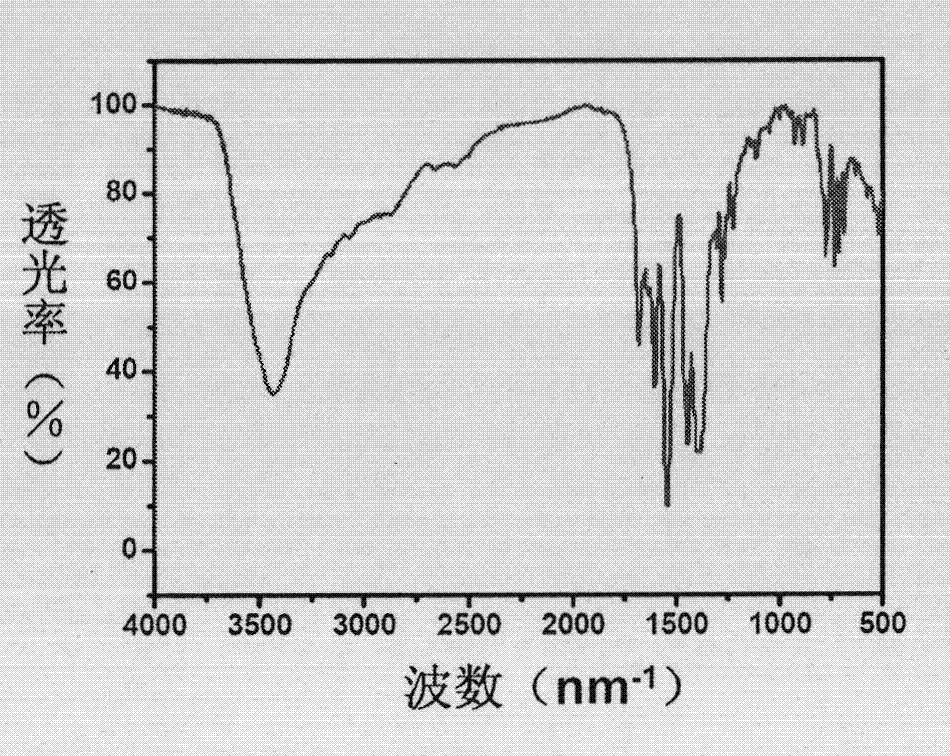
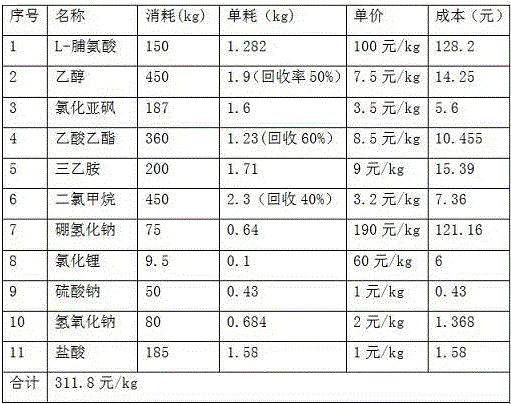
The invention discloses an L-prolinol synthetic method. The method comprises the following steps that 1, ethyl alcohol is added in a reaction kettle, thionyl chloride is dropwise added at 10-15 DEG C, then, L-prolinol is added, the temperature is increased to 40 DEG C, a heat-preservation reaction is carried out for 10-12 h, the mixture is concentrated to be thick liquid after the reaction is finished, ethyl acetate is added for dissolution, the pH value is adjusted through triethylamine to be 7-8, and salt is filtered away to obtain L-proline ethyl ester; 2, the L-proline ethyl ester is in a methyl alcohol system, lithium chloride is added at 5-10 DEG C, the sodium borohydride is added many times, a heat-preservation reaction is carried out at 20-25 DEG C for 2-2.5 h, hydrochloric acid is added, heat-preservation hydrolysis is carried out for 2 h, methyl alcohol is recycled, the pH value is adjusted through 25% sodium hydroxide to be 12, a product is extracted through dichloromethane, anhydrous sodium sulfate drying is carried out, and filtering is carried out; 3, dichloromethane is recycled from mother liquid at 30-40 DEG C and normal pressure, oily matter is obtained, pressure reduction and distillation are carried out, and therefore the pure L-prolinol is obtained. According to the L-prolinol synthetic method, the reaction temperature is mild, the safety coefficient is low, no solid waste is produced, and environmental protection is achieved.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com