Optically active 3-substituted indole derivatives as well as synthesis method and application thereof
A technology of indole derivatives and optical activity, applied in drug combination, organic chemistry, anti-tumor drugs, etc., can solve the difficulties in the preparation of chiral catalysts, the limited scope of substrate application, and the unfavorable application of 3-substituted indole derivatives Industrial synthesis and other issues, to achieve the effect of wide application range of substrates, efficient atom economy, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105]
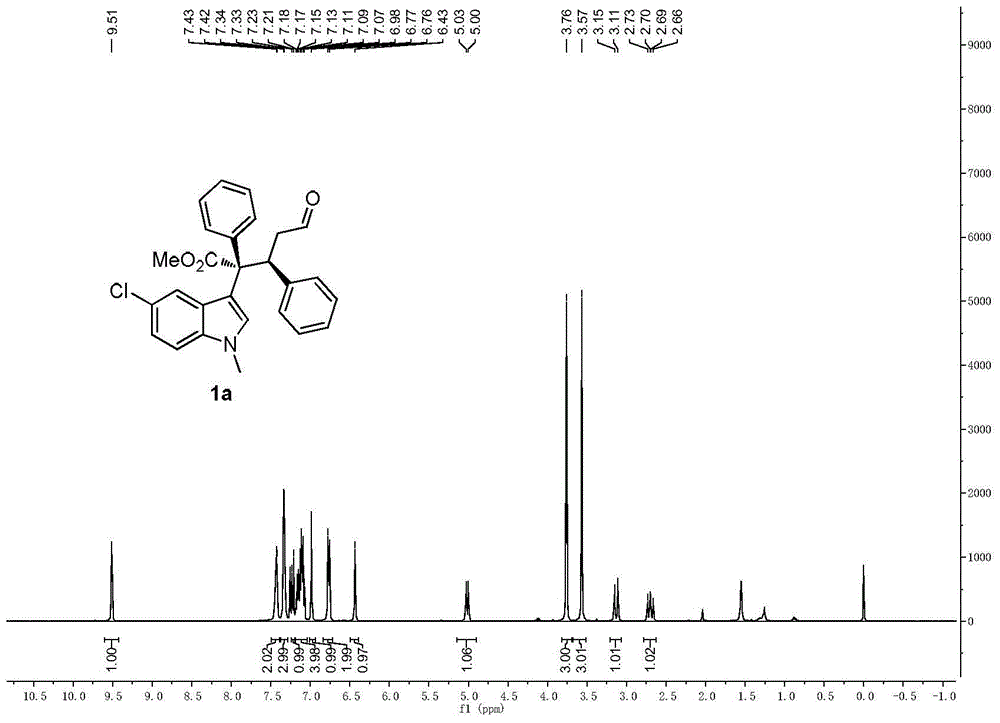
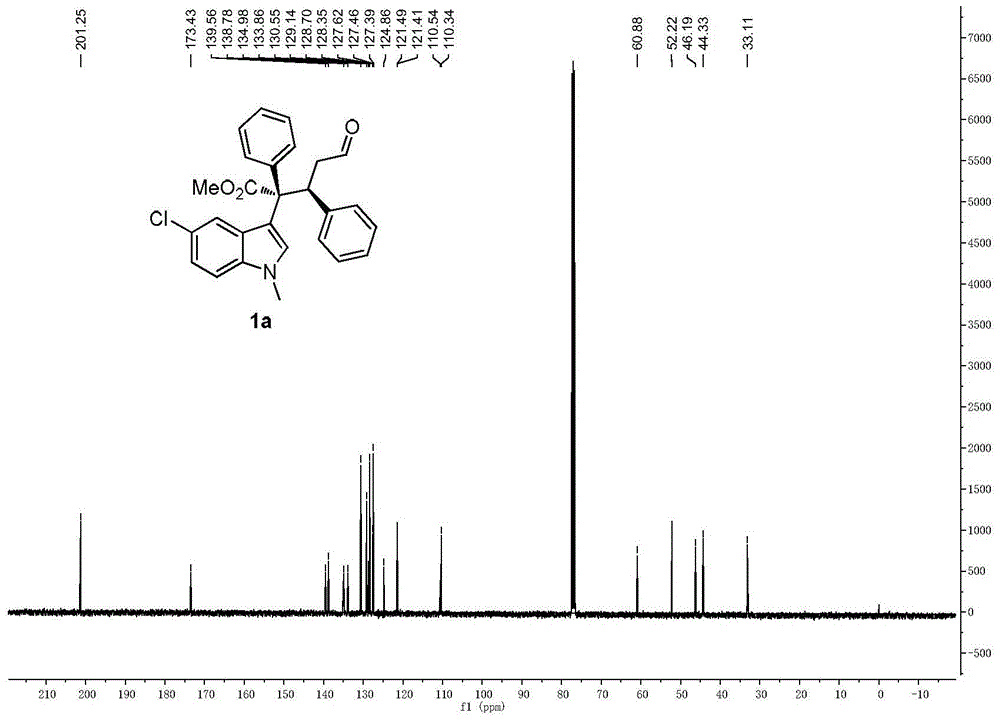
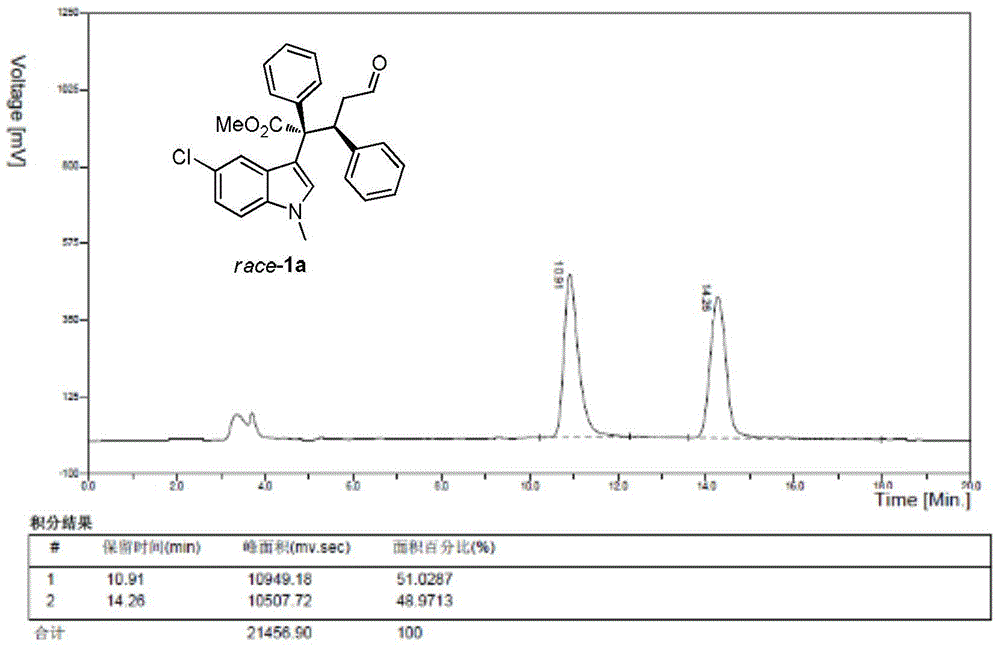
[0106] Cinnamaldehyde (1.0mmol), 1,5-cyclooctadiene iridium chloride dimer (0.05mmol), chiral diphenylprolinol trimethylsilyl ether (0.2mmol), 3,5-bis Trifluoromethylbenzoic acid (0.4mmol) and Molecular sieves (200 mg) were dissolved in 1 mL of dichloromethane to prepare a mixed solution A, which was stirred at 0° C. for 1 hour; methyl phenyldiazoacetate (2.0 mmol), N-methyl-5-chloroindole ( 2.0 mmol) was dissolved in 1 mL of dichloromethane to prepare a mixed solution B; at 0°C, the mixed solution B was added to the aforementioned mixed solution A with a syringe pump, stirred, and reacted. After the diazo decomposition is complete, after the reaction is over, the crude product is subjected to column chromatography (using ethyl acetate:petroleum ether=1:100 as eluent) to obtain a pure product with structures such as formulas (1a) and (1b) As shown, (1a) is methyl (2S,3S-)2-(5-chloro-1-methyl-3-1H-indolyl)-5-oxo-2,3-diphenylpentanoate , (1b) is (2R,3S-)2-(5-chlor...
Embodiment 2
[0116]
[0117] Cinnamaldehyde (1.0mmol), 1,5-cyclooctadiene iridium chloride dimer (0.05mmol), chiral diphenylprolinol trimethylsilyl ether (0.2mmol), 3,5-bis Trifluoromethylbenzoic acid (0.4mmol) and Molecular sieves (200 mg) were dissolved in 1 mL of dichloromethane to prepare a mixed solution A, which was stirred at 0° C. for 1 hour; methyl phenyldiazoacetate (2.0 mmol), N-methyl-5-bromoindole ( 2.0 mmol) was dissolved in 1 mL of dichloromethane to prepare a mixed solution B; at 0°C, the mixed solution B was added to the aforementioned mixed solution A with a syringe pump, stirred, and reacted. After the diazo decomposition is complete, after the reaction is over, the crude product is subjected to column chromatography (using ethyl acetate:petroleum ether=1:100 as the eluent) to obtain a pure product whose structure is as shown in formulas (2a) and (2b) As shown, (2a) is (2S,3S-)2-(5-bromo-1-methyl-3-1H-indolyl)-5-oxo-2,3-diphenylpentanoic acid methyl ester , (2b) is...
Embodiment 3
[0127]
[0128] Cinnamaldehyde (1.0mmol), 1,5-cyclooctadiene iridium chloride dimer (0.05mmol), chiral diphenylprolinol trimethylsilyl ether (0.2mmol), 3,5-bis Trifluoromethylbenzoic acid (0.4mmol) and Molecular sieves (200 mg) were dissolved in 1 mL of dichloromethane to prepare a mixed solution A, which was stirred at 0°C for 1 hour; methyl 4-bromophenyldiazoacetate (2.0 mmol), N-methylindole (2.0 mmol) was dissolved in 1 mL of dichloromethane to prepare a mixed solution B; at 0° C., add the mixed solution B to the aforementioned mixed solution A with a syringe pump, stir and react. After the diazo decomposition is complete, after the reaction is over, the crude product is subjected to column chromatography (using ethyl acetate:petroleum ether=1:100 as the eluent) to obtain a pure product whose structure is as shown in formulas (3a) and (3b) As shown, (3a) is (2S,3S-)-2-(4-bromophenyl)-2-(1-methyl-3-1H-indolyl)-5-oxo-3-phenyl Methyl valerate, (3b) as (2R,3S-)-2-(4-brom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com