Recombinant halohydrin dehalogenase, and mutant and engineering strain and applications thereof
A halohydrin dehalogenase, mutant technology, applied in application, genetic engineering, recombinant DNA technology and other directions, can solve problems such as difficult to obtain, low product ee value, and inability to industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
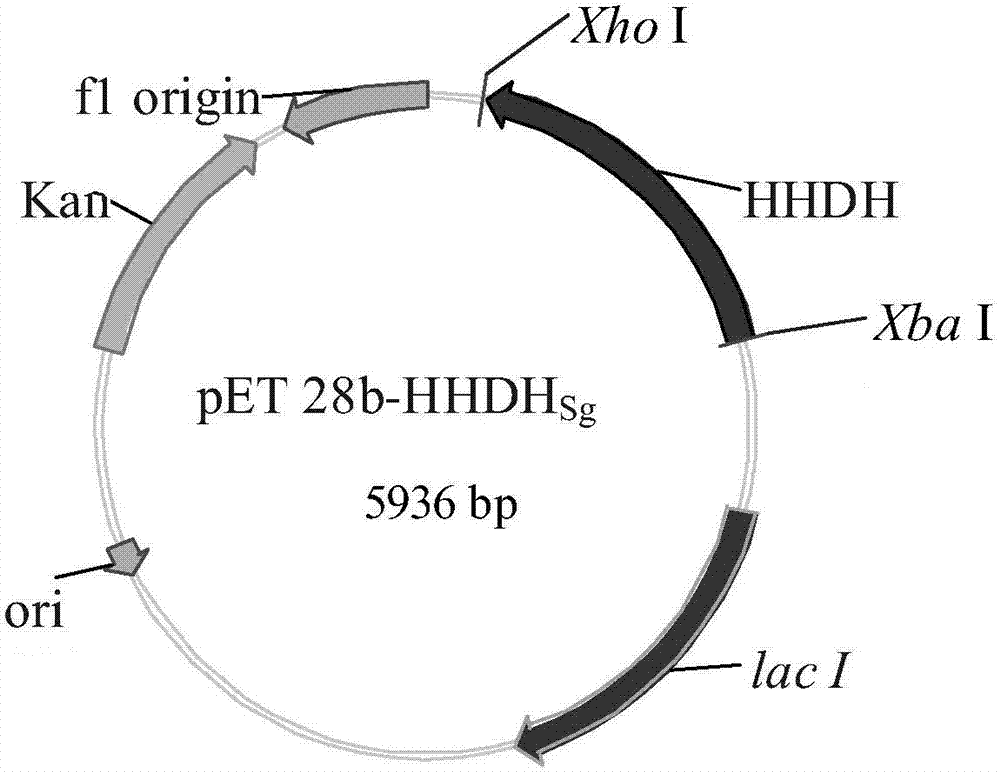
[0028] The acquisition of embodiment 1 parental gene and the preparation of recombinant expression plasmid and recombinant engineered bacteria
[0029] According to the amino acid sequence predicted to be Sneathiella glossodoripedis (Genbank No.WP_037493663) contained in GenBank, the amino acid sequence is shown in SEQ ID No.2, the codon-biased synthetic gene was designed in Escherichia coli, and submitted to Shanghai Xuguan Biotechnology Development Ltd Synthetics. The base sequence is the gene shown in SEQ ID No.1, and Xba I and Xho I restriction sites are introduced at both ends, and it is synthesized by Shanghai Xuguan Biotechnology Co., Ltd. ℃ double digestion with restriction endonucleases Xba I and Xho I for 5 h, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit (Aisijin Biotechnology (Hangzhou) Co., Ltd.). Under the action of T4 DNA ligase, the target fragment was ligated with the vector plasmid pET28b...
Embodiment 3
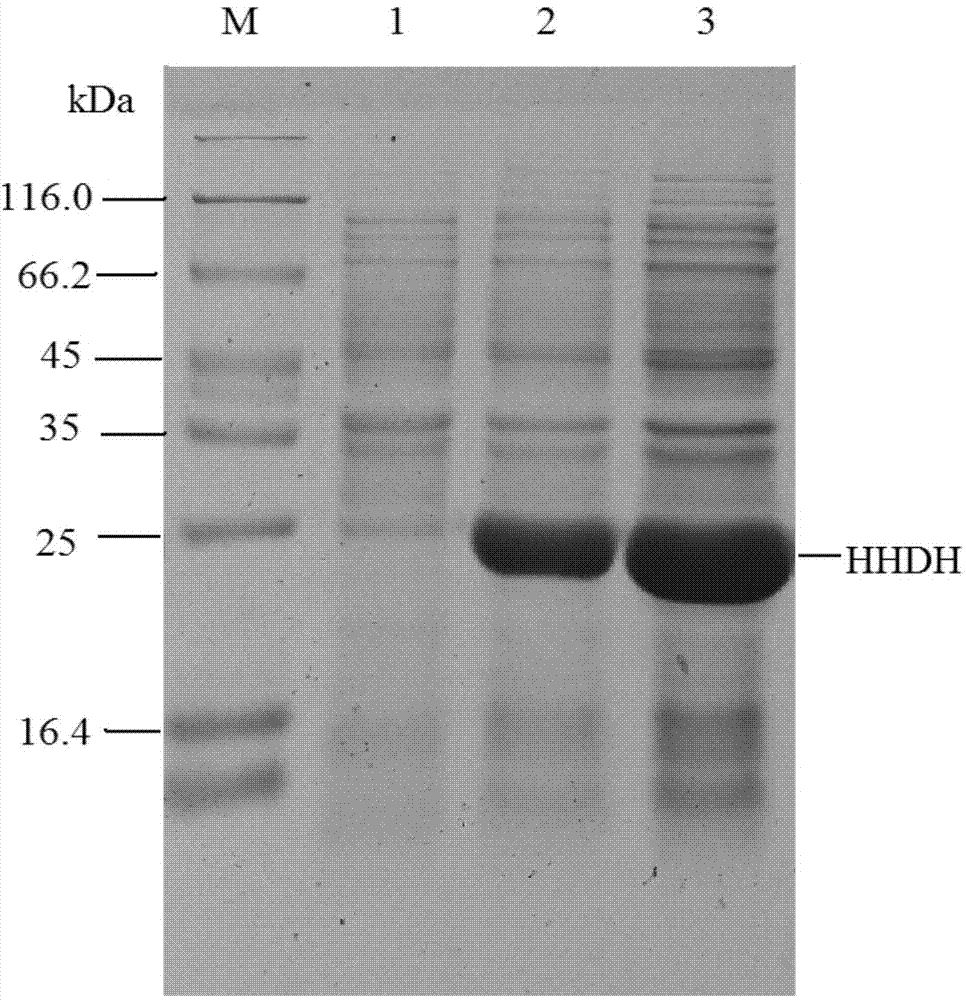
[0033] Embodiment 3 Expression of recombinant halohydrin dehalogenase
[0034]The recombinant escherichia coli obtained in Example 1 and Example 2 were inoculated into the LB medium containing kanamycin sulfate at a final concentration of 50 mg / L respectively, cultured with shaking at 37° C. overnight, and inoculated at 1% (v / v) Put the amount into a 500mL Erlenmeyer flask containing 100mL LB medium, place it on a shaker at 37°C and 180rpm for shaking culture, when the OD 600 of the culture medium reaches 0.6, add IPTG with a final concentration of 0.5mM as an inducer, and induce at 28°C After 10 hours, the culture solution was centrifuged to collect the cells (that is, wet cells), and washed twice with phosphate buffered saline to obtain resting cells. The resulting resting cells were suspended in 20 mL of pH 8.0 buffer, ultrasonically disrupted in an ice bath for 15 min, centrifuged, and the supernatant was collected, which was the crude enzyme solution of the recombinant ha...
Embodiment 4
[0035] Example 4: Halohydrin dehalogenase and mutant V137I catalyze the synthesis of (S)-epichlorohydrin from 1,3-dichloropropanol
[0036] The composition of the wild-type transformation system and the transformation operation are as follows: 0.2 g of wild-type halohydrin dehalogenase wet cells (prepared in Example 3) and 30 mM of 1,3 - Dichloropropanol, reacted on a shaking table at 35°C and 150r / min. After reacting for 2.5 minutes, the reaction solution was extracted with 2 times the volume of ethyl acetate, extracted twice, the extracts were combined, and 1-chloro For n-hexane, the conversion rate and ee value of the substrate were determined by gas chromatographic analysis. After reacting for 2.5 minutes, the conversion rate of 1,3-dichloropropanol reached over 95%, the yield of (S)-epichlorohydrin reached 88.6%, and the ee value reached 84.5%.
[0037] The composition of the mutant transformation system and the transformation operation are as follows: 0.2 g of mutant V1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com