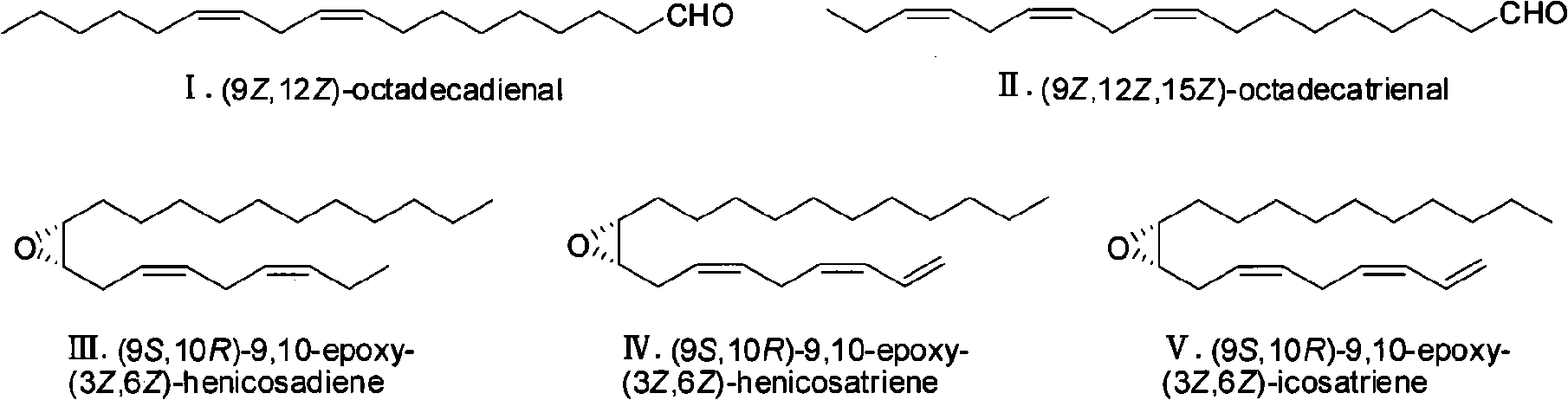
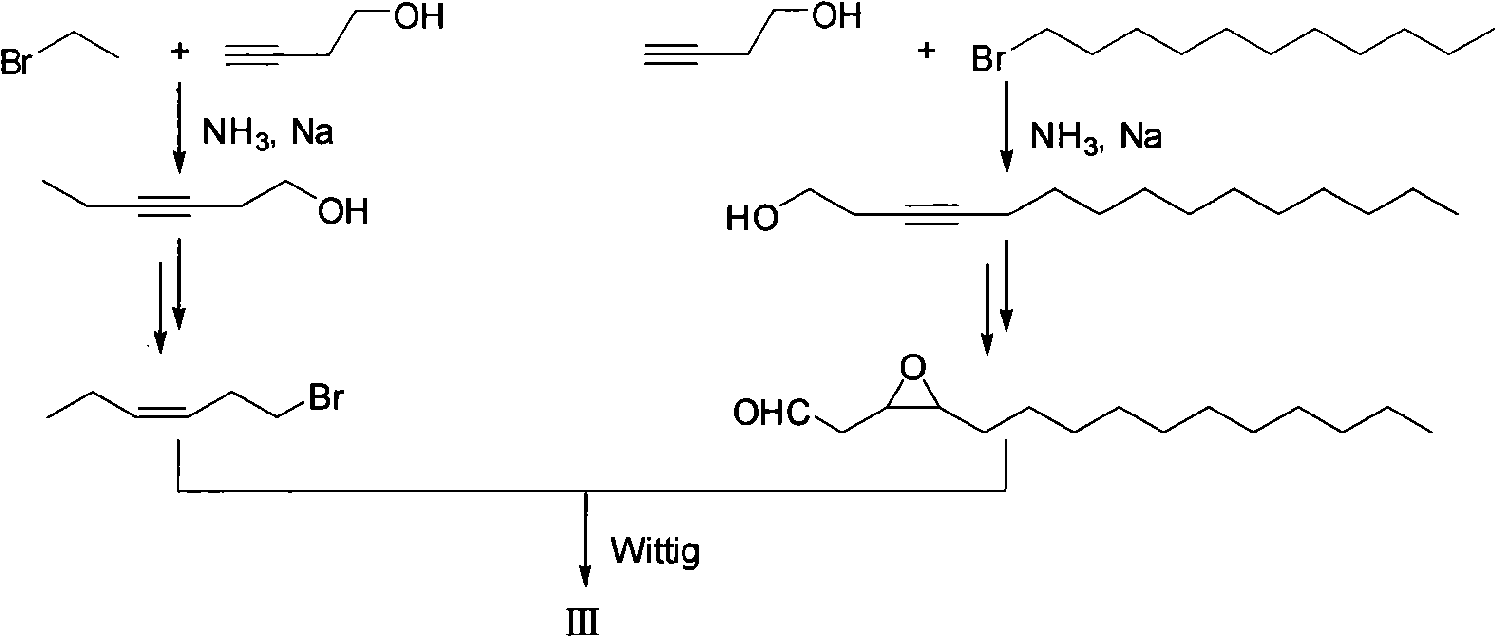
Fall webworm sex pheromone synthesizing method
A technology of American white moth and synthesis method, which is applied in the fields of botanical equipment and methods, chemicals for biological control, animal repellents, etc., and can solve the problems of complex synthesis of pheromone molecules, low efficiency and low yield, etc. , to achieve the effect of high enantioselectivity, simple operation and separation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
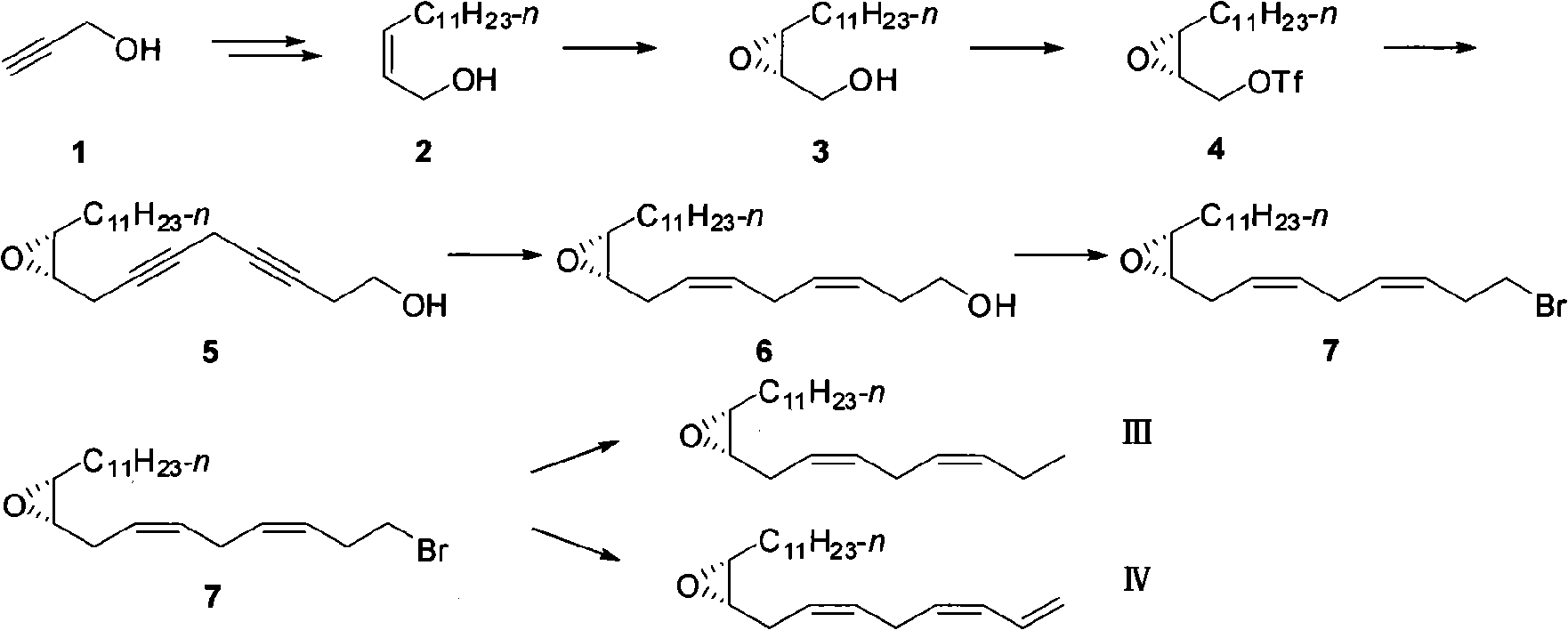
[0043] Step 1 Synthesis of (2S, 3R)-2,3-epoxy-1-14 carbon alcohol (3)
[0044] Under the protection of argon atmosphere, 200 mg of silica gel was mixed with 150 mg of calcium hydride, and 280 mL of dry dichloromethane solvent was injected, followed by 10.9 mL (35.8 mmol) of tetraisopropyltitanium oxide, and stirred at -35°C for 20 min. Slowly inject a solution of L-(+)-diisopropyl tartrate (9825 mg, 41.1 mmol) in dichloromethane (15 mL), and stir at -35°C for 20 min. Slowly inject a solution of (Z)-2-alkyne tetradecyl alcohol 2 (6.6 g, 31.13 mmol) in dichloromethane (15 mL), and stir at -35°C for 20 min. A solution of tert-butanol peroxide (68.49 mmol) in nonane (12.5 mL) was slowly injected, and stirred at -30°C for 3 days. 80 mL of 10% tartaric acid aqueous solution was added to quench the reaction, the temperature was raised slowly, the layers were separated, the aqueous phase was extracted with dichloromethane, and the organic layer was washed three times with saturated b...
Embodiment 2
[0058] Step 1 Synthesis of (2S, 3R)-2,3-epoxy-1-14 carbon alcohol (3)
[0059] Under the protection of argon atmosphere, the activated 3 300mg of molecular sieve powder was injected into 280mL of dry dichloromethane solvent, 1.09mL (3.58mmol) of tetraisopropyltitanium oxide was injected, and stirred at -35°C for 20min. Slowly inject a solution of L-(+)-diisopropyl tartrate (983mg, 4.11mmol) in dichloromethane (5mL) and stir at -35°C for 20min. Slowly inject a solution of (Z)-2-alkyne tetradecyl alcohol 2 (6.6 g, 31.13 mmol) in dichloromethane (15 mL), and stir at -35°C for 20 min. A solution of tert-butanol peroxide (68.49 mmol) in nonane (12.5 mL) was slowly injected, and stirred at -30°C for 3 days. Add 50mL of 10% tartaric acid aqueous solution to quench the reaction, raise the temperature slowly, separate the liquid, and use CH 2 Cl 2After extraction, the organic layer was washed three times with saturated brine (3×20 mL). Drying over anhydrous sodium sulfate, concen...
Embodiment 3
[0073] Step 1 Synthesis of (2S, 3R)-2,3-epoxy-1-14 carbon alcohol (3)
[0074] Under the protection of argon atmosphere, 200 mg of silica gel was mixed with 150 mg of calcium hydride, and 280 mL of dry dichloromethane solvent was injected, followed by 10.9 mL (35.8 mmol) of tetraisopropyltitanium oxide, and stirred at -35°C for 20 min. Slowly inject a solution of L-(+)-diethyl tartrate (8650 mg, 41.1 mmol) in dichloromethane (15 mL), and stir at -35°C for 20 min. Slowly inject a solution of (Z)-2-alkyne tetradecyl alcohol 2 (6.6 g, 31.13 mmol) in dichloromethane (15 mL), and stir at -35°C for 20 min. A solution of tert-butanol peroxide (68.49 mmol) in nonane (12.5 mL) was slowly injected, and stirred at -30°C for 3 days. 80 mL of 10% tartaric acid aqueous solution was added to quench the reaction, the temperature was raised slowly, the layers were separated, the aqueous phase was extracted with dichloromethane, and the organic layer was washed three times with saturated brine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com