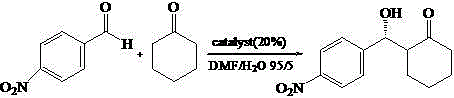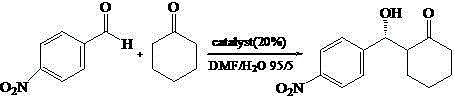Nanogel modified by ionic liquid and loaded with chiral catalyst and preparing method and application thereof
A chiral catalyst, ionic liquid technology, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve the problems of difficult recovery of homogeneous reactions and expensive chiral catalysts, and improve catalytic activity. , the effect of adjustable size and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of O-methacryloyl-L-hydroxyproline chiral monomer:
[0022] (1) Preparation of O-methacryloyl-L-hydroxyproline hydrochloride:
[0023] Add 30mL of trifluoroacetic acid into a 100mL round-bottomed flask, add 6g of L-hydroxyproline in batches in an ice-water bath, stir and react at room temperature for 3 hours, and wait until the L-hydroxyproline is completely dissolved, then place in an ice-water bath Add 9.6mL of newly prepared methacryloyl chloride, react at room temperature for 3 hours, stir magnetically, slowly add cold diethyl ether dropwise to the reaction bottle, a white substance will precipitate, filter it with suction and wash it with diethyl ether three times, and dry the product in a drying oven to obtain a white powder shape substance.
[0024] (2) Preparation of O-methacryloyl-L-hydroxyproline:
[0025] Weigh 3.97g of O-methacryloyl-L-hydroxyproline hydrochloride and add it to a 250mL round bottom flask, add 4.4mL triethylamine, 100mL dichloro...
Embodiment 2
[0029] Preparation of proline derivative monomer:
[0030] Preparation of chiral monomer N-p-vinylbenzenesulfonyl-L-prolineamide: add 85mL tetrahydrofuran, 2.50g L-proline, 1.23g p-vinylbenzenesulfonamide, 3.52g 4-dimethylamino Pyridine and 5.04g of dicyclohexylcarbodiimide, stirred at room temperature for 48h, then added 8g of protonated Amberlyst-15, 20mL of ethyl acetate, and continued to stir for 5h, a large amount of white precipitate was produced, and the precipitate was filtered off with a silica gel column, acetic acid After washing with ethyl ester, the resulting solution was concentrated by rotary evaporation, precipitated twice in ether, filtered, dried under vacuum at 30°C, and weighed to obtain 0.7334 g of the product, with a yield of 30%.
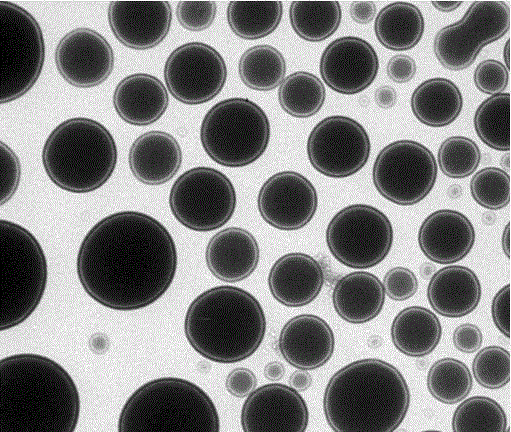
[0031] Preparation of proline derivative nanogels:
[0032] Weigh the chiral monomer N-p-vinylbenzenesulfonyl-L-prolinamide (0.2mmol, 0.056g), imidazole ionic liquid (n=7 and X=Br - ) (0.1005mmol, 0.02163g), sodium dodecylsu...
Embodiment 3
[0034] Add the nanogel ball of the load chiral catalyst that the embodiment 1 that adds the amount of substrate p-nitrobenzaldehyde 20% as catalyst 4.9mg, 0.1mmol p-nitrobenzaldehyde, 1mL cyclohexanone and 1 mL DMF / H 2 O (v / v, 95 / 5), reacted at room temperature for 48 hours, and tracked the reaction to the end point by thin-layer chromatography. After the reaction was completed, centrifuged, the supernatant was extracted three times with ethyl acetate (3×10mL), and the organic phases were combined. MgSO 4 Drying, suction filtration, concentration, CDCl 3The conversion rate was calculated by solvent proton nuclear magnetic resonance spectroscopy. The measured conversion rate was 99%, and the anti / syn was 95 / 5. The pure product was obtained by column chromatography (petroleum ether: ethyl acetate = 4:1), and was passed through AD- H chiral column separation chiral substance, high performance liquid chromatography (HPLC) records ee value to be 95%, and reaction equation is as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com