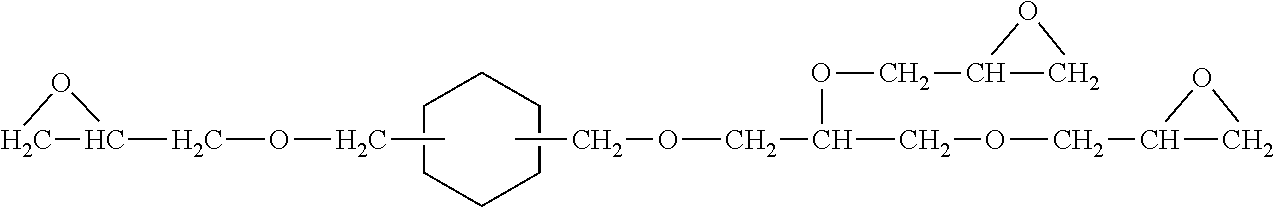
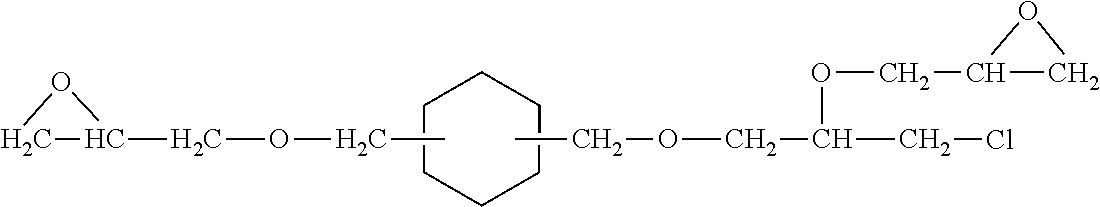
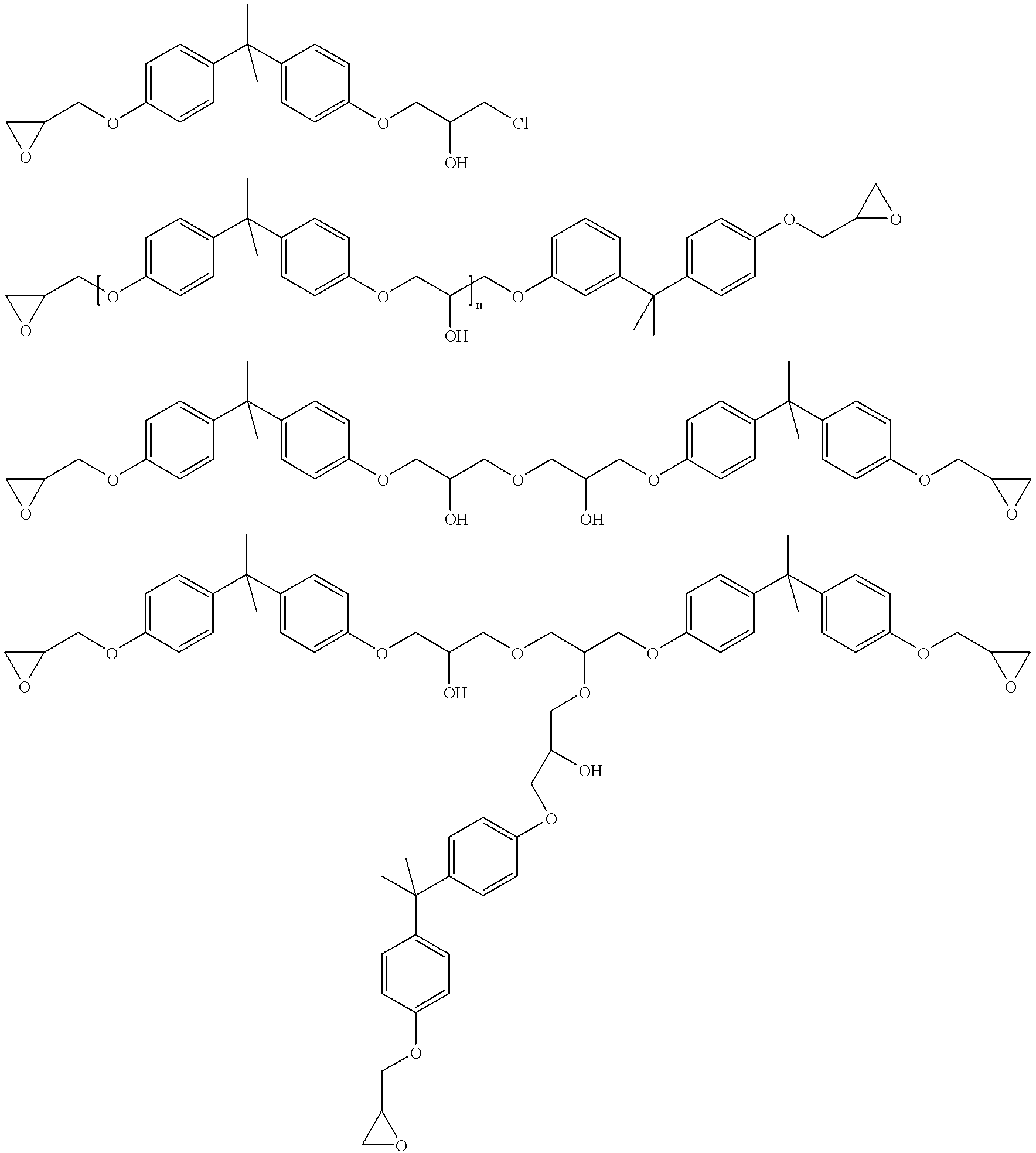
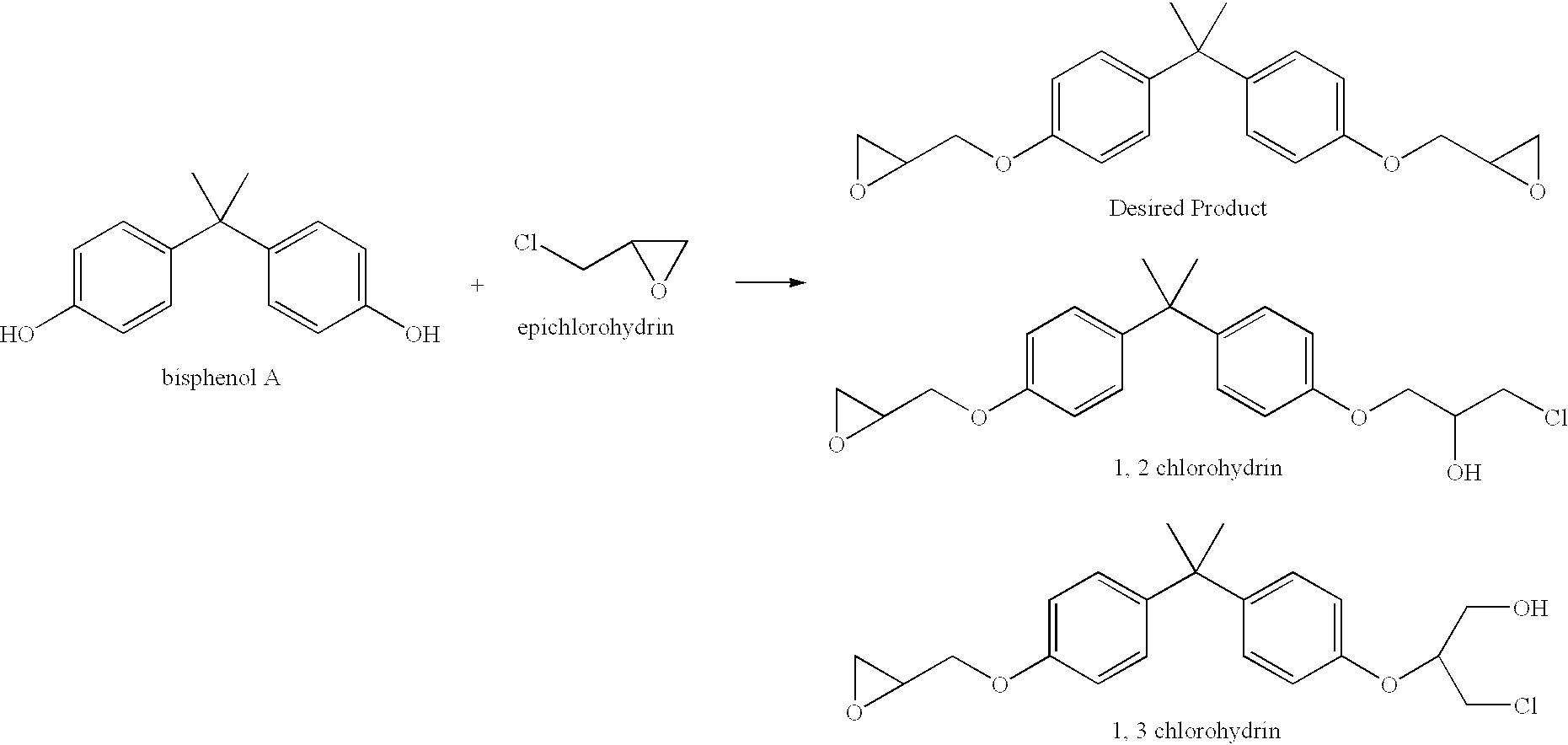
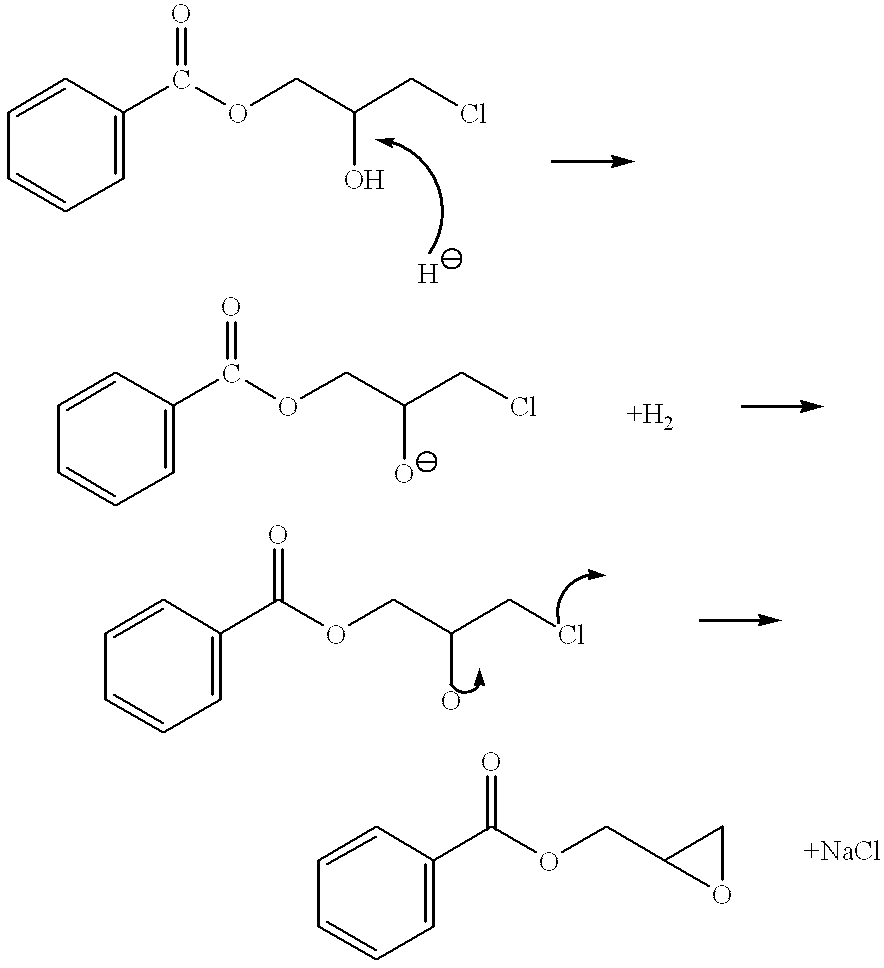
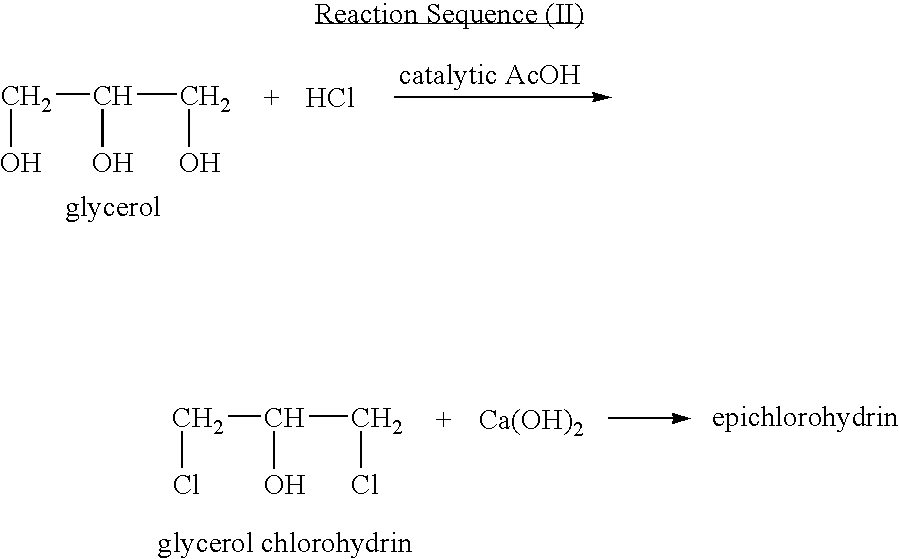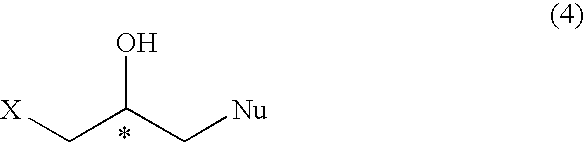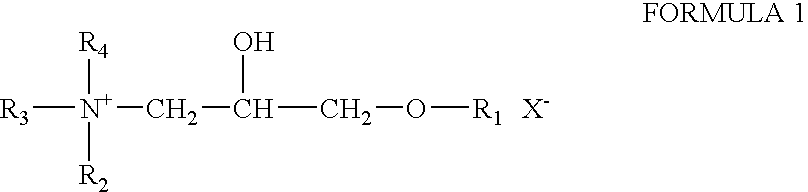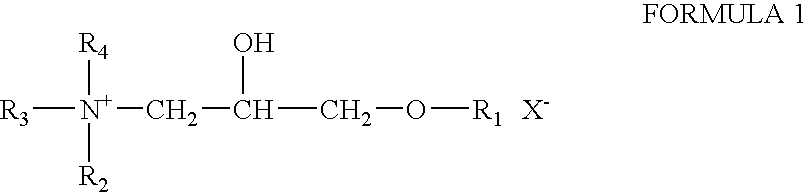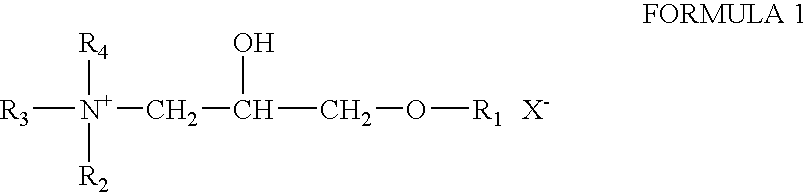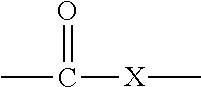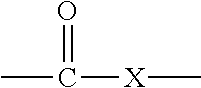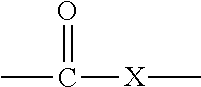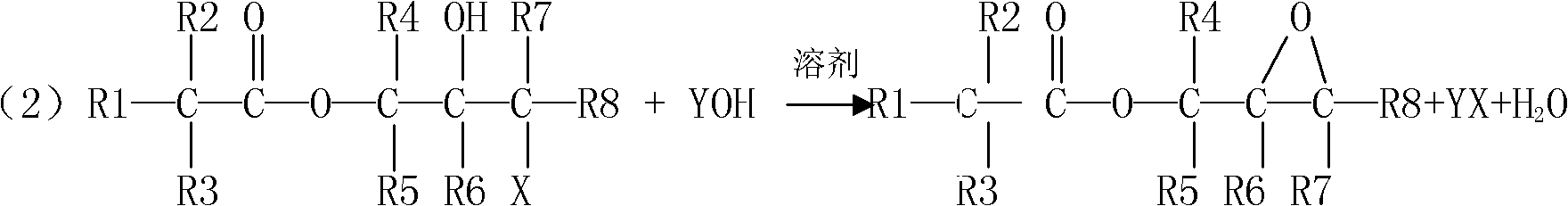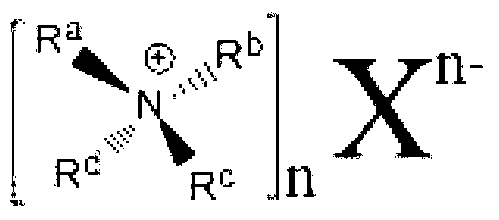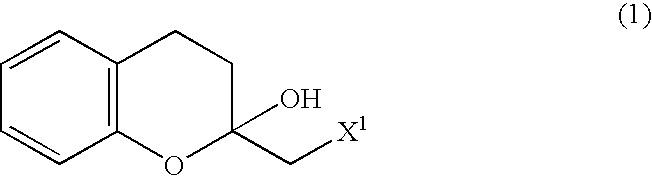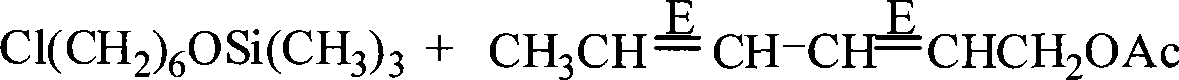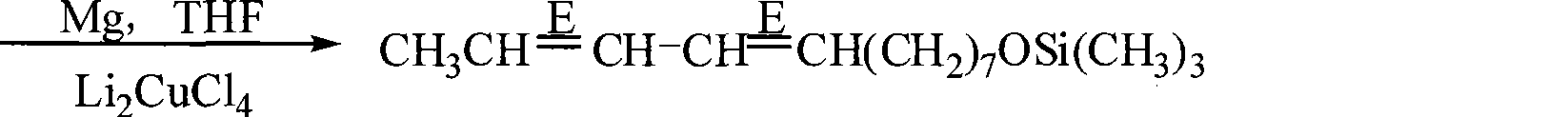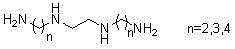Patents
Literature
167 results about "Halohydrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
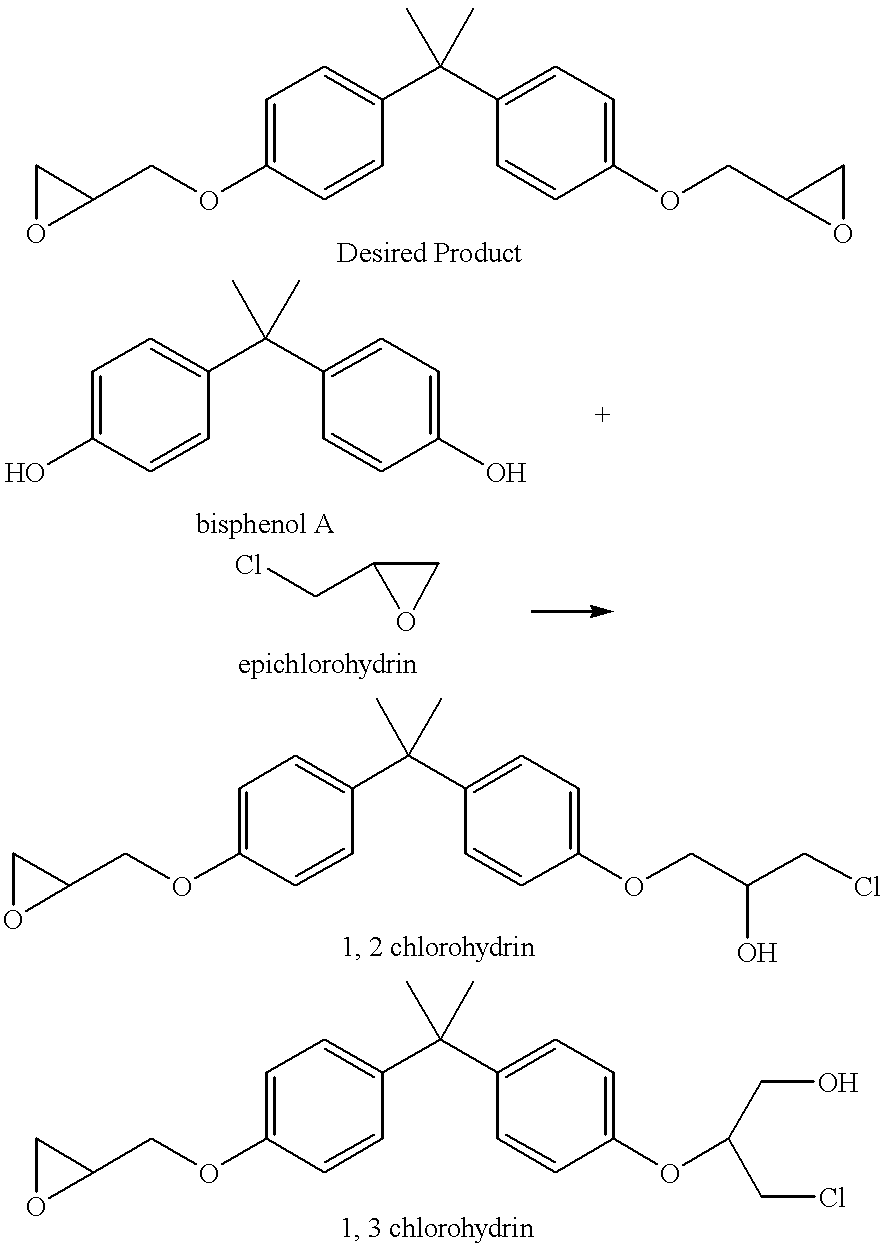
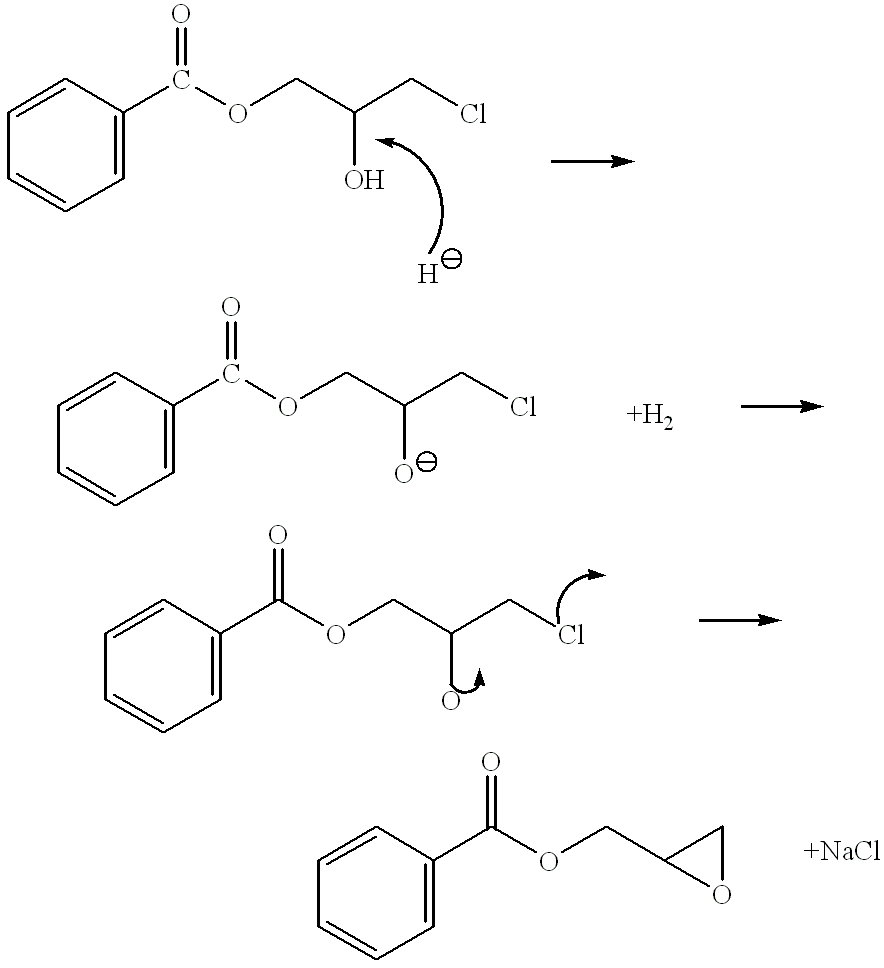
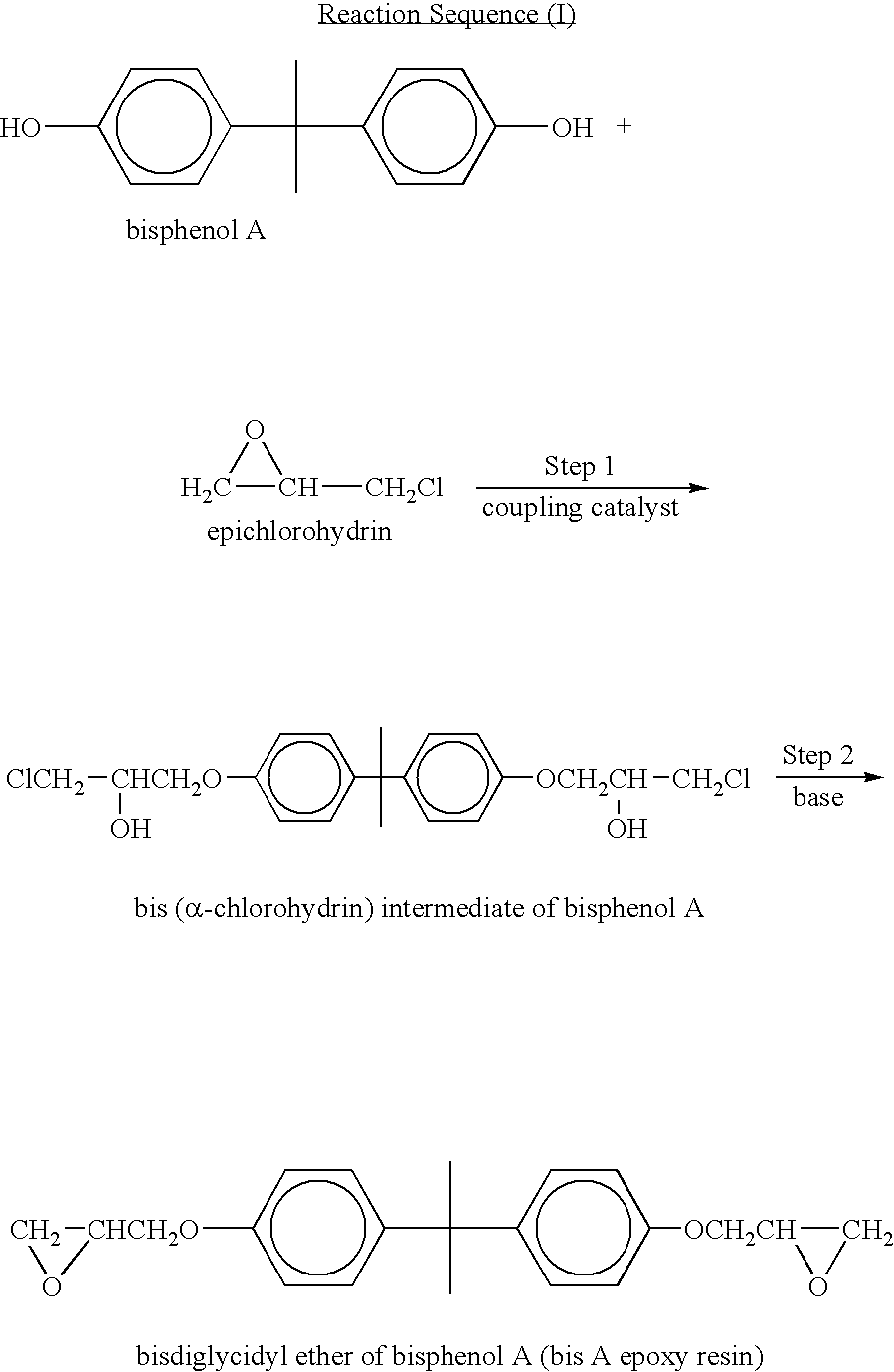
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol, 3-chloropropane-1,2-diol). The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers.
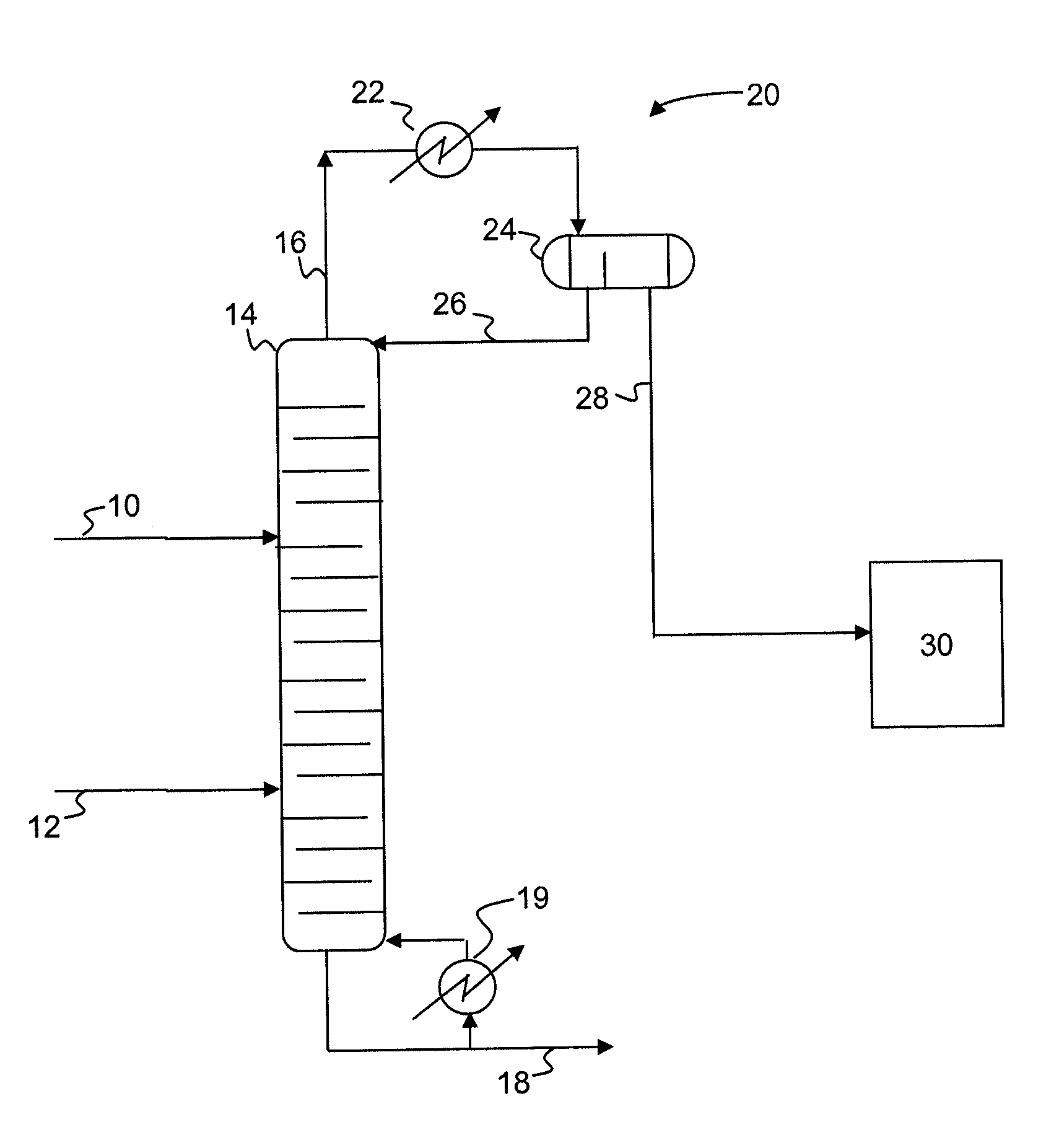
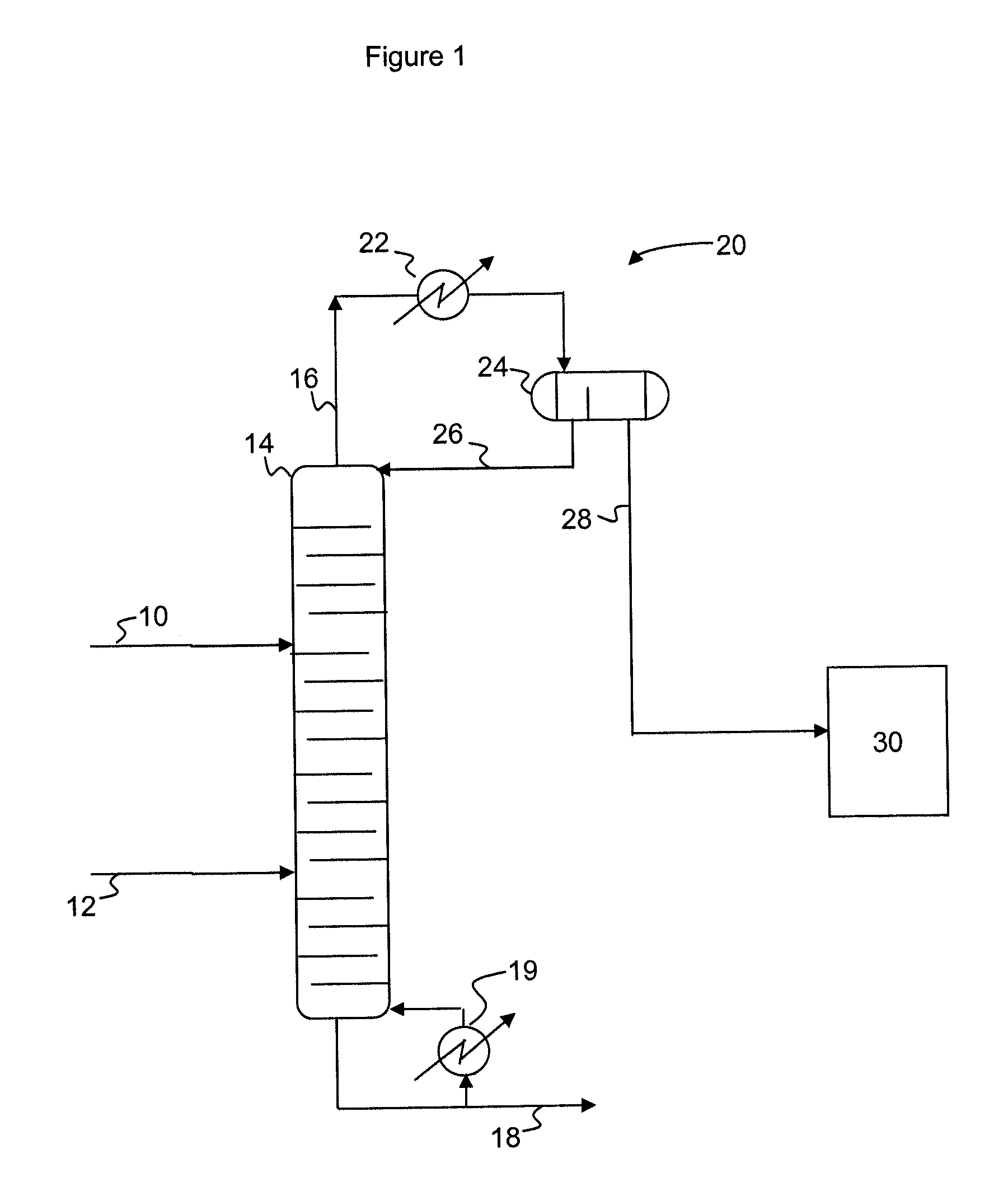
Process for producing epoxides
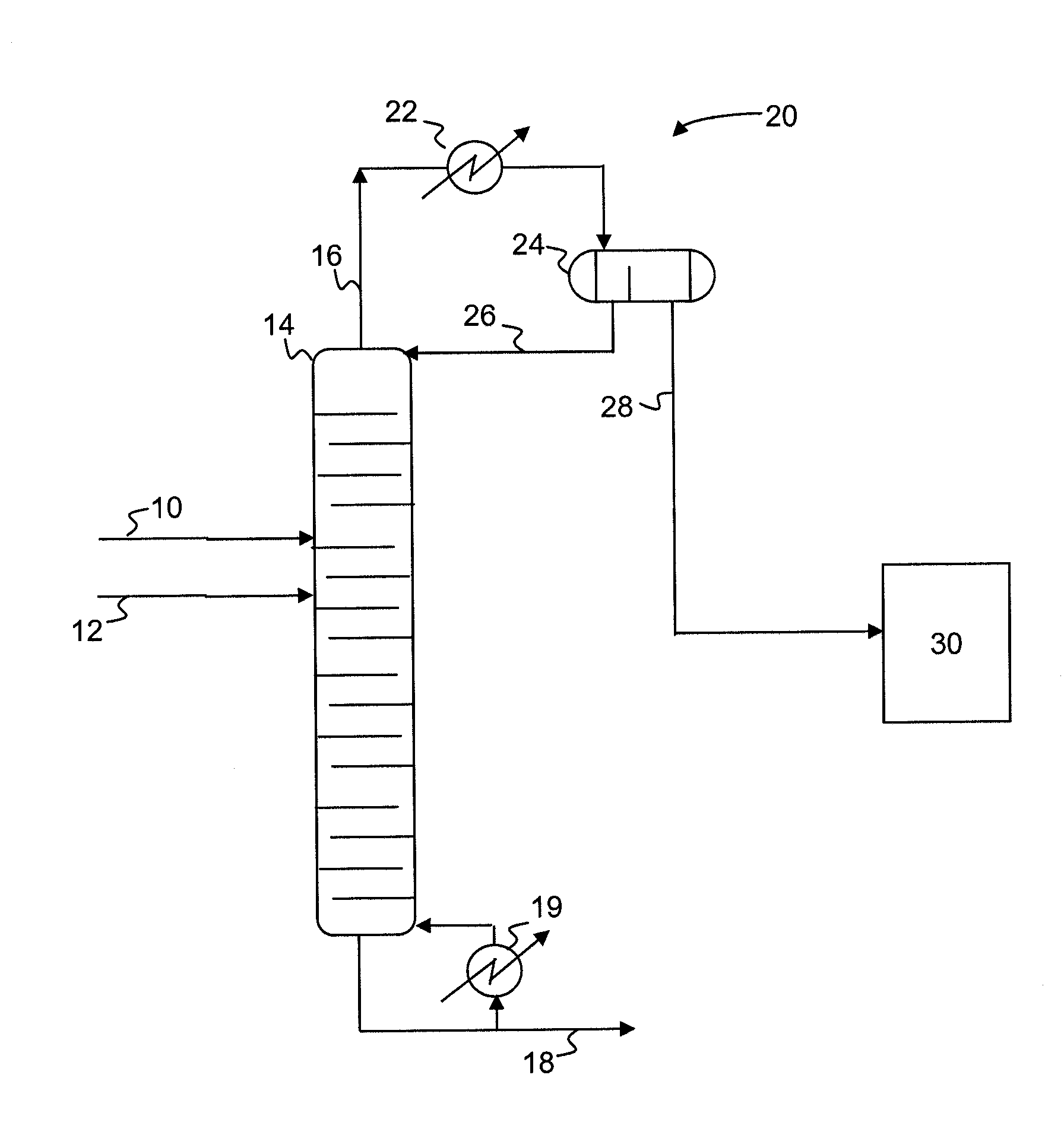
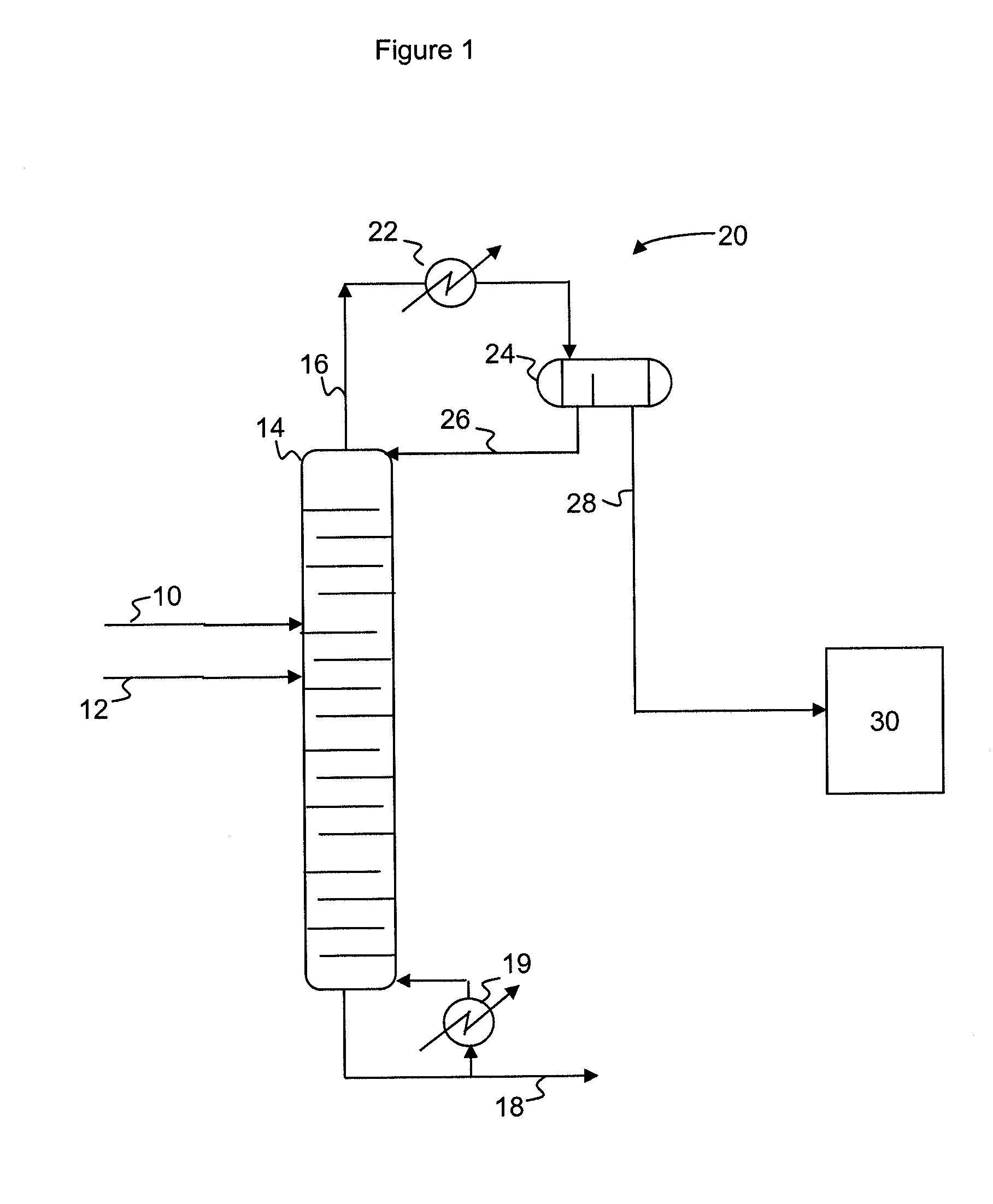
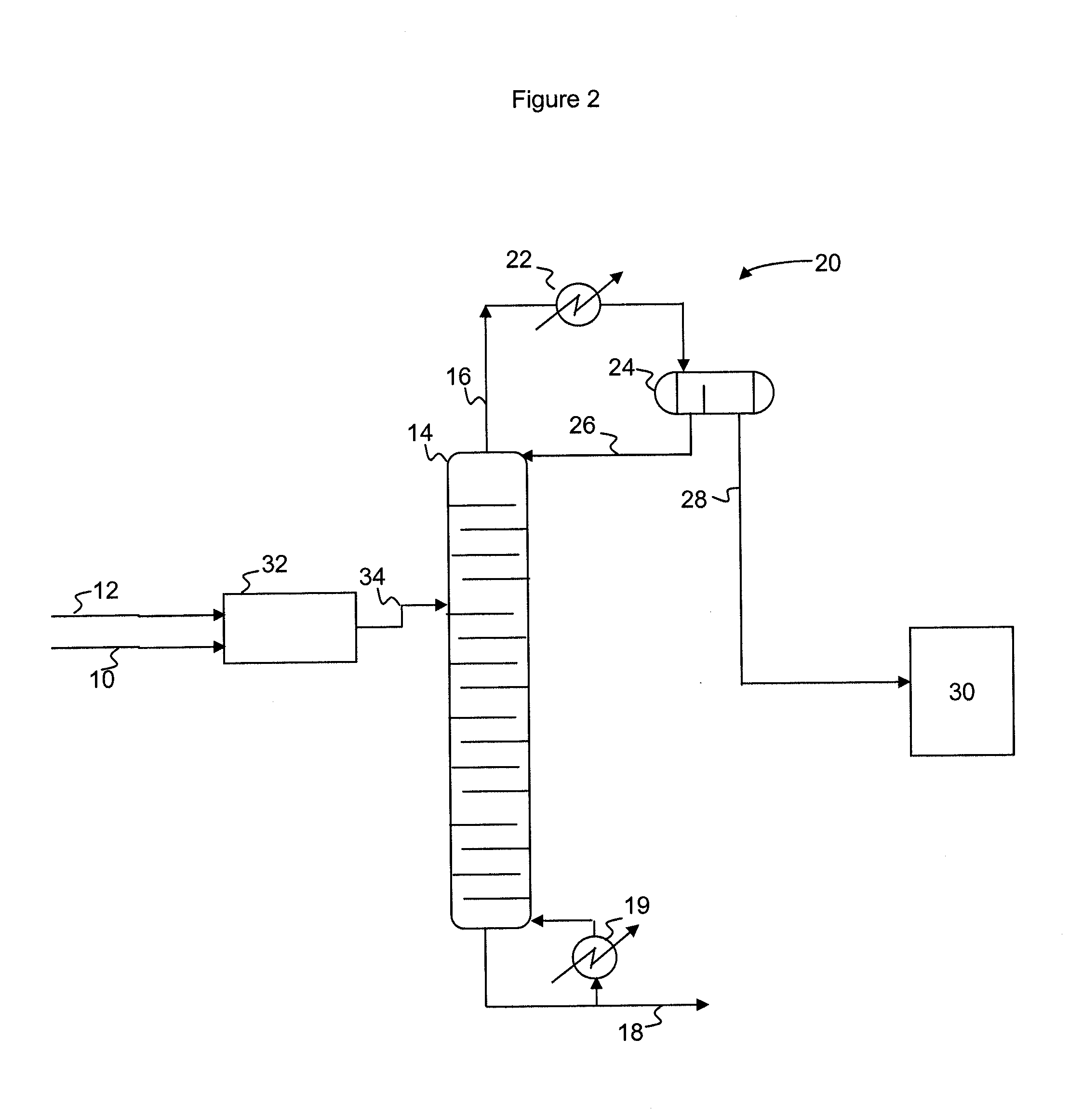
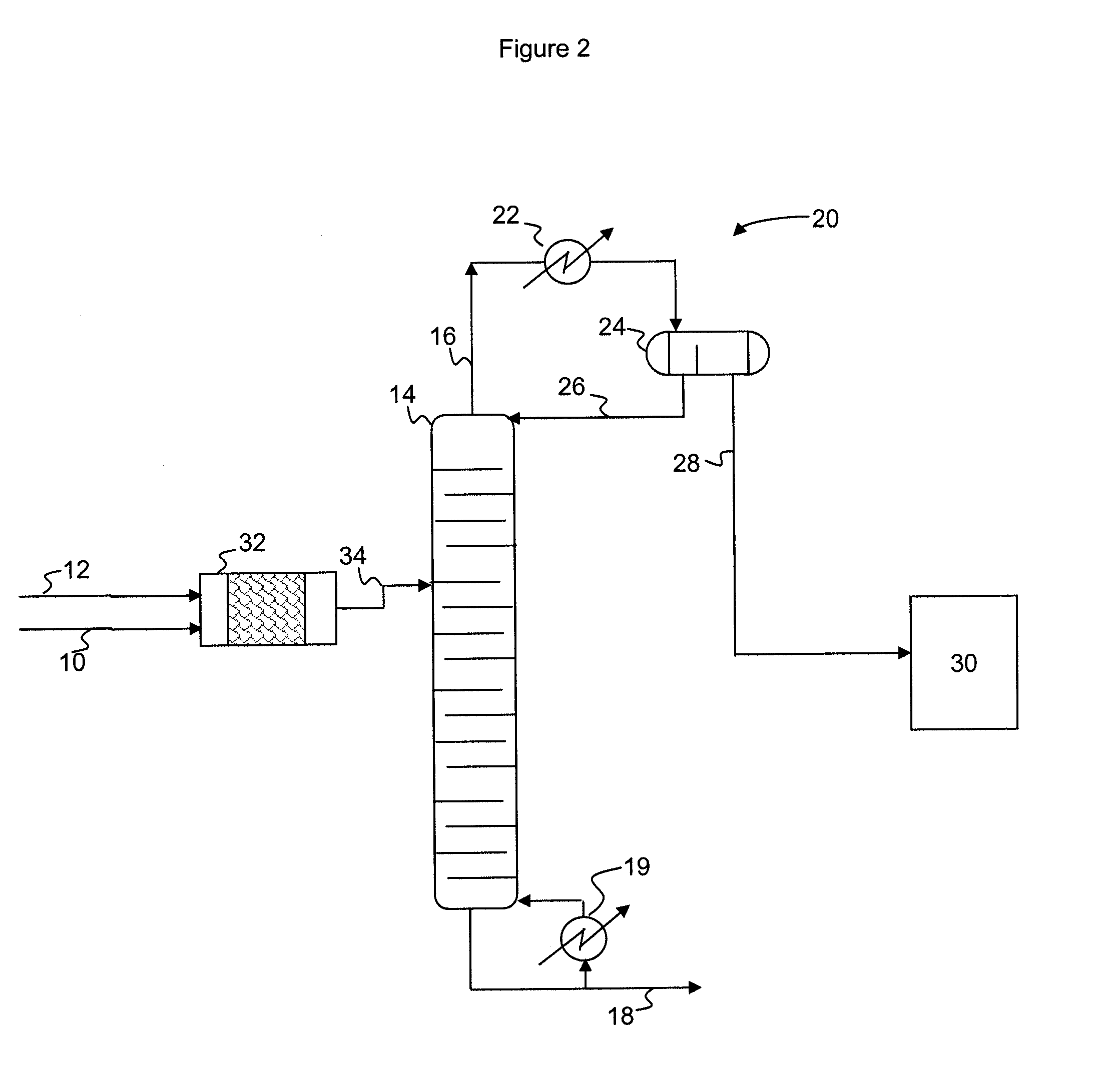
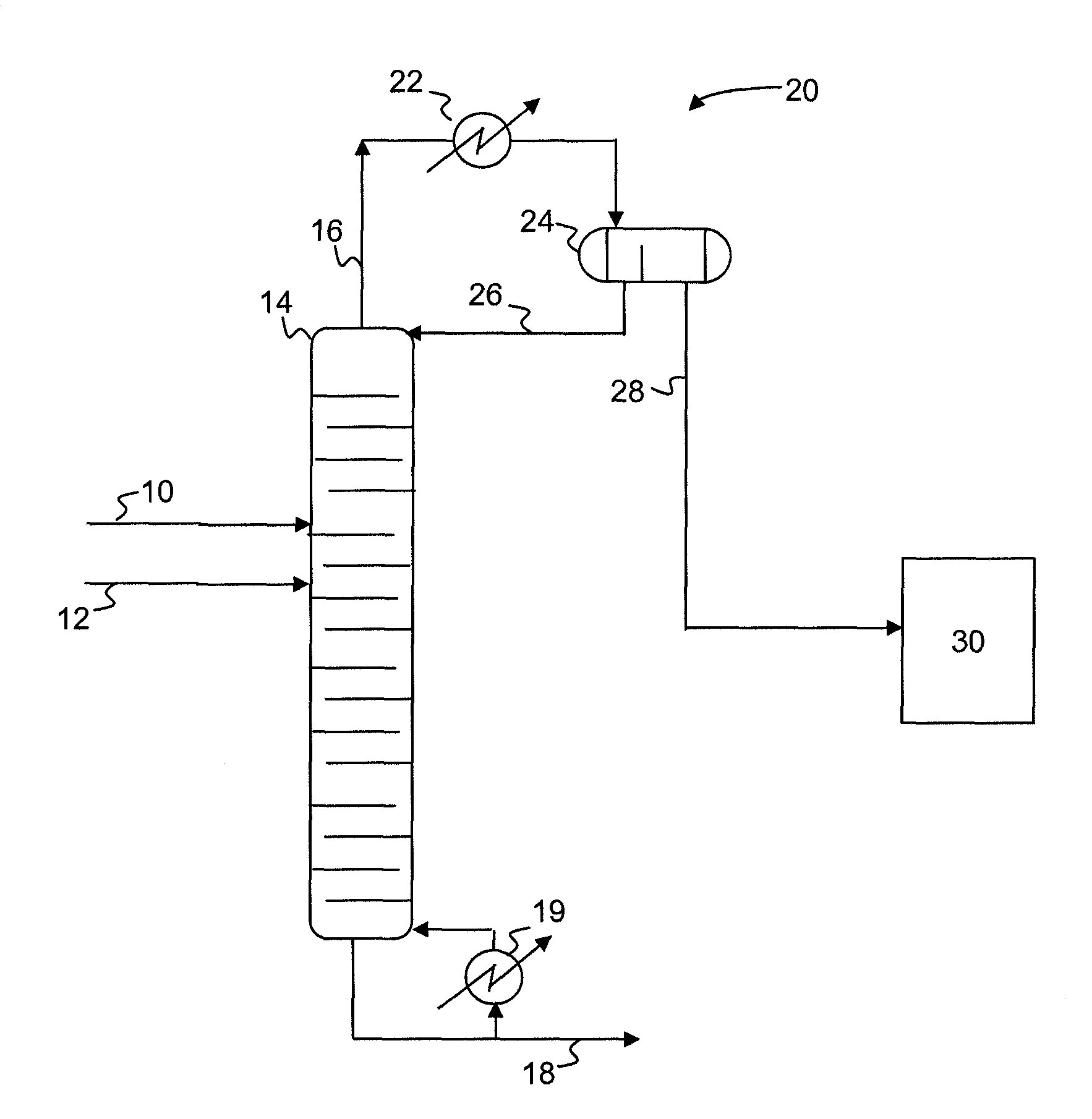
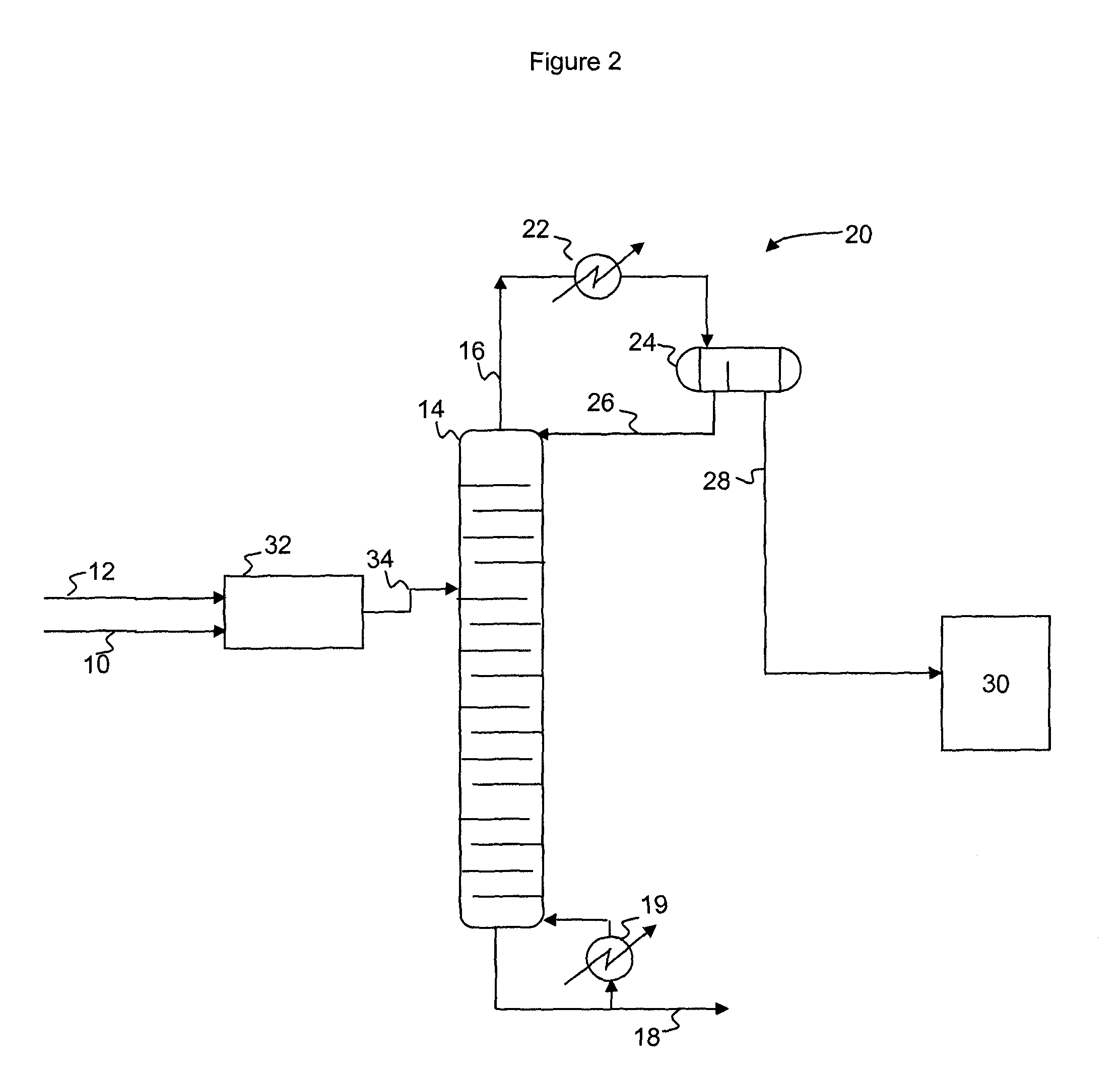
A process for producing epoxides, the process including: (a) feeding at least one aqueous alkali and at least one halohydrin to a reactive distillation column, wherein the reactive distillation column includes a feed zone, a top zone disposed above the feed zone, and a bottom zone disposed below the feed zone; (b) concurrently in the reactive distillation column: (i) reacting at least a portion of the halohydrin with the alkali to form an epoxide; and (ii) stripping water and the epoxide from a basic aqueous residue; (c) recovering the water and the epoxide from the reactive distillation column as an overheads fraction; (d) condensing and phase separating the overheads fraction to form an organic overheads fraction including the epoxide and an aqueous overheads fraction including water; and (e) maintaining a liquid holdup per plate in the feed zone at a residence time of 10 seconds or less.
Owner:BLUE CUBE IP
Process for the elimination of materials containing hydrolyzable halides and other high molecular weight materials from epihalohydrin derived epoxy resins
The present invention provides a process for eliminating contaminants from epihalohydrin-derived epoxy resins. Another embodiment of the present invention is an epoxy product formed using said process. Yet another embodiment of the present invention is an epoxy derived in part from epihalohydrin wherein said epoxy is has a hydrolyzable halogen content of less than 10 ppm and an epoxide equivalent weight within 2 percent of the theoretical epoxide equivalent weight.
Owner:3M INNOVATIVE PROPERTIES CO
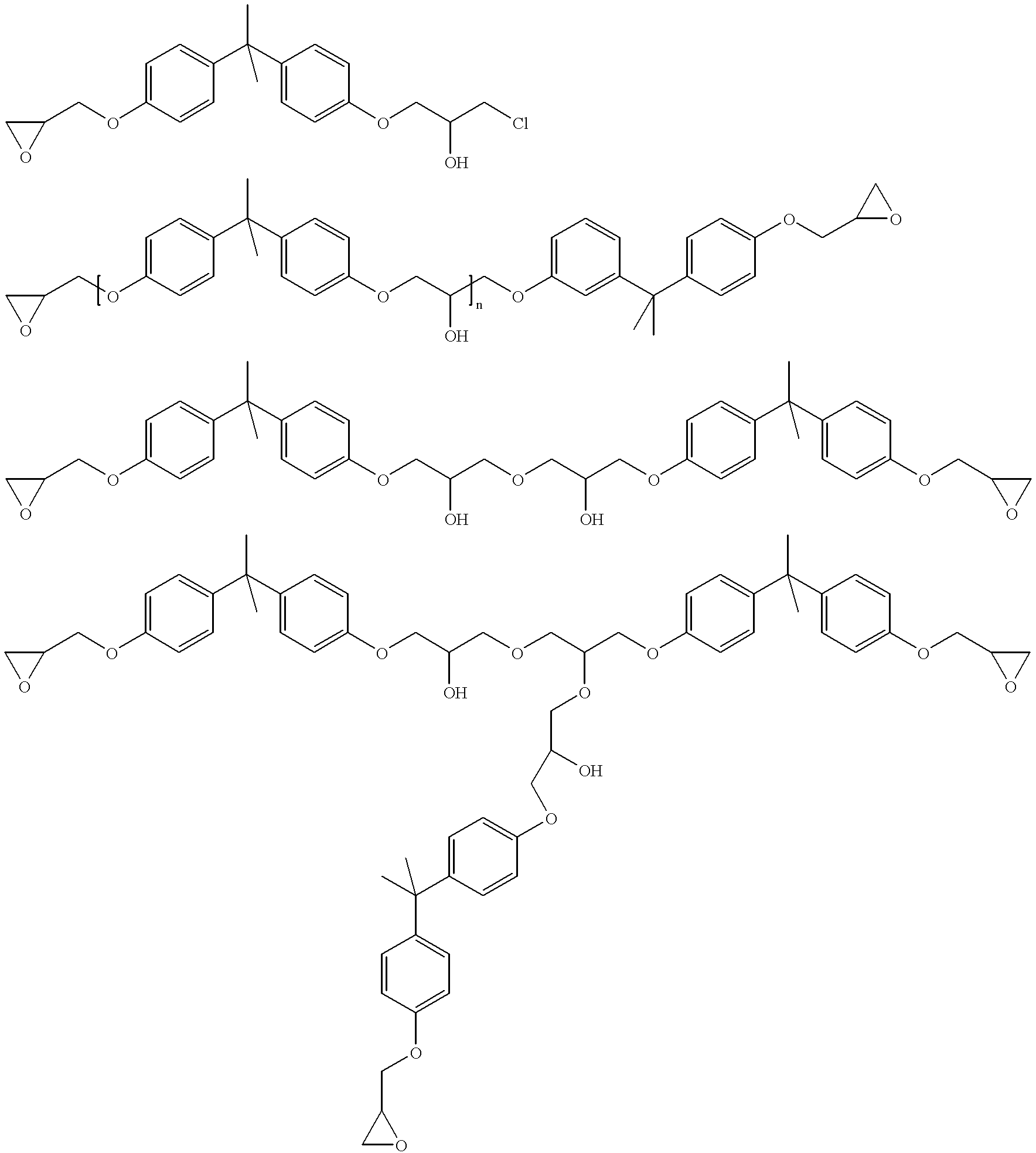
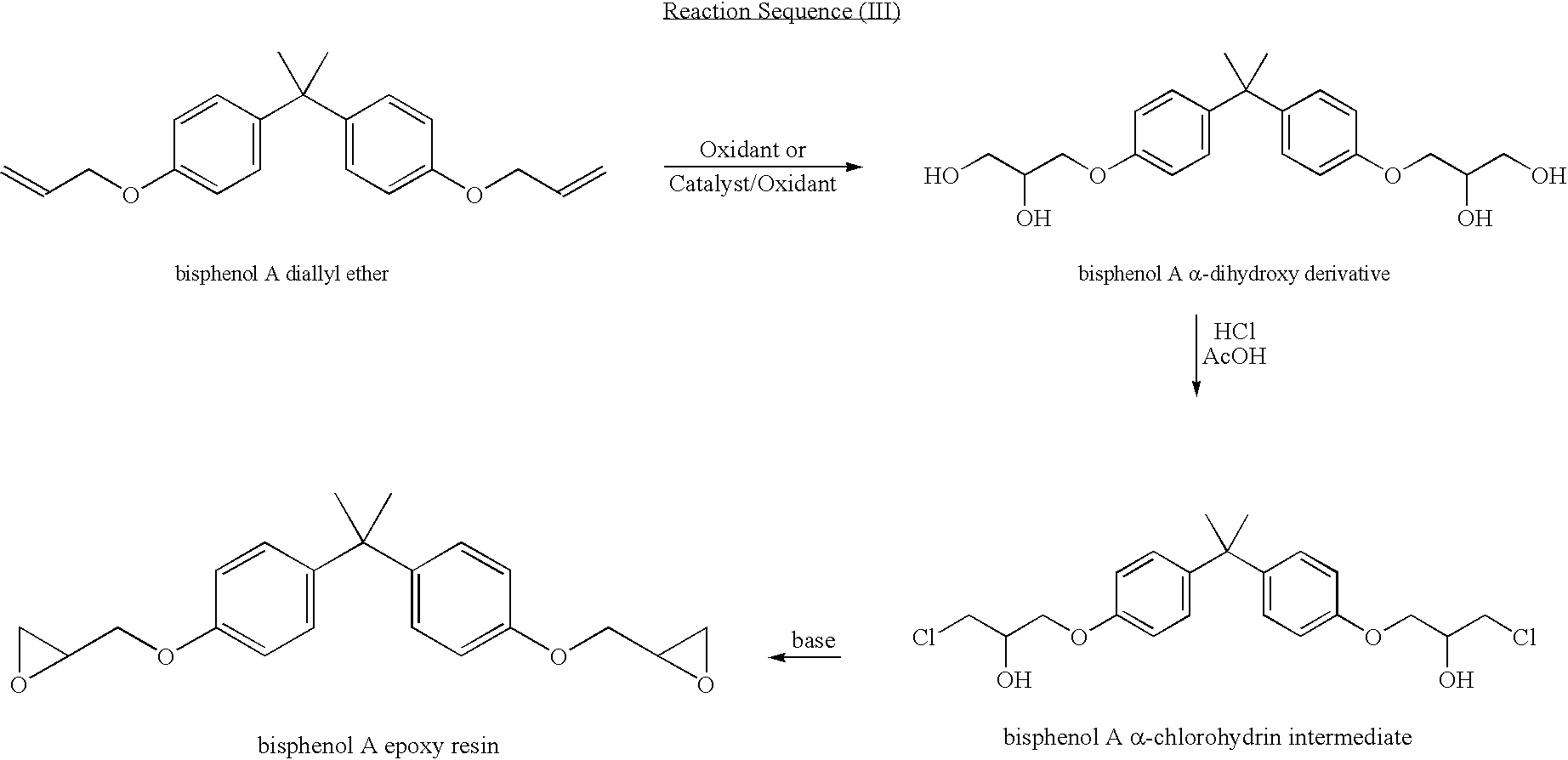
Process for manufacturing an alpha-dihydroxy derivative and epoxy resins prepared therefrom
A process for manufacturing an α-dihydroxy derivative from an aryl allyl ether wherein such α-dihydroxy derivative can be used to prepare an α-halohydrin intermediate and an epoxy resin prepared therefrom including epoxidizing an α-halohydrin intermediate produced from a halide substitution of an α-dihydroxy derivative which has been obtained by a dihydroxylation reaction of an aryl allyl ether in the presence of an oxidant or in the presence of an oxidant and a catalyst.
Owner:DOW GLOBAL TECH LLC
Creping adhesives comprising blends of high and low molecular weight resins
A composition comprising one or more high molecular weight resins and one or more low molecular weight resins in a ratio of about 1:99 to about 99:1 based on polymer actives, wherein said high molecular weight resins are selected from the group consisting of glyoxylated polyacrylamide, crosslinked polyaminoamide and polyaminoamide-epihalohydrin resins having a molecular weight of about 100,000 to about 5,000,000 Dalton and the low molecular weight resins are selected from the group consisting of glyoxylated polyacrylamide, crosslinked polyaminoamide and polyaminoamide-epihalohydrin resins having a molecular weight of less than about 100,000 Dalton and wherein the mole ratio of epihalohydrin to secondary nitrogen atoms in the high and low molecular weight polyaminoamide-epihalohydrin resins is less than about 0.5. and use of the composition for creping paper webs.
Owner:ECOLAB USA INC
Process for preparation of optically active halogeno hydroxypropyl compound and glycidyl compound
A process for preparing regioselectively an optically active 1-halogeno-2-hydroxypropyl compound of the following formula; wherein X is halogen atom and Nu is a heteroatom having a substituent,and an optically active glycidyl compound of the formula; which comprises reacting an optically active epihalohydrin of the formula; with a neucleophilic agent,in the presence of a metal complex of the formula; wherein n is an integer of 0, 1 or 2, Y1, Y2 and Y3 are hydrogen atom, etc., and Y2 and Y3 may form a ring such as benzene, A is a counterion and M is a metal ion, and further subjecting the compound (4) to reaction with a base to prepare the optically active glycidyl compound (5).
Owner:DAISO CO LTD
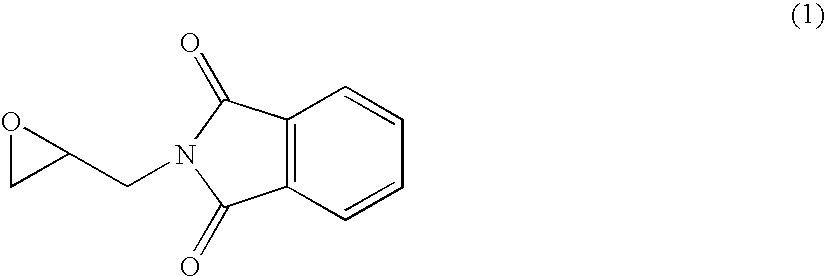
Process for preparing glycidylphthalimide
A process for preparing glycidylphtalimide or it optically active compound by reacting a phthalimide alkalimetal salt with an epihalohydrin or an optically active epihalohydrin in an alcohol solvent; or by reacting phthalimide with an epihalohydrin or an optically active epihalohydrin in the presence of an alkali metal carbonate, an alkali metal hydrogencarbonate or a quaternary ammonium salt to obtain a N-(3-halogeno-2-hydroxypropyl)phthalimide and then by cyclizing the product with an alkali metal alkoxide.
Owner:OSAKA SODA CO LTD
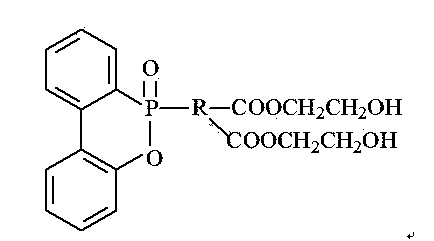
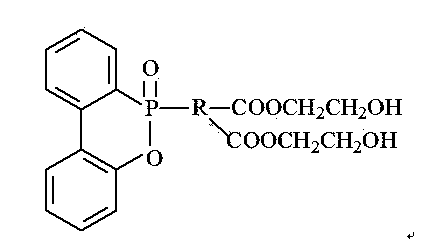
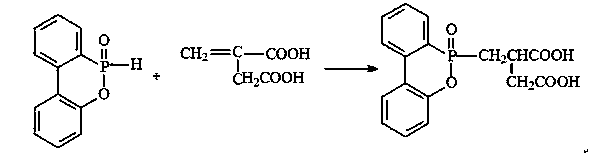
Phosphorus-containing flame retardant and preparation method thereof
ActiveCN103772740AIncrease profitLow viscosityGroup 5/15 element organic compoundsPtru catalystOrganic synthesis
The invention relates to the field of organic synthesis and provides a phosphorus-containing flame retardant and a preparation method thereof. The phosphorus-containing flame retardant has a chemical formula shown as a formula (i). The preparation method comprises the following steps: (1) adding DOPO and dicarboxylic acid into toluene, stirring and heating to 110+ / -2 DEG C, reacting for 2-6 hours, stopping heating, performing suction filtration on the reacted solution, obtaining white solids, drying, and preparing a DOPO dicarboxylic acid derivative; (2) dissolving the DOPO dicarboxylic acid derivative in a 2+ / -0.2 mol / L of potassium hydroxide solution at the temperature of 0-40 DEG C, stirring for 1.5-2.5 hours, and generating sylvite of the DOPO dicarboxylic acid derivative; (3) adding a phase transfer catalyst and halohydrin into the solution prepared in the step (2), reacting for 4-8 hours, layering the solution in a separating funnel, taking the lower layer, and obtaining the phosphorus-containing flame retardant. (img file='2014100188078100004dest-path-image001.TIF' wi='366' he='207' / ) (i).
Owner:SHOUGUANG WEIDONG CHEM
Method for the emulsification of ASA with polyamidoamine epihalohydrin (PAE)
The invention is directed towards methods and compositions for improving the sizing of paper using PAE. Proper sizing of paper requires that a sizing agent adequately disperse so as to evenly distribute on the paper. Proper sizing also requires that the agent not cause runnability or chemical problems with other materials or equipment used in the papermaking process. PAE displays unexpected attributes that allow it to disperse sizing agents. Best of all it is not subject to the same overdose effect of typical emulsifiers such as cationic starch. As a result PAE is an excellent option for use in sizing paper.
Owner:ECOLAB USA INC
Method for inhibiting hydrate formation
A sterically hindered quaternary ammonium composition is prepared by contacting a solvent having hydroxyl functionality, a halohydrin, and a sterically hindered tertiary amine, under reaction conditions sufficient to produce a sterically hindered quaternary ammonium compound. The reaction proceeds with excellent yield. The resulting compounds are particularly useful for inhibiting formation of hydrates in hydrocarbon reservoirs and pipelines. Novel compositions of matter include sterically hindered quaternary ammonium compounds conforming to the formulas C27H58XNO2 and C29H63XNO2, wherein X is a halogen.
Owner:BAKER HUGHES INC
Polymerizable type thioxanthone visible light initiator containing acrylate or methacrylate and preparation method
The invention discloses a polymerizable type thioxanthone visible light initiator containing acrylate or methacrylate and a preparation method of the polymerizable type thioxanthone visible light initiator. Thioxanthone containing an aromatic amino group can be directly reacted with various types of halogenated alcohol to generate thioxanthone containing an aliphatic hydroxyl group; the thioxanthone containing the aromatic amino group can also be reacted with 4-benzoyloxy benzyl chloride, and then is subjected to alcoholysis to generate thioxanthone containing a phenolic hydroxyl group; the thioxanthone containing the phenolic hydroxyl group can be reacted with the various types of halogenated alcohol to generate thioxanthone containing the aliphatic hydroxyl group; then the thioxanthone containing the phenolic hydroxyl group or the thioxanthone containing the aliphatic hydroxyl group is reacted with acryloyl chloride or methacryloyl chloride to form the polymerizable type thioxanthone visible light initiator containing an acrylate structure or a methacrylate structure. The compound disclosed by the invention has very good compatibility with a light curing system; in an application process, any additive does not need to be added, and any auxiliary agent does not need to be added; the polymerizable type thioxanthone visible light initiator is high in initiation efficiency, green and environment-friendly and low in energy consumption, meets the requirements of green chemistry, and has a wide application prospect in the field of visible light curing.
Owner:WUHAN UNIV
Process for producing epoxides
InactiveUS20100029960A1Promote conversionGood epoxide selectivityOrganic chemistryChemical industryReactor systemHalohydrin
A process for producing epoxide, the process including contacting an organic phase including at least one halohydrin(s) with at least one aqueous phase including a base in a plug-flow mixer / reactor system to disperse the organic phase in the aqueous phase via a mixing device imparting a power-to-mass ratio of at least 0.2 W / kg to convert at least a portion of the at least one halohydrin to an epoxide.
Owner:BLUE CUBE IP
Active Energy Ray Curable Aqueous Emulsions
The present invention relates to (meth)acrylated compounds (A) prepared from (a) at least one cyclic ether polyol, (b) at least one linking compound (b1) and / or (b2), wherein the linking compound (b1) is selected from cyclic compounds (b11) containing at least one (I) group in the cycle where X=O or NH, from hydroxy acids (b12) and / or from alkylene oxides (b13) containing from 2 to 4 carbon atoms and the linking compound (b2) is selected from epihalohydrins or polyisocyanates, (c) a (meth)acrylating compound; and to their use in radiation curable compositions for the coatings, inks, overprint varnishes, adhesives and composites.
Owner:CYTEC SURFACE SPECIALTIES INC +1
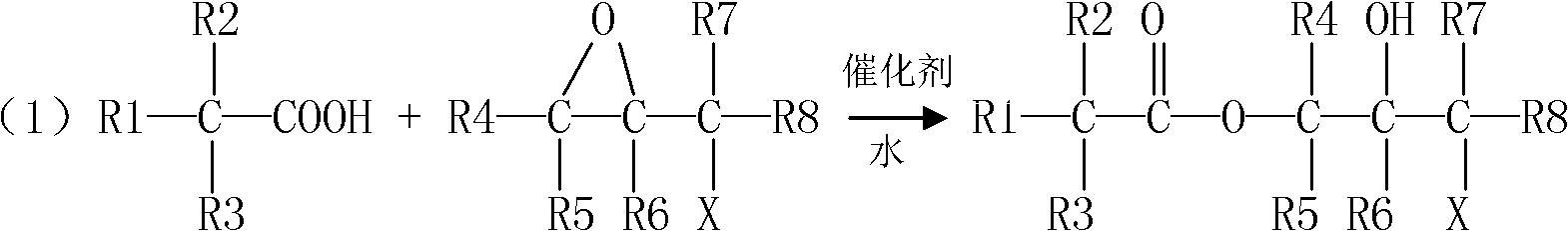
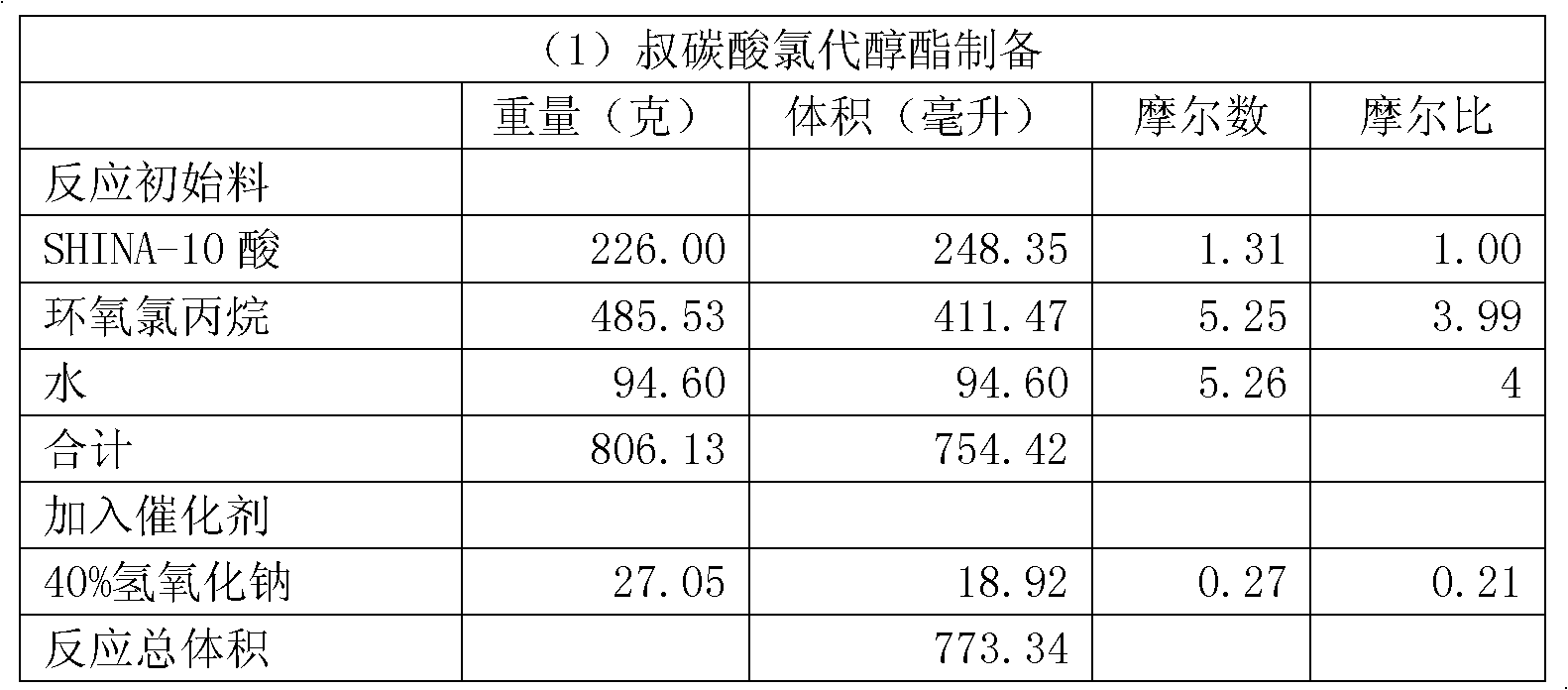
Preparation method for tert-carbonic acid glycidyl ester
ActiveCN103183652AHigh purityInitial color lightOrganic compound preparationCarboxylic acid esters preparationEpoxyGlycerol ester of wood rosin
The invention provides a preparation method for tert-carbonic acid glycidyl ester. The tert-carbonic acid glycidyl ester is synthesized through two steps, i.e., a first step of subjecting tert-carbonic acid and a halogenated epoxy compound to a reaction under the action of a catalyst to produce tert-carbonic acid halohydrin ester and a second step of removing halogen hydride from the tert-carbonic acid halohydrin ester to form the tert-carbonic acid glycidyl ester, wherein in the first step of synthesis of the tert-carbonic acid halohydrin ester, the tert-carbonic acid reacts with the halogenated epoxy compound only in the presence of water and the catalyst and the water comprises water added in the reaction in advance. With the preparation method provided by the invention, product output per unit volume can be substantially increased, and the method is especially applicable to large-scale industrial production of tert-carbonic acid glycidyl ester with low cost, high purity and a light and stable color.
Owner:河北四友卓越科技有限公司
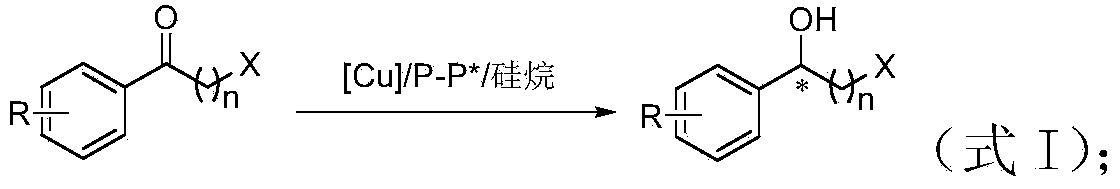
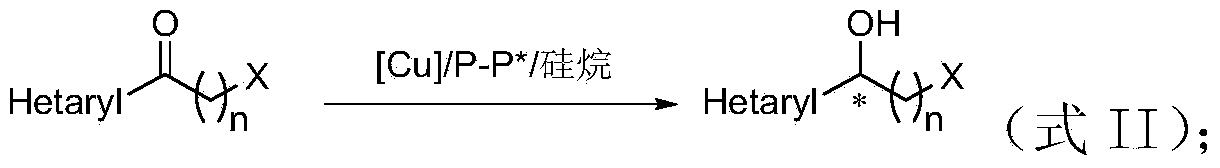
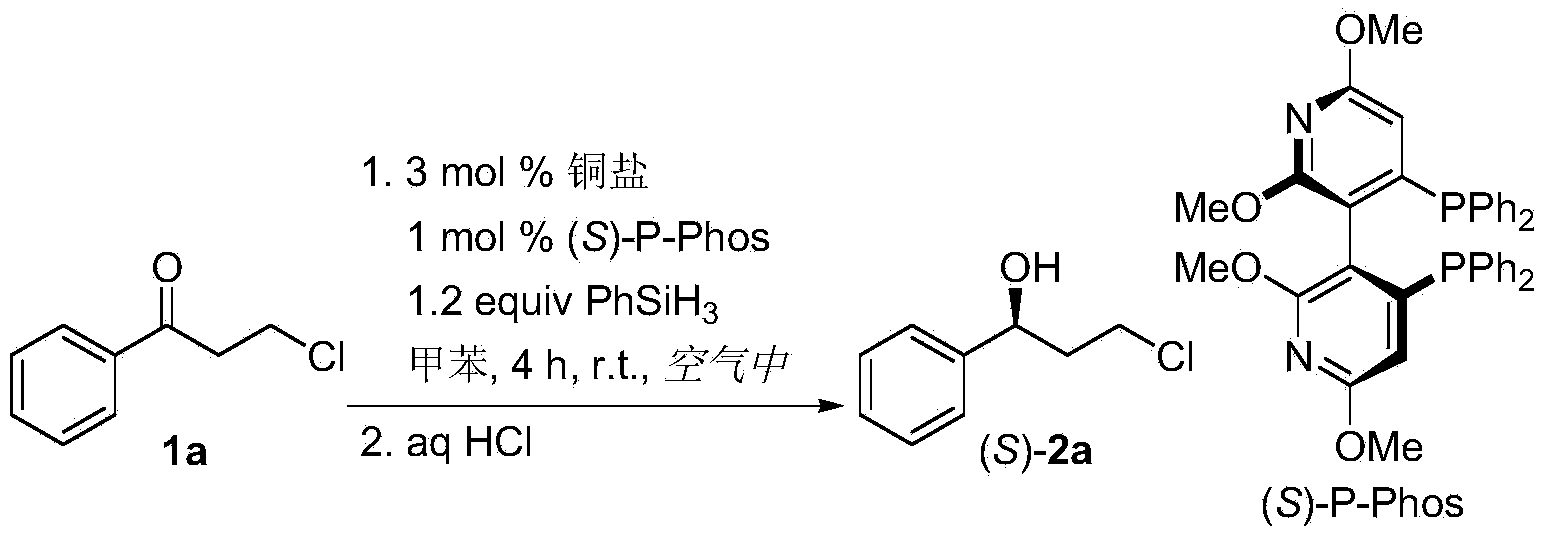
Method for preparing chirality halohydrin in copper-catalyzed asymmetry hydrosilation mode
InactiveCN103524307AHigh yieldHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholNitrogen
The invention discloses a method for preparing chirality halohydrin in a copper-catalyzed asymmetry hydrosilation mode. In the prior art, mostly a noble metal catalyst is needed, the research is generally limited in catalysis asymmetry reduction of alpha-haloketone, and documents about catalysis asymmetry reduction of beta-, gamma-, epsilon- or other haloketone substrates are less related. The method disclosed by the invention comprises preparing chirality gamma-, delta- or zeta-haloalkyl aryl alcohol from beta-, gamma-, epsilon-haloalkyl aryl ketone in a copper catalysis asymmetry hydrosilation reduction mode, or preparing chirality beta-, gamma- or delta-haloalkyl aryl alcohol from alpha-, beta- or gamma-haloalkyl aryl ketone in the copper catalysis asymmetry hydrosilation reduction mode. The method adopts a non-noble metal catalyst, the raw material is easy to obtain, nitrogen protection is not needed, the reaction condition is mild, the operation is simple, and the yield and the enantioselectivity of the reaction are high.
Owner:HANGZHOU NORMAL UNIVERSITY
Epoxy resin adducts and thermosets therefrom
An epoxy resin adduct including the reaction product of (A) at least one polyfunctional aliphatic or cycloaliphatic epoxy resin; and (B) at least one reactive compound; wherein the polyfunctional aliphatic or cycloaliphatic epoxy resin is isolated from the epoxy resin formed by the epoxidation of (i) an aliphatic or cycloaliphatic hydroxyl-containing material using (ii) an epi-halohydrin, (iii) a basic-acting substance, (iv) a non-Lewis acid catalyst, and (v) optionally one or more solvents; and wherein the re-active compound (B) comprises one or more compounds having two or more reactive hydrogen atoms per molecule and the reactive hydrogen atoms are reactive with epoxide groups. A curable epoxy resin composition includes the adduct described above. A cured epoxy resin is prepared by a process of curing the curable epoxy resin composition containing the adduct described above.
Owner:BLUE CUBE IP
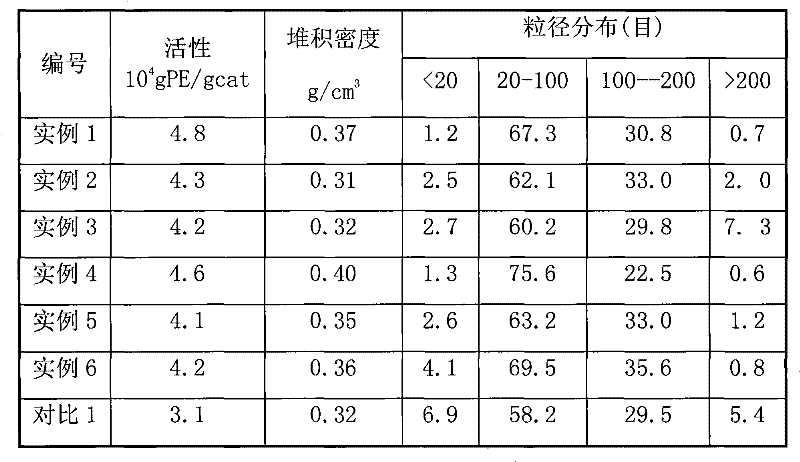
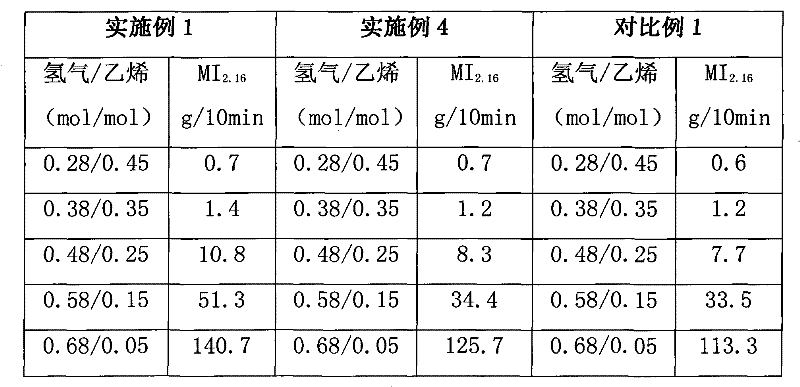
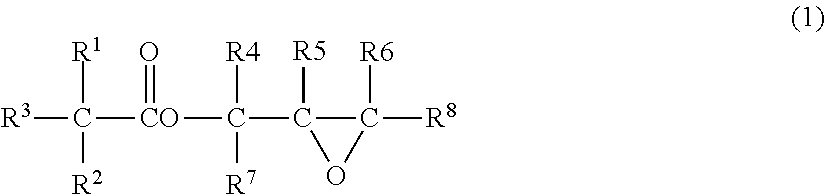
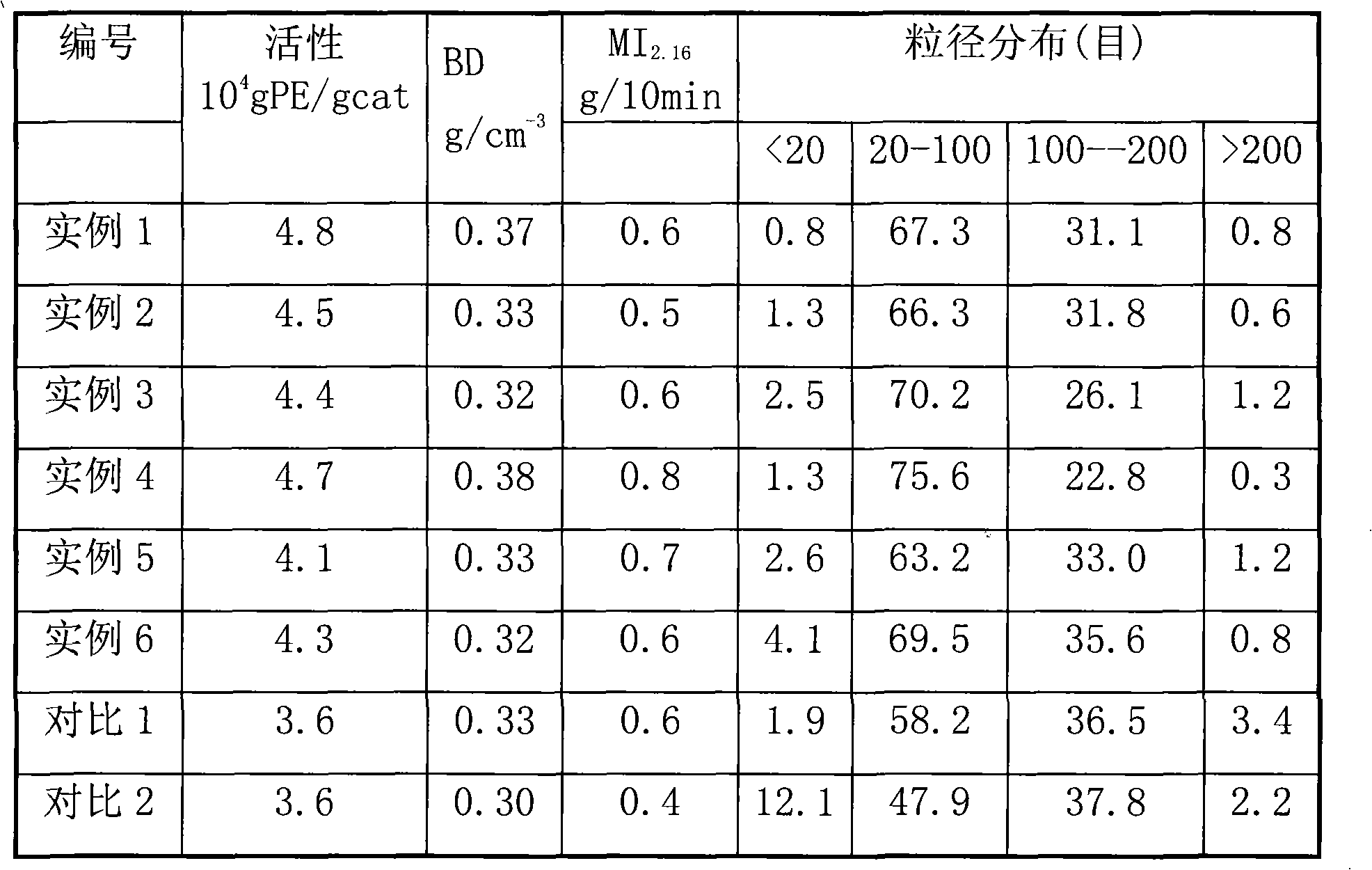
A kind of catalyst component and catalyst for ethylene polymerization
The present invention is a catalyst component for ethylene polymerization, which is formed by dissolving magnesium halide in organic epoxy compounds and organic phosphorus compounds to form a uniform solution, adding halogenated alcohols and alcohols, and in the presence of organosilicon compounds without active hydrogen in the precipitation aid Under the role of titanium compounds obtained. Due to the addition of halohydrin compounds, the catalyst exhibits higher polymerization activity, better hydrogen adjustment sensitivity and better bulk density when used in ethylene polymerization. And adopt the organosilicon compound without active hydrogen as precipitation aid, help to improve the activity of catalyst and the improvement of the particle form of catalyst, the catalyst particle form of preparation is better; Do not need to use a large amount of titanium tetrachloride to promote precipitation Precipitation and repeated use of titanium tetrachloride to deal with the precipitate resulted in a significant reduction in the amount of titanium tetrachloride added.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthetizing hydrogenated bisphenol A epoxy resin under catalysis of ammonium salt
The invention discloses a method for synthetizing a hydrogenated bisphenol A epoxy resin under the catalysis of ammonium salt. According to the method, epihalohydrin such as hydrogenated bisphenol A, epichlorohydrin and the like are taken as raw materials, simultaneously, an ammonium salt catalyst and alkali are added, a ring-opening etherification reaction is performed at the temperature of 30 DEG C-100DEG C, and halogen alcohol ether is obtained; then, the alkali is continuously added in the same reaction system, and a ring-closing epoxidation reaction is performed at the temperature of 20 DEG C-80 DEG C; and the hydrogenated bisphenol A epoxy resin is obtained through posttreatment processes such as filtering, washing, separation, reduced pressure distillation and the like. A product is measured to be superior to number one with a GB-T12007.1-1989 epoxy resin color measuring method-Gardner colorimetric method.
Owner:NANJING UNIV
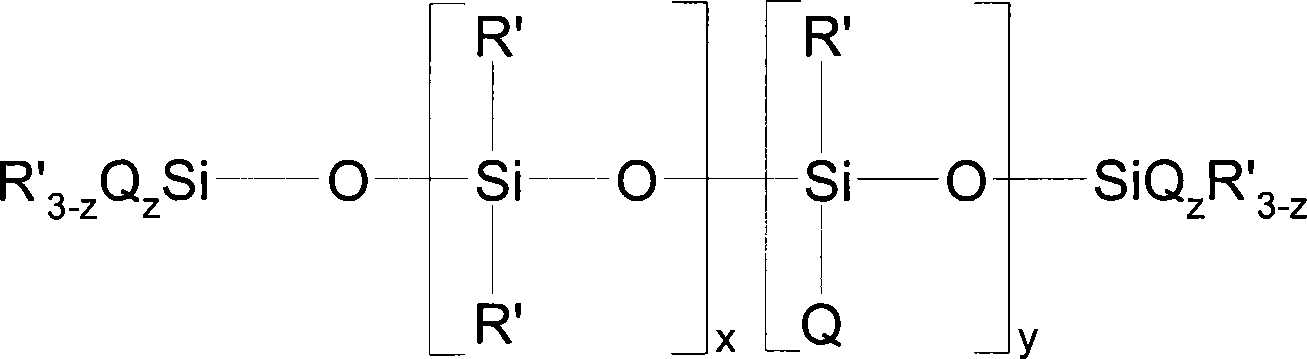
Method for preparing organosilicon emulsion containing elastomer polysiloxane with quaternary ammonium radical
InactiveCN101386678AGood spreadabilityCosmetic preparationsHair cosmeticsElastomerAmmonium compounds
The invention relates to an organosilicon emulsion containing elastic body polyorganosiloxane with quaternary ammonium functionality and polydiorganosiloxane with a non-reactivity organic group. The organosilicon emulsion is obtained through the following method: in the presence of the polydiorganosiloxane with the non-reactivity organic group not reacting with other components (such as polyorganosiloxane with an amino group, an organic quaternary ammonium compound and a crosslinking agent) and a surfactant, the polyorganosiloxane with the amino group and the organic quaternary ammonium compound with an epoxy group or a halohydrin group react simultaneously, the crosslinking agent is used to crossly link the polyorganosiloxane with the amino group, and the components are dispersed in a polarity water phase. The organosilicon emulsion can be applied to various fields of textile fabrics, personnel nursing articles, and the like.
Owner:DOW CORNING SHANGHAI
Process for preparing glycidyl esters of branched monocarboxylic acids
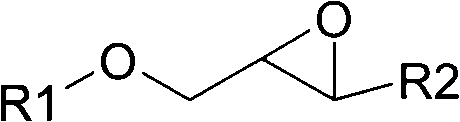
Accordingly, the invention relates to a process for the preparation of a glycidyl ester of a branched monocarboxylic acid by reacting an aliphatic monocarboxylic acid of the formula R1R2R3COOH, wherein R1, R2, and R3 each independently represent an alkyl radical of normal or branched structure containing from 1 to 20 carbon atoms and an epoxyalkyl halide containing from 3 to 13 carbon atoms in the presence of a catalyst, whereina greater than stoichiometric amount of epoxyalkyl halide is reacted with the acid(e.g., preferably in the molar ratio of epoxyalkyl halide to acid that is in the range of from 1.02:1 to 1.50:1) to form an intermediate reaction product comprising a halohydrin,the epoxyalkyl halide is added to the acid with appropriate cooling of the reactants and / or the reaction mixture to keep the temperature of the reaction mixture below 80° C., whereupon the epoxyalkyl halide and the acid are reacted at a temperature below 80° C. (preferably in the range of from 55 to 75° C.) for a time sufficient to reduce the amount of acid to no more than 2 wt % but no less than 0.1 wt % calculated on the initial amount of acid,optionally removing any excess epoxyalkyl halide from the reaction product prior to the ring closure reaction,subjecting the reaction product to a ring closure reaction (DHC) and optionally to one or more after treatments (ADHC) for removal of any remaining halo functionality.
Owner:HEXION INC
Catalyst component for ethylene polymerization and catalyst
The invention discloses a catalyst component for ethylene polymerization, which is prepared by dissolving magnesium halide in an organic epoxy compound and an organic phosphorous compound to form uniform solution, adding a halohydrin compound and an alcohol, and reacting with a titanium compound in the presence of an active hydrogen-free organic silicon compound auxiliary precipitator. In the invention, because of the use of the active hydrogen-free organic silicon compound as the auxiliary precipitator and the inter-molecular construction function of halohydrin, the catalyst particles can precipitate easily in the preparation process of the catalyst component without using a large amount of titanium tetrachloride to promote the precipitation of the precipitate nor using titanium tetrachloride to treat the precipitate for many times, so the consumption of titanium tetrachloride is reduced greatly. Meanwhile, the addition of the organic silicon compound helps to improve the activity and particle form of the catalyst, and when used for ethylene polymerization, the catalyst shows high polymerization activity and high hydrogen adjustment sensitivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for producing epoxides
A process for producing epoxides, the process including: (a) feeding at least one aqueous alkali and at least one halohydrin to a reactive distillation column; (b) concurrently in the reactive distillation column: (i) reacting at least a portion of the halohydrin with the alkali to form an epoxide; and (ii) stripping water and the epoxide from a basic aqueous residue; (c) recovering the water and the epoxide from the reactive distillation column as an overheads fraction; and, (d) condensing and phase separating the overheads fraction at a temperature of 50° C. or less to form an organic overheads fraction including the epoxide and an aqueous overheads fraction including water.
Owner:BLUE CUBE IP
Process for producing optically active chroman derivative and intermediate
InactiveUS20050014818A1Efficient productionHighly practicalBiocideSulfonic acid esters preparationHemiacetalHalohydrin
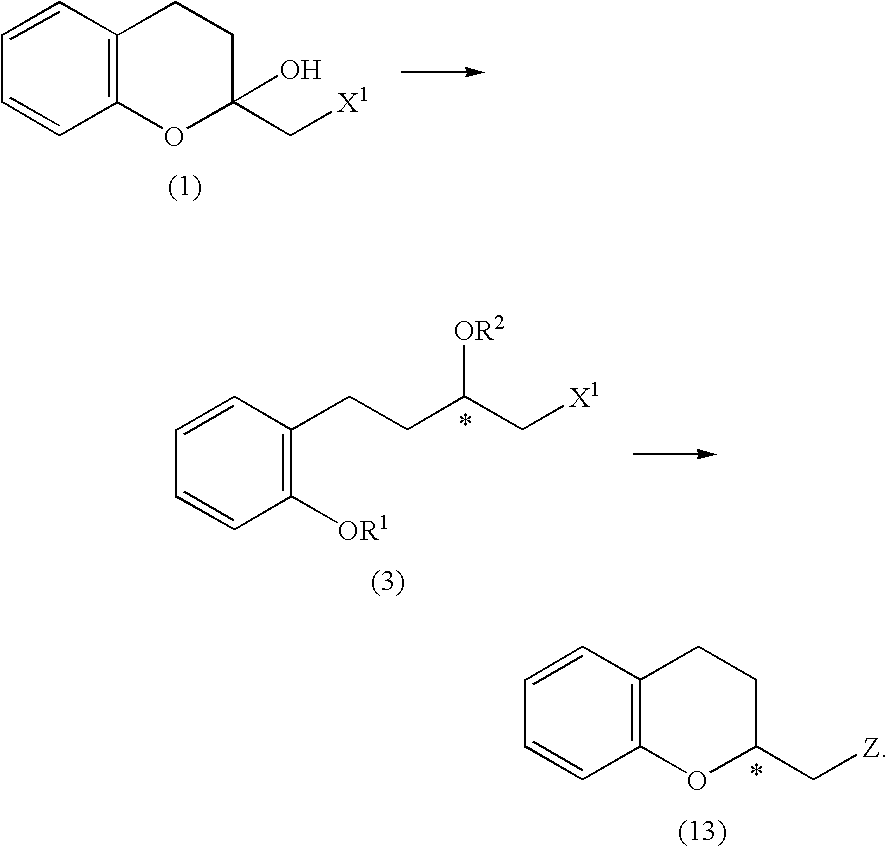
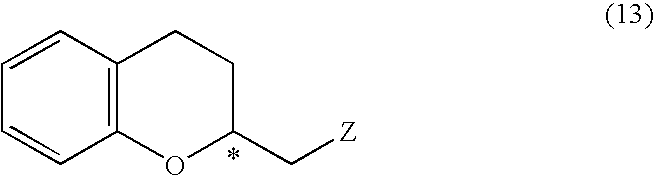
A process for easily producing various optically active chroman derivatives that are useful as pharmaceutical intermediates from inexpensive starting materials is provided. Cyclic hemiacetal (1) obtained from dihydrocoumarin through one step is asymmetrically reduced to produce an optically active halohydrin derivative (3), and the optically active halohydrin derivative (3) is cyclized to produce an optically active chroman derivative (13):
Owner:KANEKA CORP
Preparation of (8E, 10E)-8,10- dodecadienol-1-alcohol
The invention discloses a method for preparing (8E, 10E)-8, 10-dodecyl diene-1-alcohol. The method comprises the following steps: chlorine hexanol and trimethyl chlorosilane are reacted under alkaline conditions to form chlorine hexyloxy trimethyl silicane which is subjected to Grignard reaction to obtain Grignard reagent, (2E, 4E)-2, 4-hexadiene-1-alcohol acetic ester and the Grignard reagent are subjected to coupling reaction in the presence of an Li2CuCl4 catalyst to from (8E, 10E)-8, 10-dodecyl diene-1-O-trimethyl silane which is dissolved in solution of methanol and water, and paratoluenesulfonic acid is added into the solution to react to obtain the (8E, 10E)-8, 10-dodecyl diene-1-alcohol. The method uses the trimethyl chlorosilane which has advantages of low price and easy purchase in market and can lower cost as a hydroxyl protecting agent of halohydrin. The method does not produce malodor during reaction, can absorb by-products, has no pollution to environment, can not influence health of human body, and is suitable for industrialized production.
Owner:JIANGSU INST OF ECOMONES +1
Clean fracturing fluid for oil fields and preparation method thereof
ActiveCN105820805AImprove performanceLow temperature resistanceDrilling compositionOrganic acidFracturing fluid
The invention discloses clean fracturing fluid for oil fields and a preparation method thereof, belonging to the technical field of oil production engineering in the petroleum industry. The clean fracturing fluid can be obtained by synthesizing a gemini surfactant by using organic acids, an N,N-dimethylamino compound, an N,N'-diaminoalkylethylenediamine compound and halohydrin as raw materials and then stirring a water solution of the gemini surfactant and an ammonium chloride solution according to a certain proportion. Compared with traditional fracturing fluid, the clean fracturing fluid has good temperature and shear resistance and good sand-carrying property, dispenses with gel breakers and reverse discharge and is convenient to use.
Owner:HIGH & NEW TECH RES CENT OF HENAN ACAD OF SCI
Process for the elimination of materials containing hydrolyzable halides and other high molecular weight materials from epihalohydrin derived epoxy resins
The present invention provides a process for eliminating contaminants from epihalohydrin-derived epoxy resins. Another embodiment of the present invention is an epoxy product formed using said process. Yet another embodiment of the present invention is an epoxy derived in part from epihalohydrin wherein said epoxy is has a hydrolyzable halogen content of less than 10 ppm and an epoxide equivalent weight within 2 percent of the theoretical epoxide equivalent weight.
Owner:3M INNOVATIVE PROPERTIES CO
Synthetic method for fluoromethylation of halogenated alcohols
A method for fluoromethylation of a halogenated alcohol. The method includes refluxing a halogenated alcohol with a dihalomethane under basic conditions in a first solvent to form a halomethyl ether and fluorinating the halomethyl ether in the presence of a fluorinating agent.
Owner:ABBOTT LAB INC
Process for producing epoxides
A process for producing epoxides, the process including: (a) feeding at least one aqueous alkali and at least one halohydrin to a reactive distillation column, wherein the reactive distillation column includes a feed zone, a top zone disposed above the feed zone, and a bottom zone disposed below the feed zone; (b) concurrently in the reactive distillation column: (i) reacting at least a portion of the halohydrin with the alkali to form an epoxide; and (ii) stripping water and the epoxide from a basic aqueous residue; (c) recovering the water and the epoxide from the reactive distillation column as an overheads fraction; (d) condensing and phase separating the overheads fraction to form an organic overheads fraction including the epoxide and an aqueous overheads fraction including water; and (e) maintaining a liquid holdup per plate in the feed zone at a residence time of 10 seconds or less.
Owner:BLUE CUBE IP
Creping adhesives and methods for making and using same
InactiveUS20170204565A1Creping adhesives additionNon-macromolecular organic additionAdhesiveHalohydrin
Creping adhesives and methods for making and using same are provided. The creping adhesive can include a first thermosetting polyamidoamine-epihalohydrin resin that includes a reaction product of a first epihalohydrin and a first polyamidoamine containing one or more secondary amine groups, a first thermoplastic polyamidoamine-epihalohydrin resin that includes a reaction product of a second epihalohydrin and a second polyamidoamine containing one or more secondary amine groups, and one or more re-wetting agents. The first thermosetting polyamidoamine-epihalohydrin resin can have a weight average molecular weight of about 800,000 to about 1,200,000 and a molar ratio of the first epihalohydrin to the secondary amine groups of about 0.002:1 to about 0.1:1. The first thermoplastic polyamidoamine-epihalohydrin resin can have a weight average molecular weight of about 40,000 to about 200,000 and a molar ratio of the second epihalohydrin to the secondary amine groups of about 0.001:1 to about 0.1:1.
Owner:ECOLAB USA INC
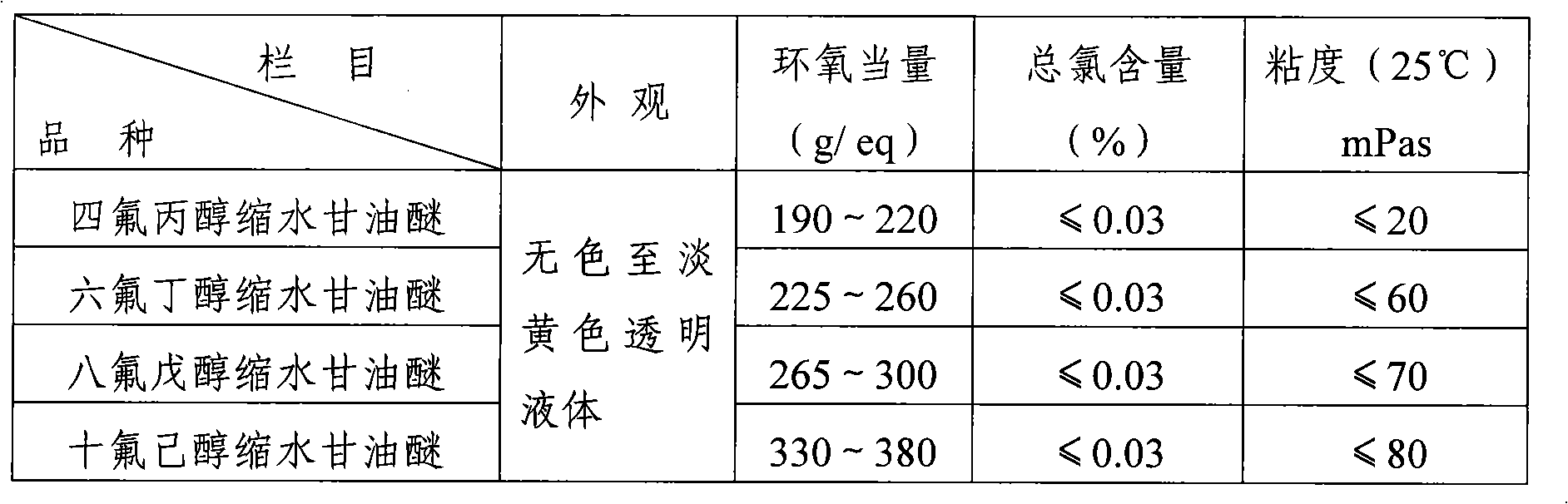
Fluorine-containing epoxide resin reactive diluent and preparation thereof
InactiveCN101353331AImprove featuresImprove overall performanceOrganic chemistrySolventCombinatorial chemistry
The invention provides a novel reactive diluent of a fluoro-epoxy resin and a preparation method thereof. In the invention, fluoro-alcohol and epihalohydrin are adopted as the materials; Lewis acid is used as a catalyst for carrying out a ring-opening etherification; then alkaline matter is added for carrying out a ring-closing reaction; after the post-treatment techniques of washing, filtering and solvent removing, etc., the novel reactive diluent of the fluoro-epoxy resin is obtained. The diluent provided by the invention has no thrill taste; the diluting effect is better than common reactive diluents; in addition, the reactive diluent can effectively improve the defects of poor weatherability, incomplete penetration, poor fire resistance and being incapable of resisting high temperature; the reactive diluent has the advantages of innocuity, non-corrosiveness, convenient operation, and safe use, etc.
Owner:ZHONGHAO CHENGUANG RES INST OF CHEMICALINDUSTRY CO LTD
Method for preparing gefitinib intermediate
The invention relates to a method for preparing a gefitinib intermediate, in particular to a method for preparing 4-chlorine-6-(3-chlorine propoxy)-7-methoxy quinazoline. A formula II compound 6-(3-hydroxy propoxy)-7-methoxy quinazoline-4 (3H)-ketone and thionyl chloride are reacted to obtain the gefitinib intermediate. The formula II compound uses 5-hydroxy-4-methoxy group-2-nitrobenzene methyl formate as a raw material to be condensed with halohydrin shown in a formula VI, nitro is reduced, and formamidine acetate cyclization and thionyl chloride chlorination are reacted to obtain the gefitinib intermediate. The synthesis scheme provided by the invention reduces the generation of by-products by introduction of hydroxyl, the production cost is reduced, meanwhile, the environment pollution is reduced, the quality of products is increased, and the purity of the prepared 4-chlorine-6-(3-chlorine propoxy)-7-methoxy quinazoline is over 98 percent. Compared with the prior art, the method is greatly increased and improved, wherein, X is halogen.
Owner:浙江瑞博制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com