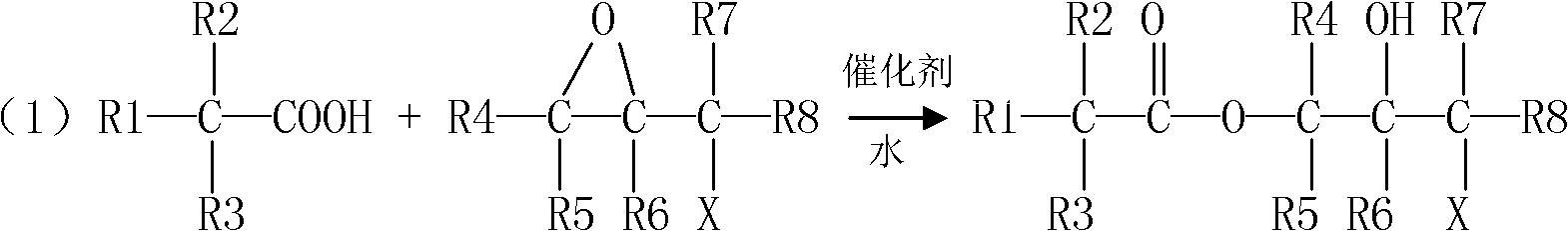
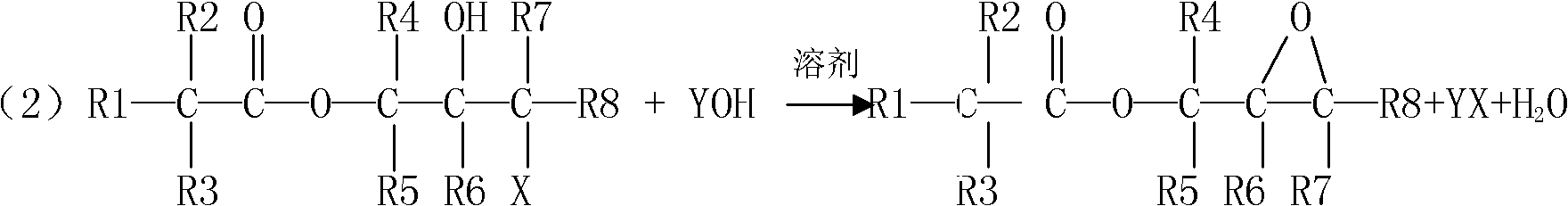Preparation method for tert-carbonic acid glycidyl ester
A technology of glycidyl ester and tertiary carbonic acid, which is applied in the field of organic compound synthesis, can solve problems such as loss of raw materials and products, formation of waste water or solid waste, and decline in product quality, and achieve the goals of reducing raw material consumption, improving product quality, and reducing emissions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
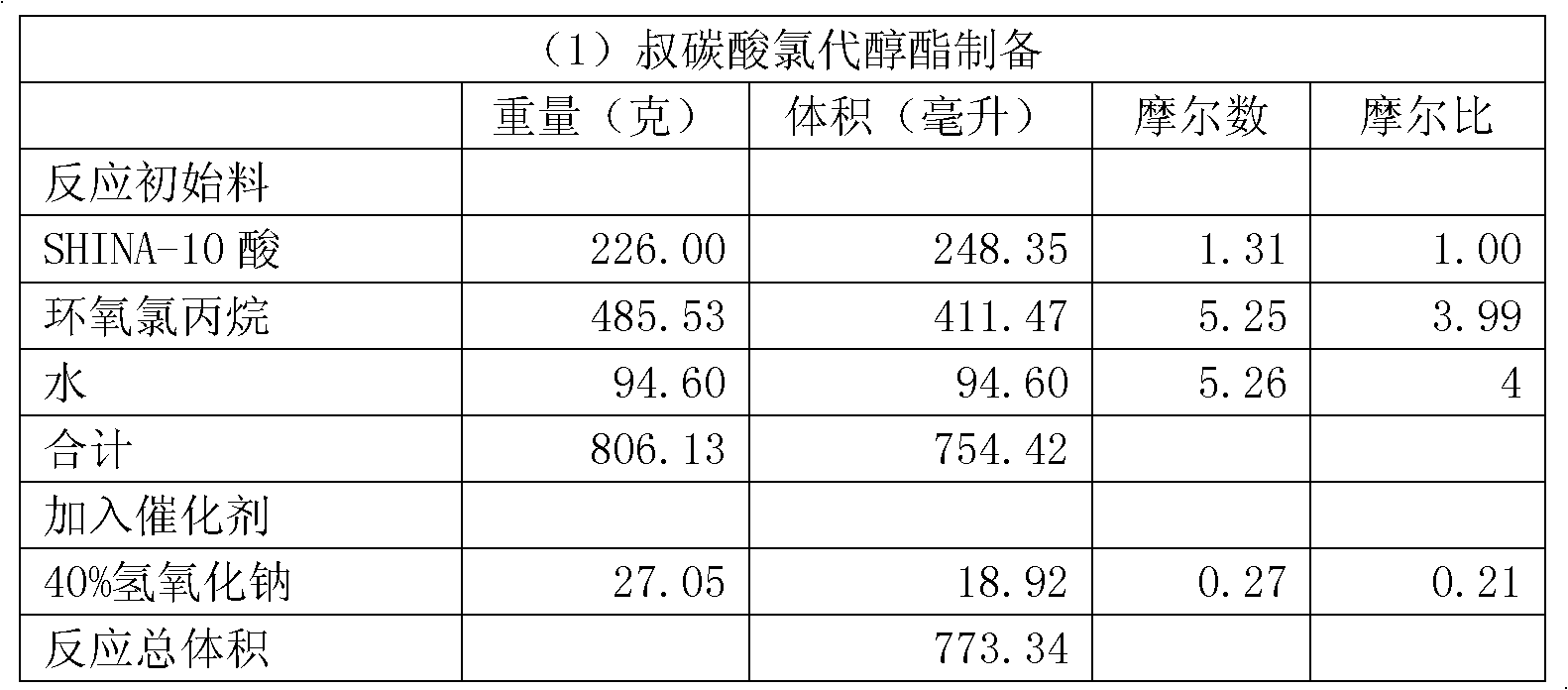
[0069] (1) Preparation of chlorohydrin tertiary carbonic acid ester
[0070] Add the reaction starting material to a 1-liter reactor equipped with a mechanical stirrer, a water bath heater and a reflux device.
[0071]
[0072] The SHINA-10 acid mentioned in the above table and below is neodecanoic acid, which is purchased from the open market, wherein SHINA is a trademark of Hebei Siyou Excellence Technology Co., Ltd.
[0073] It should be noted that the "water" listed in the table in this embodiment and the following embodiments is the amount of water added in advance when the first step reaction starts.
[0074] Start stirring, and under rapid stirring, the initial reaction material is heated to 50-55° C., and then 40% (by weight) sodium hydroxide aqueous solution is added within 20-25 minutes, adding a total of 27.05 grams.
[0075] In the present embodiment, tertiary carbonic acid: total water = 1 mole: 4.91 moles, wherein, tertiary carbonic acid: water added: water b...
Embodiment 2
[0097] Repeat the process of Example 1, change the addition of raw materials, specifically refer to the following table.
[0098] In this embodiment, tertiary carbonic acid: total water = 1 mole: 3.24 moles. Wherein, tertiary carbonic acid: the water that adds: the water that sodium hydroxide brings in: the water that acid-base reaction produces=1 mole: 2.33 mole: 0.7 mole: 0.21 mole.
[0099]
[0100]
Embodiment 3
[0103] Embodiment 3 repeats the process of embodiment 1, but keeps tertiary carbonic acid and epichlorohydrin mol ratio=1: 2, tertiary carbonic acid: total water amount=1 mole: 3.24 moles, the tertiary carbonic acid in the synthetic tertiary carbonic acid chlorohydrin ester process Molar ratio with water=1:2.33. Specifically, tertiary carbonic acid: added water: water brought in by sodium hydroxide: water produced by acid-base reaction=1 mole: 2.33 mole: 0.7 mole: 0.21 mole.
[0104] The mol ratio of tertiary carbonic acid and Virahol=1:1 in the synthesis process of tertiary carbonic acid glycidyl ester.
[0105]
[0106]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com