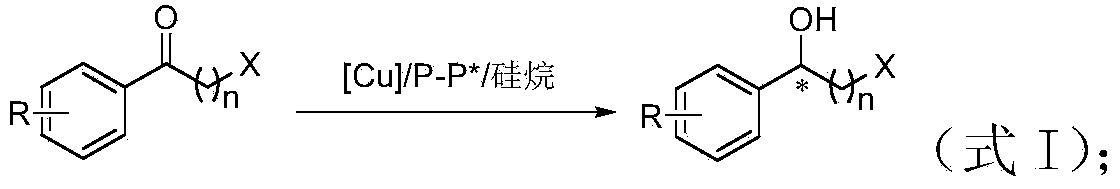
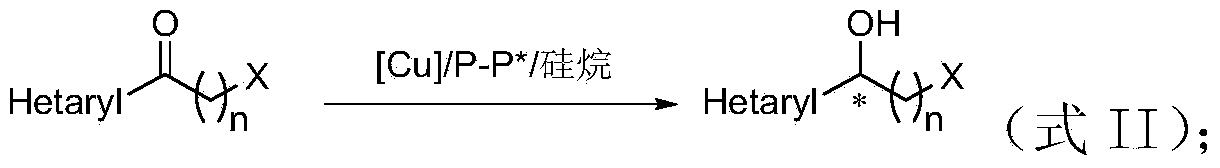
Method for preparing chirality halohydrin in copper-catalyzed asymmetry hydrosilation mode
An asymmetric, hydrosilation technology, applied in organic chemistry methods, chemical instruments and methods, preparation of hydroxyl compounds, etc., to achieve atomic economy and environmental friendliness, mild reaction conditions, high yield and enantioselectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
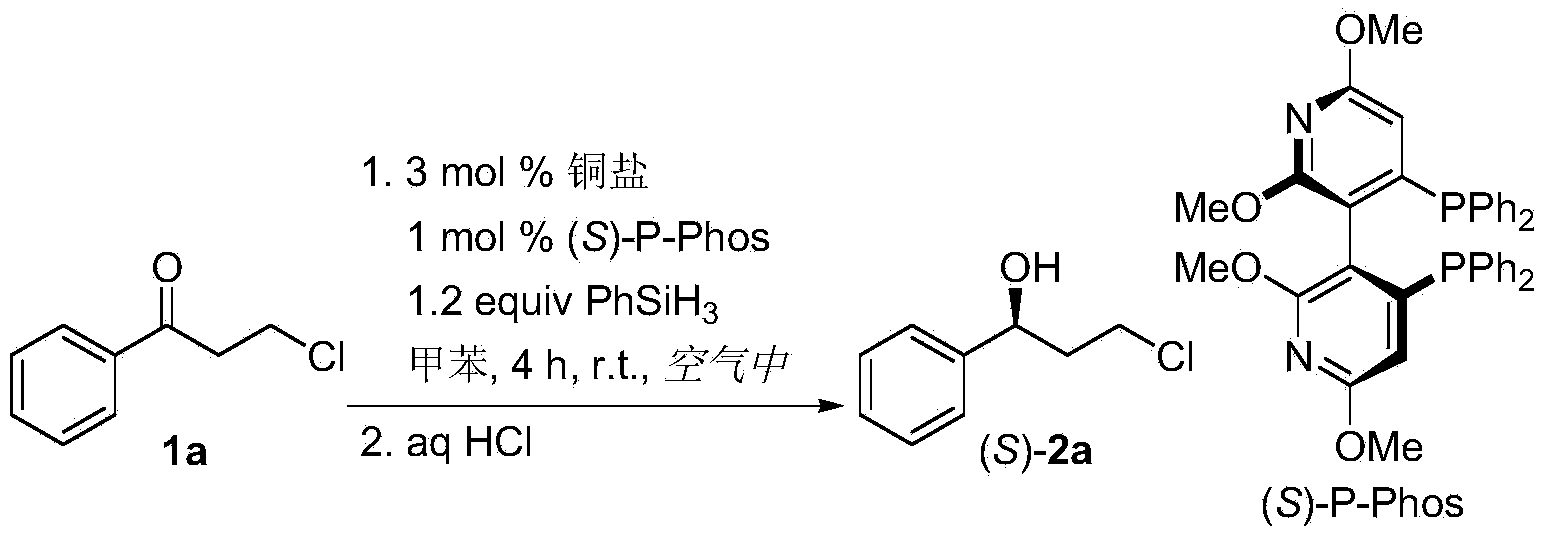
[0026] Embodiment 1: the screening of copper salt
[0027] Using the asymmetric hydrosilylation reaction of 3-chloro-1-phenyl-1-propanone (1a) as a model reaction, using (S)-P-Phos as a chiral ligand, toluene as a solvent, PhSiH 3 A screening of different copper salts was carried out for silanes with the following reaction:
[0028]
[0029] The specific operation of the catalytic asymmetric hydrosilylation reaction is as follows: Cu salt (9.0×10 –3 mmol) and the chiral ligand (S)-P-Phos (1.9mg, 3.0×10 –3 mmol) into a 25mL reaction tube with a stirring bar, add 0.5mL toluene, stir at room temperature for 15 minutes, then add PhSiH 3 (45μL, 3.6mmol), continue to stir for 10 minutes, add 3-chloro-1-phenyl-1-propanone (1a, 50.6mg, 0.3mmol) in toluene (0.5mL) solution, stir vigorously, and use TLC to detect the reaction process . After reacting for 4 hours, add 10% hydrochloric acid solution (2mL), extract with ether (3×3mL), combine the organic phases, wash with water, anh...
Embodiment 2
[0034] Example 2: Screening of Chiral Dual Phosphorous Ligands
[0035] Using the asymmetric hydrosilylation reaction of 3-chloro-1-phenyl-1-propanone (1a) as a model reaction, Cu(OAc) 2 ·H 2 O is the catalyst precursor, toluene is the solvent, PhSiH 3 Screening of different chiral ligands for silane, the reaction formula and the structure of each chiral diphosphorus ligand are as follows:
[0036]
[0037] The specific operation of catalytic asymmetric hydrosilylation reaction is as follows: Cu(OAc) 2 ·H 2 O (1.8mg, 9.0×10 –3mmol) and chiral bisphosphorus ligand (3.0×10 –3 mmol) into a 25mL reaction tube with a stirring bar, add 0.5mL toluene, stir at room temperature for 15 minutes, then add PhSiH 3 (45μL, 3.6mmol), continue to stir for 10 minutes, add 3-chloro-1-phenyl-1-propanone (1a, 50.6mg, 0.3mmol) in toluene (0.5mL) solution, stir vigorously, and use TLC to detect the reaction process . After reacting for 4 hours, add 10% hydrochloric acid solution (2mL), ex...
Embodiment 3
[0042] Embodiment 3: the influence of solvent and temperature of reaction
[0043] Using the asymmetric hydrosilylation reaction of 3-chloro-1-phenyl-1-propanone (1a) as a model reaction, Cu(OAc) 2 ·H 2 O is the catalyst precursor, (S)-P-Phos or (S)-Xyl-P-Phos is the chiral diphosphorus ligand, PhSiH 3 Screening of different solvents and optimization of reaction temperature were carried out for silane, and the reaction formula is as follows:
[0044]
[0045] The specific operation of catalytic asymmetric hydrosilylation reaction is as follows: Cu(OAc) 2 ·H 2 O (1.8mg, 9.0×10 –3 mmol) and chiral bisphosphorus ligand (3.0×10 –3 mmol) into a 25mL reaction tube with a stirring bar, add 0.5mL solvent, stir at room temperature for 15 minutes, then add PhSiH 3 (45μL, 3.6mmol), continue to stir for 10 minutes, add 3-chloro-1-phenyl-1-propanone (1a, 50.6mg, 0.3mmol) in a solvent (0.5mL) solution, stir vigorously, and use TLC to detect the reaction process . After reacting f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com