Patents
Literature
33results about How to "Atomic economy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
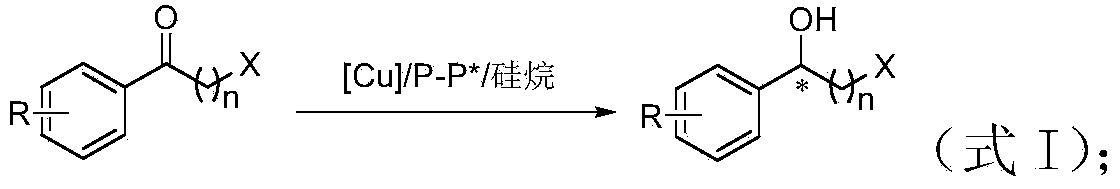
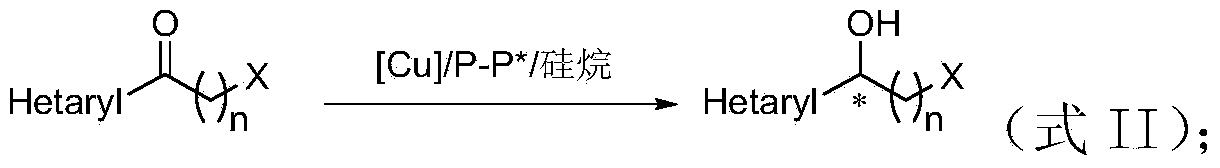
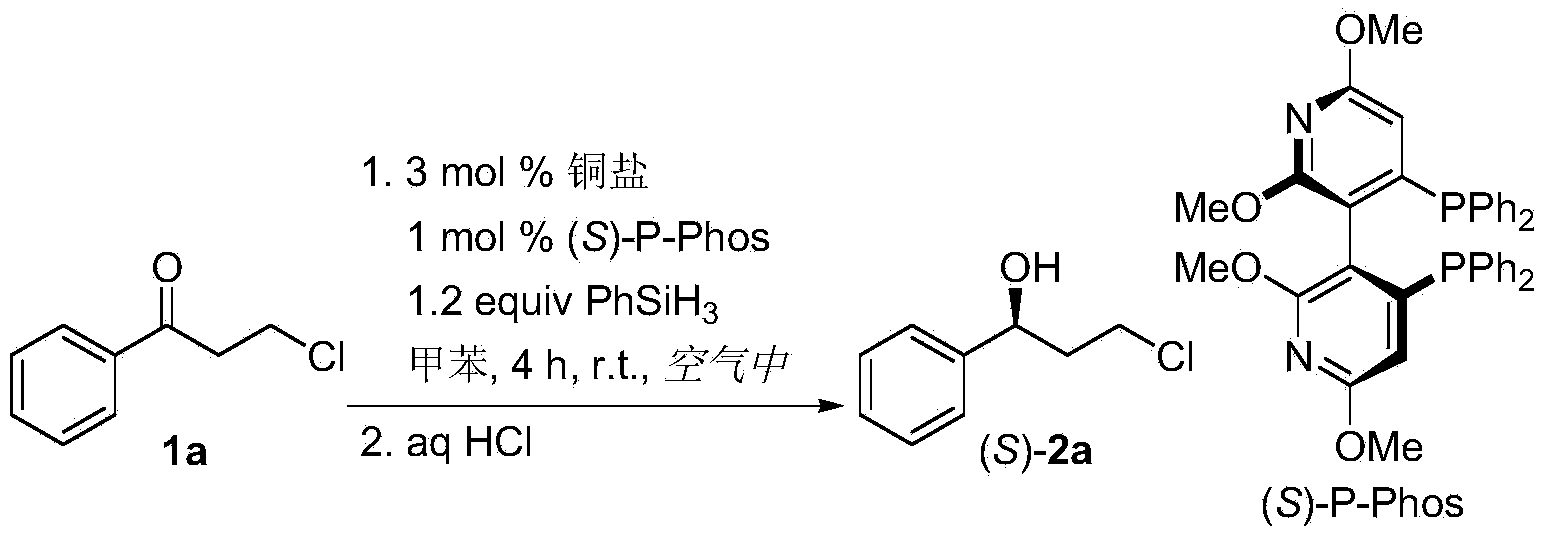
Method for preparing chirality halohydrin in copper-catalyzed asymmetry hydrosilation mode
InactiveCN103524307AHigh yieldHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholNitrogen
The invention discloses a method for preparing chirality halohydrin in a copper-catalyzed asymmetry hydrosilation mode. In the prior art, mostly a noble metal catalyst is needed, the research is generally limited in catalysis asymmetry reduction of alpha-haloketone, and documents about catalysis asymmetry reduction of beta-, gamma-, epsilon- or other haloketone substrates are less related. The method disclosed by the invention comprises preparing chirality gamma-, delta- or zeta-haloalkyl aryl alcohol from beta-, gamma-, epsilon-haloalkyl aryl ketone in a copper catalysis asymmetry hydrosilation reduction mode, or preparing chirality beta-, gamma- or delta-haloalkyl aryl alcohol from alpha-, beta- or gamma-haloalkyl aryl ketone in the copper catalysis asymmetry hydrosilation reduction mode. The method adopts a non-noble metal catalyst, the raw material is easy to obtain, nitrogen protection is not needed, the reaction condition is mild, the operation is simple, and the yield and the enantioselectivity of the reaction are high.
Owner:HANGZHOU NORMAL UNIVERSITY
Method for preparing p-hydroxy-cinnamate by using ionic liquid to catalyze lignin
ActiveCN107602383AEasy to makeAtomic economyPreparation by ester-hydroxy reactionOrganic compound preparationMetal chlorideDepolymerization
The invention discloses a method for preparing p-hydroxy-cinnamate by using ionic liquid to catalyze lignin. The method comprises the following steps of: (1) preparation of the metal halide salt ionicliquid, wherein an alkyl imidazole chloride salt and metal chloride are heated and stirred at 30-80 DEG C so as to obtain the metal halide salt ionic liquid; (2) catalytic depolymerization of the lignin, wherein the metal halide salt ionic liquid, the lignin and alcohol are mixed, and a mixture obtained after N2 replacement is heated and stirred at 140-200 DEG C for a reaction for 4-8 hours, so that high value-added chemicals which mainly comprise the p-hydroxy-cinnamate are obtained. The method for preparing the p-hydroxy-cinnamate by using the ionic liquid to catalyze the lignin has the advantages of a simple process, mild conditions, environment protection and high selectivity to the main product, the preparation of the ionic liquid which can be recycled and have economical atoms is simple, and the yield of the p-hydroxy-cinnamate can reach 40-80 mg / g under a preferred condition.
Owner:SOUTH CHINA UNIV OF TECH
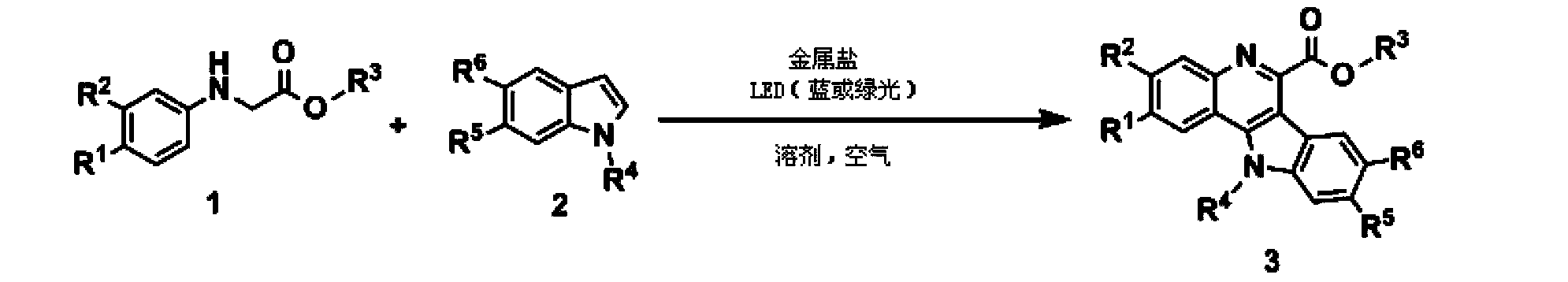
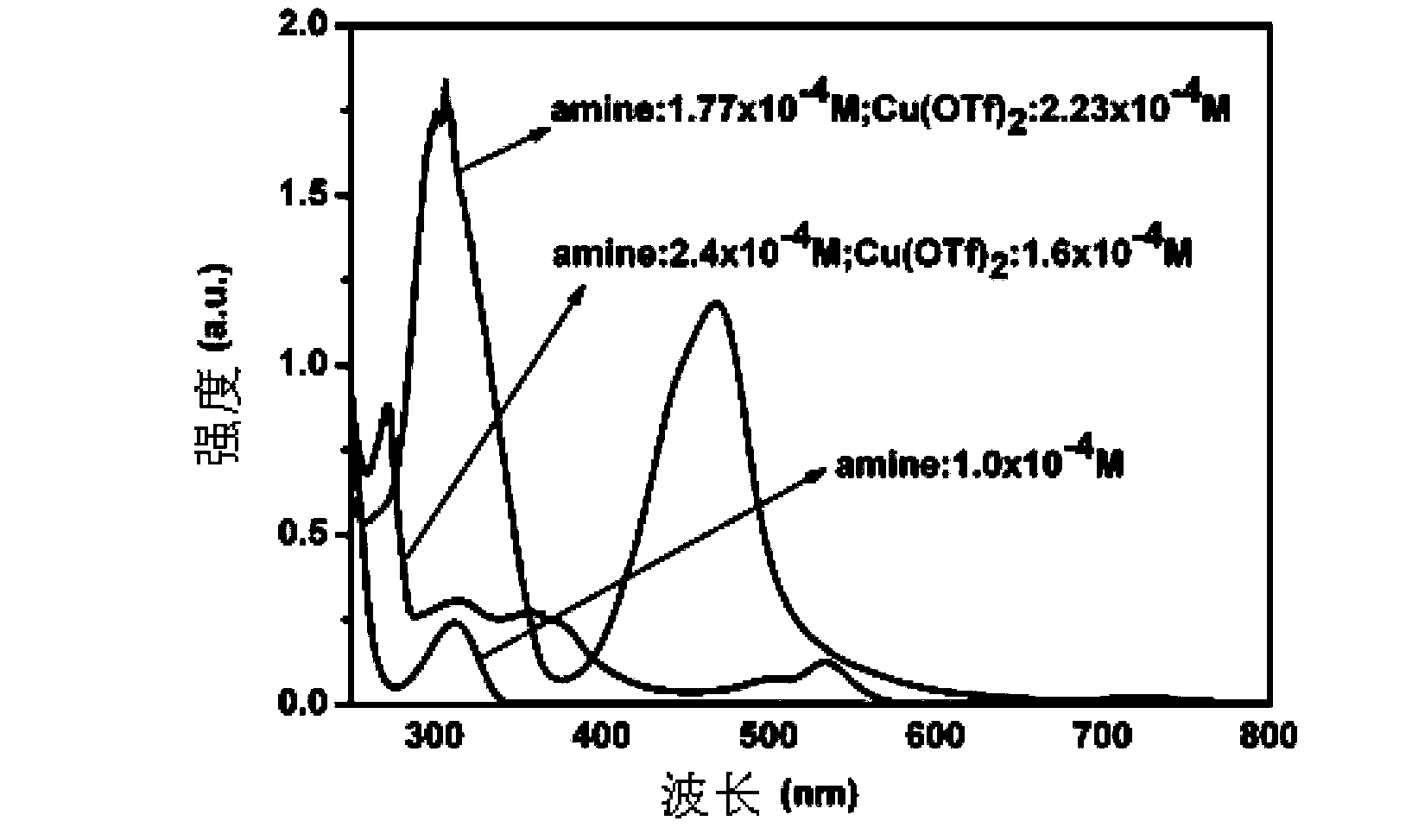
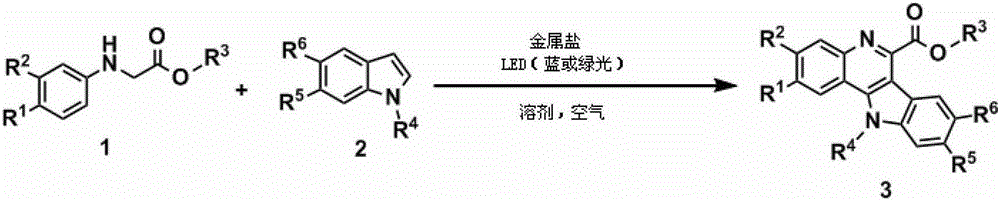
Method for synthesizing quindoline derivative by visible light catalysis
ActiveCN103910723ASynthetic reaction conditions are mildEasy to operateOrganic chemistryPhotosensitizerCoupling
The invention discloses a method for synthesizing a quindoline derivative by visible light catalysis. The method uses metal salt to synthesize the quindoline derivative through photocatalysis. According to the invention, extra photosensitizer is not required, cheap transition metal salt and secondary amine enable in-situ coordination to generate a compound which is taken as a photocatalyst, oxygen in air is taken as a terminal oxidizing agent, so that oxidation coupling of secondary amine and an indole derivative can be realized through a photochemical method, and the quindoline derivative enables one-step synthesis. The whole synthesis reaction has the advantages of mild condition, high efficiency, simple operation and atom economy.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing compound zolpidem
ActiveCN103360387AAvoid the disadvantages of long and high costHigh yieldOrganic chemistryChemical synthesisBiochemical engineering
The invention belongs to the field of chemical synthesis, and relates to a method for preparing a compound zolpidem. The invention provides an effective new method for preparing zolpidem by a trimaceral serially reaction. The method for preparing the zolpidem provided by the invention has the advantages that the reaction step is short, the condition is moderate, atom economy and environmentally friendly are achieved, the yield is high, the cost is low, industrialized production is applicable, and the defects in the prior art that the synthetic route is long and the cost is high are overcome.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing aniline derivative through visible light catalysis of acyl migration
ActiveCN104163768ASynthetic reaction conditions are mildEasy to operateOrganic compound preparationAmino-carboxyl compound preparationEnamineGreen-light
The invention discloses a method for preparing an aniline derivative through visible light catalysis of acyl migration. The method comprises the following step: (1) adding enamine and alcohol into an organic solvent to obtain a solution A; (2) adding a photosensitizer into the solution A to obtain a solution B; and (3) in an air or oxygen atmosphere, irradiating the solution B with visible light to obtain a 1,2-acyl migrated aniline derivative. An acyl functional group in a substrate undergoes a 1,2-migration reaction under visible light catalysis; acyl-substituted, ester-substituted, arylamino-substituted and alkyl-substituted quaternary carbon compounds are synthesized at one step through a photochemical method, the whole synthesis reaction is mild in condition, operation is easy, and atom economy is realized; oxygen in the air is converted into an oxygen-containing functional group; and LED (Light-Emitting Diode) blue light or green light is taken as a visible light source, and operation is easy.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
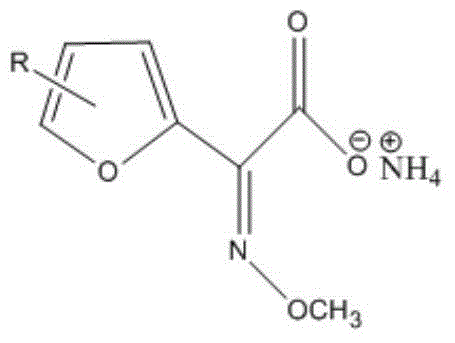
Industrial production method of furan derivative
The invention provides an industrial production method of a furan derivative. The industrial production method is characterized in that a compound A and a cyanide are subjected to an addition reaction, and an oxidation reaction and a hydrolysis reaction are performed to obtain the target furan derivative. According to the present invention, the production method is simple and different from the complex production way and the stringent production conditions in the prior art; the synthesis of the target product can be achieved through the one step; and the production process and the synthesis route are optimized, such that the advantages of less side reaction, convenient post-treatment and mild production condition during the production process are provided, and the method is suitable for the industrial production mode.
Owner:王志训
Catalyst for alcohol alcohol condensation reaction, preparation method and application thereof
ActiveCN107983328AAlleviate the crisis of overcapacityRealize comprehensive utilizationHeterogenous catalyst chemical elementsPreparation by OH group eliminationAlcoholCerium
The invention discloses a catalyst alcohol alcohol condensation reaction. The catalyst comprises a mixed metallic oxide of M1, Mg, Al and M2, and the molar ratio of catalyst elements is as follows: M1: Mg: Al: M2 is equal to (1-5): (70-80): (15-25): (1-5). M1 is one or more in copper, nickel, cobalt or zinc, and M2 is one in iron, cerium, zirconium or lanthanum. The catalyst has the advantages ofhigh activity and good selectivity and stability.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
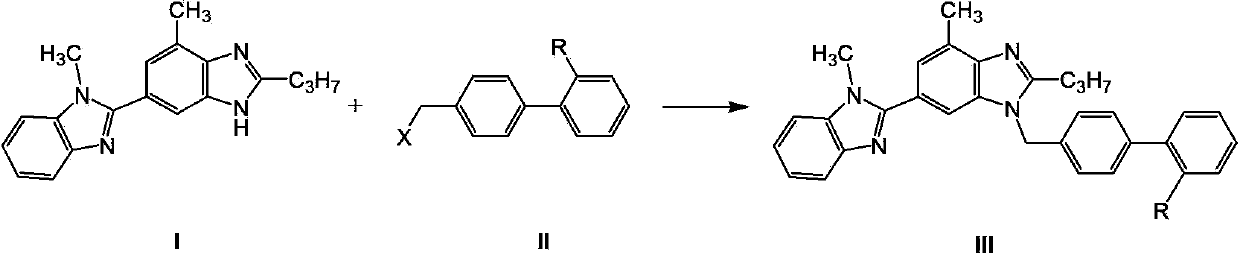
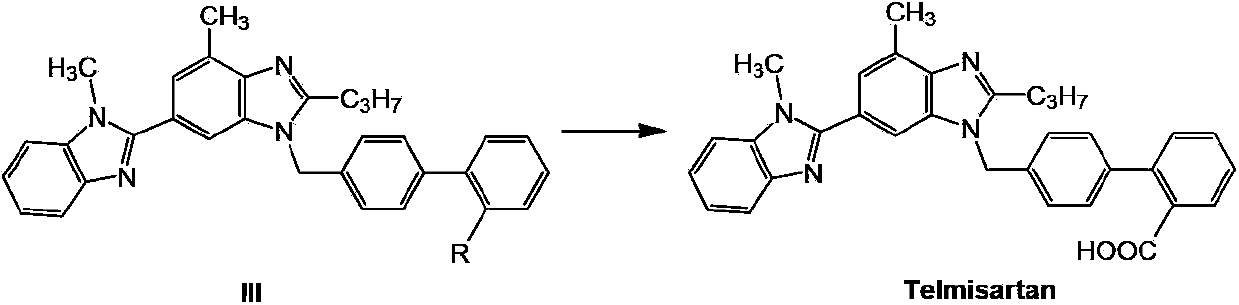
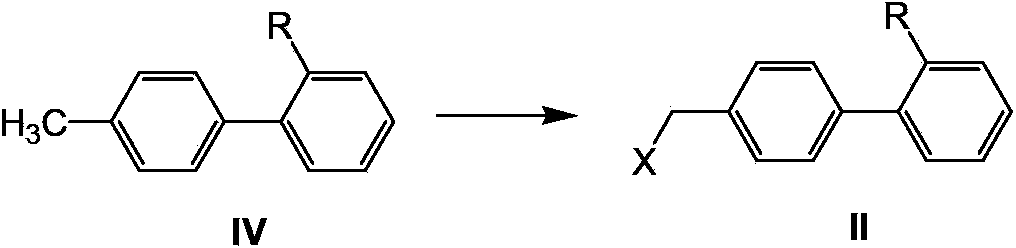
Telmisartan preparation method and intermediate of telmisartan
InactiveCN103787982ASimple and fast operationReduce one step reactionCarboxylic acid nitrile preparationOrganic compound preparationCombinatorial chemistryAlkyl substitution
The invention relates to a telmisartan preparation method. The telmisartan preparation method is characterized in that 2-n-propyl-4-methyl-6-(1-methyl benzimidazole-2-yl) benzimidazole (compound I) and 4'-halomethyl diphenyl-2-substituted compound (compound II) carry out a nucleophilic substitution reaction to obtain a compound III; when R is COOH, the compound III is telmisartan; when R is COOR' or CN, the compound III is hydrolyzed to obtain the telmisartan.
Owner:TOPHARMAN SHANGHAI +2
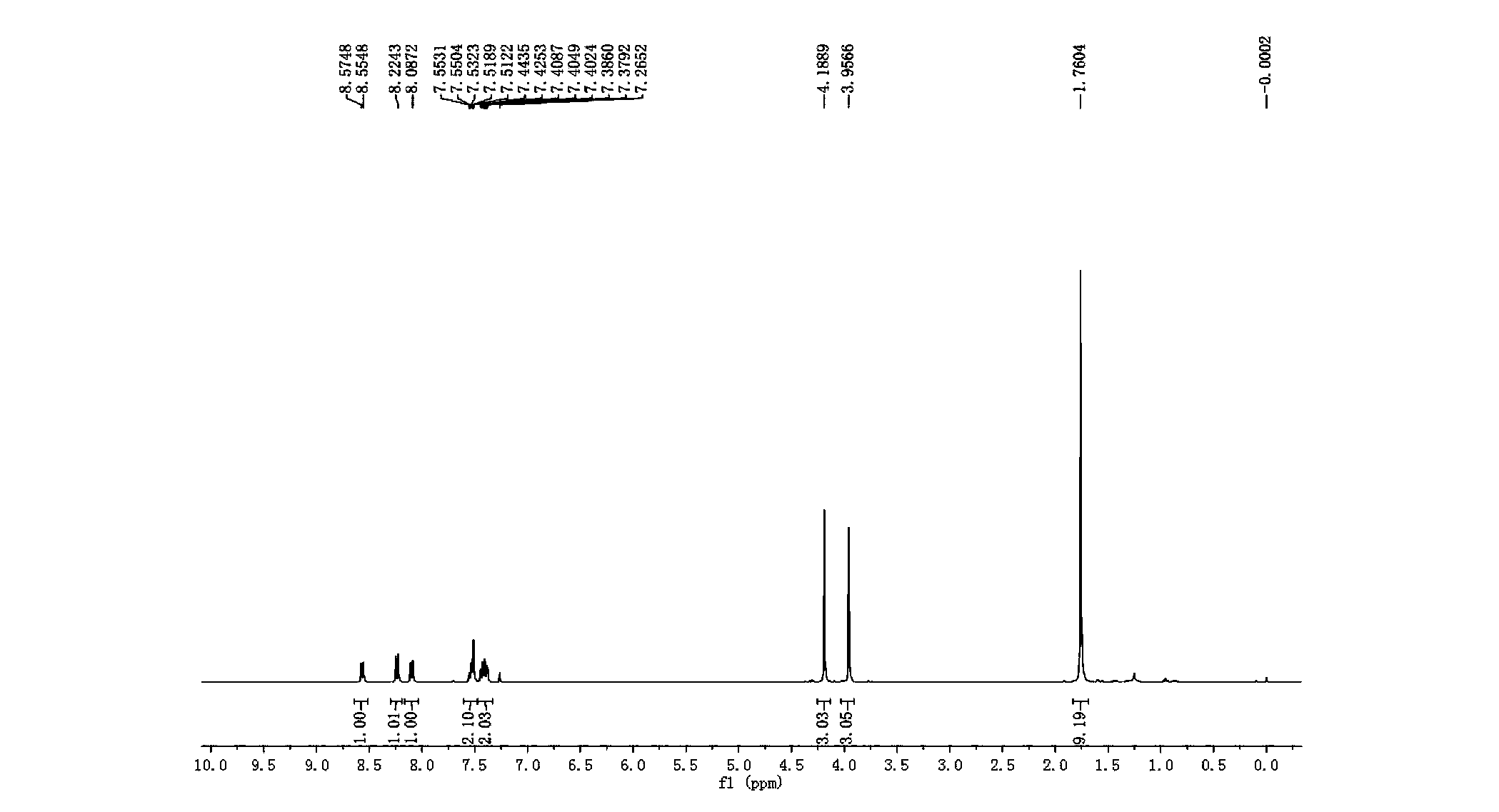
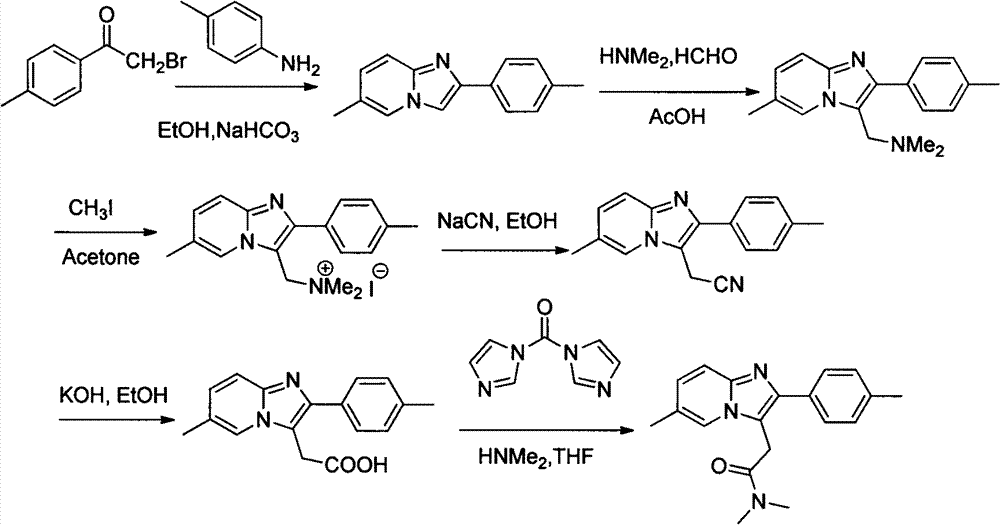
3-imidazole[1,2-a]pyridine compound
The invention discloses a 3-imidazole(1,2-a)pyridine compound which is prepared by an oxidation cross-coupling reaction of a visible-light photocatalytic N-aryl glycine ester and a imidazo(1,2-a)pyridine compound. In the preparation method, eosin Y serves as a photosensitizer, citric acid serves as an additive, and N-aryl glycine ester and imidazo(1,2-a)pyridine compound are directly dehydrogenated and cross-linked to form 3-imidazole(1,2-a)pyridine compound in an organic solvent at room temperature after being irradiated by visible light. Accoding to the preparation method, eosin Y serves asphotosensitizer, citric acid monohydrate serves as additive, ethanol serves as solvent, and the 3-imidazole(1,2-a)pyridine compoundand is effectively prepared by visible light photocatalysis at room temperature. The method has the advantages of simple operation, mild reaction conditions, good selectivity and atomic economy.
Owner:EAST CHINA UNIV OF TECH
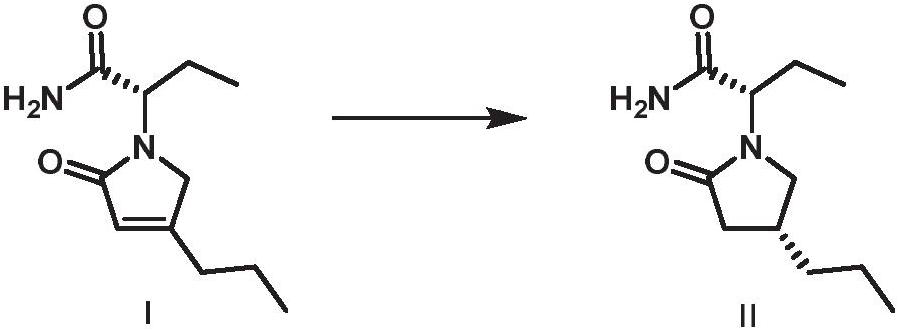
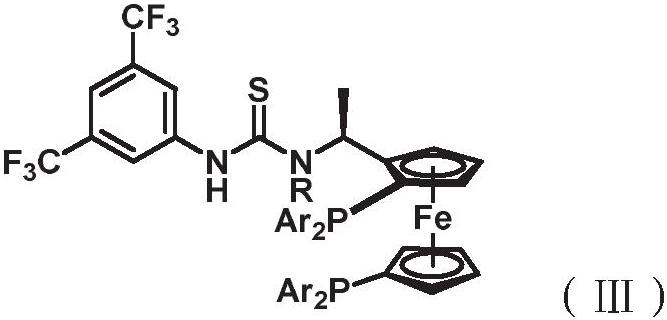
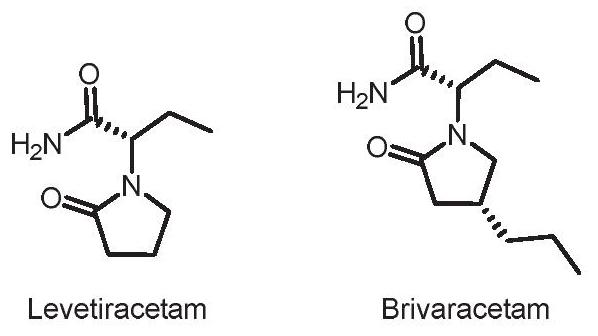
Asymmetric catalytic preparation method of brivaracetam
ActiveCN111848483AAchieve asymmetric preparationMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsCombinatorial chemistryBrivaracetam
The invention discloses a preparation method of brivaracetam, which adopts cheap and accessible hydrogen as a hydrogen source, can implement asymmetric preparation of brivaracetam in a catalytic system with rhodium (I) as a metal center, and has the advantages of mild reaction conditions, simplicity, controllability, high yield, high enantioselectivity, environment friendliness, favorable atom economy, low cost and the like. In addition, the invention also provides the brivaracetam compound prepared by the method.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
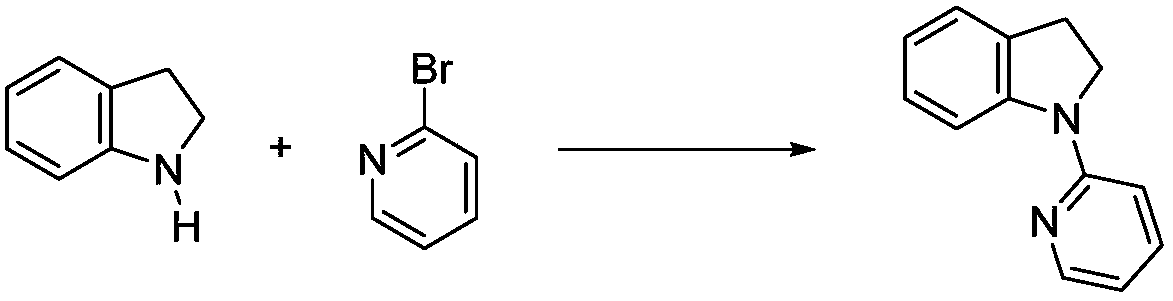
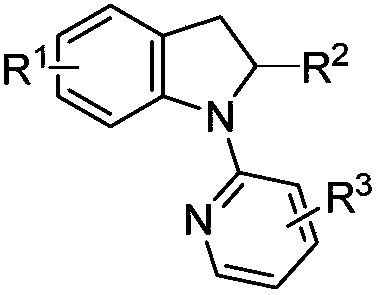
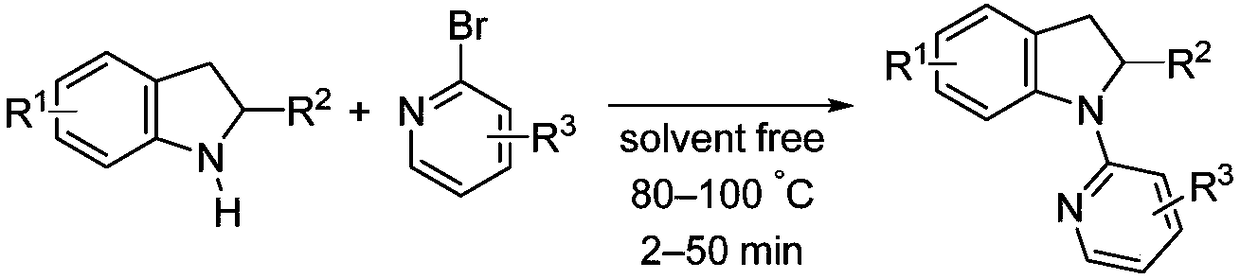
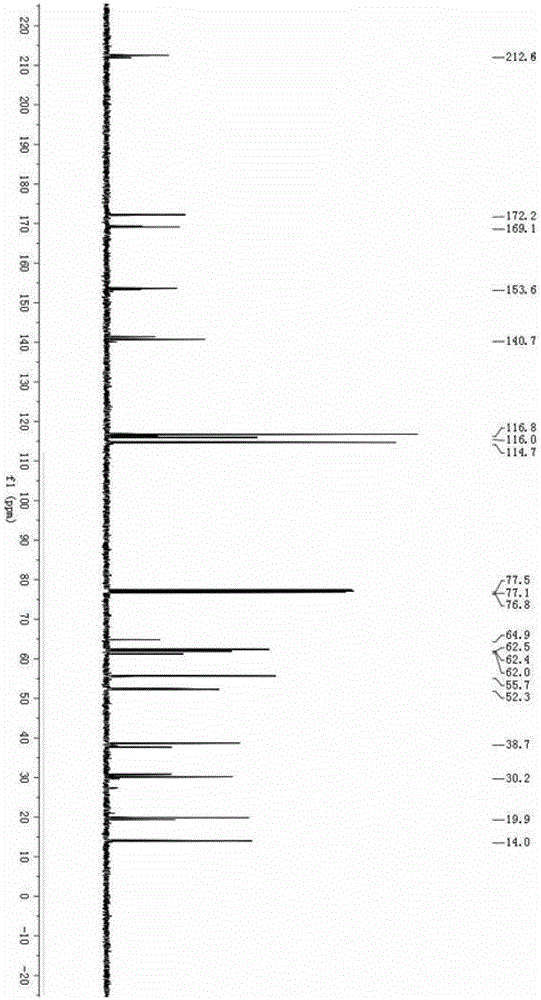
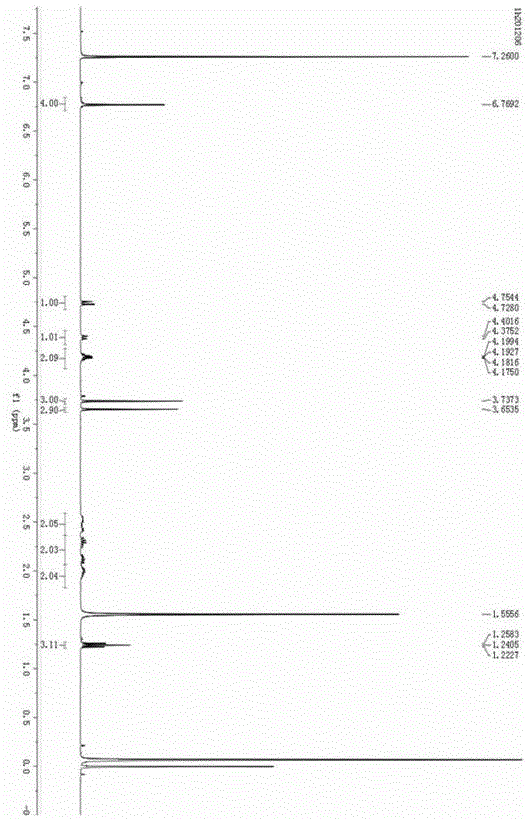
Solvent-free synthesis method of 1-(2-pyridyl)indoline derivative
A solvent-free synthesis method of a 1-(2-pyridyl)indoline compound is provided. The method includes adding an indoline derivative and a pyridine derivative to a container, stirring uniformly, curingat 80-100 DEG C and then cooling to the room temperature; then adding a NaHCO3 solution to dissolve the solid, adding ethyl acetate, shaking, separating and concentrating; dissolving a concentrated solution in ethanol, refluxing for 5-20 min, cooling, filtering and drying to obtain the 1-(2-pyridyl)indoline compound. The method has the advantages of being simple in operation, low in cost, convenient to post treat, easy to amplify and good in atomic economy.
Owner:NORTHWEST UNIV(CN)
A synthetic method for α-position alkylation of secondary amines catalyzed by visible light
ActiveCN104230734BSynthetic reaction conditions are mildEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAir atmosphereAmine alkylation
The invention discloses a synthesis method for α-position alkylation of a secondary amine catalyzed by visible light, comprising the following steps: 1) adding an inorganic metal salt and N-aryl glycine ester into an organic solvent to obtain a solution A; 2 ) adding β-keto ester to solution A to obtain solution B; 3) irradiating solution B with visible light in an air atmosphere to obtain an alkylated product of N-aryl glycinate. In the invention, the compound formed by in-situ coordination of cheap inorganic metal salt and secondary amine is used not only as a photosensitizer, but also as a catalyst to realize the α-position alkylation reaction of the secondary amine. The system of the present invention uses LED blue light or green light as a visible light source, does not need to add additional photosensitizers, does not need to add additional electron acceptors, uses oxygen in the air as the terminal oxidant, and has mild synthesis reaction conditions, simple operation, and atom economy. .
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing clioquinol and diiodohydroxyquinoline by one-pot method
PendingCN113292492AOvercoming many shortcomings, harsh reaction conditions, cumbersome post-processing steps, etc.Solve the problem of high ratioOrganic chemistryClioquinolQuinoline
The invention discloses a method for preparing clioquinol and diiodohydroxyquinoline by a one-pot method, and effectively solves the following problems: (1) the problem that the proportion of isomers in a reaction product is too high due to the fact that hydroxyl is an ortho- / para-positioning group and the purification difficulty is increased when no guiding group exists and C5 halogenation is performed firstly and then C7 iodination is performed is solved; and (2) the problem of low yield (66%) of the first-step C7-site iodination reaction when no guiding group exists and C7 iodination is performed firstly and then C5 halogenation is performed is solved, and the total yield is only about 60% although a C5-site halogenation product can be obtained subsequently. The method effectively overcomes the ubiquitous defects of poor atom economy, harsh reaction conditions, tedious post-treatment steps and the like in the two methods, and is suitable for industrial production. The invention provides an efficient synthesis method for preparing the clioquinol and diiodohydroxyquinoline, and the method has the characteristics of greenness, environmental protection, atom economy, simplicity in operation, easiness in amplification and the like.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
Visible light-catalyzed acyl transfer method for preparation of aniline derivatives
ActiveCN104163768BSynthetic reaction conditions are mildEasy to operateOrganic compound preparationAmino-carboxyl compound preparationEnamineAniline
The invention discloses a method for preparing aniline derivatives by visible light-catalyzed acyl transfer; the method comprises the following steps: 1) adding enamine and alcohol into an organic solvent to obtain solution A; 2) adding a photosensitizer into solution A, Obtain solution B; 3) In air or oxygen atmosphere, irradiate solution B with visible light to obtain 1,2-acyl-transferred aniline derivatives. The present invention uses visible light to catalyze the 1,2-transfer reaction of the acyl functional group in the substrate; the present invention synthesizes quaternary carbon compounds containing acyl, ester, arylamino, and alkyl substitutions in one step through a photochemical method. The reaction conditions are mild, the operation is simple, and the atom is economical; the invention converts oxygen in the air into oxygen-containing functional groups; the invention uses LED blue light or green light as a visible light source, and the operation is simple.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
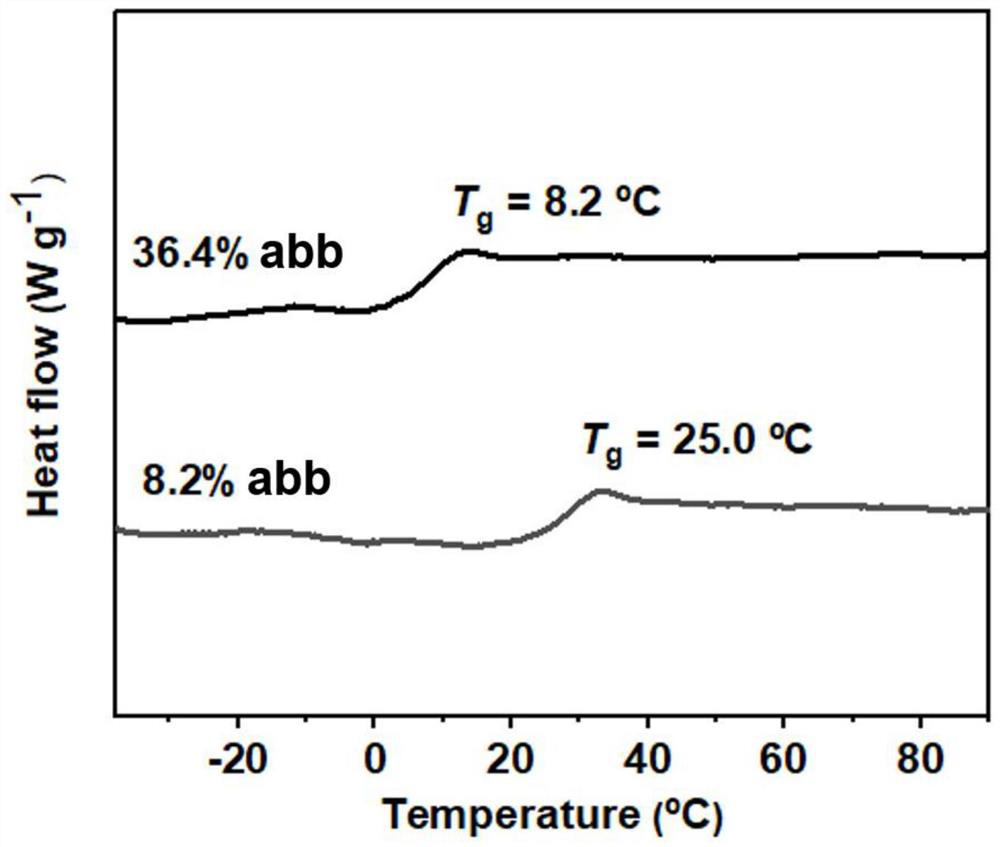
Carbon dioxide-based polycarbonate containing abb structure and synthesis method thereof
The invention discloses a carbon dioxide-based polycarbonate containing an abb structure and a synthesis method thereof, under the action of a difunctional organic boron catalyst, carbon dioxide and alkylene oxide as shown in a formula I are subjected to a copolymerization reaction, the carbon dioxide is used as a structural unit a, the alkylene oxide is subjected to ring opening to serve as a structural unit b to be inserted into a polymer chain, and the carbon dioxide-based polycarbonate containing the abb structure is obtained. Copolymerizing to form carbon dioxide-based polycarbonate containing an abb structure as shown in a formula II; the ab structural unit represents a carbonate unit formed by ring-opening copolymerization of carbon dioxide and one alkylene oxide, the abb structural unit is formed by ring-opening copolymerization of carbon dioxide and two alkylene oxides in sequence, and the bbb structural unit represents an ether unit formed by ring-opening auto-polymerization of alkylene oxides; the molar fraction of the abb structure is 1%-100%. The composition of the polycarbonate can be selectively regulated and controlled by changing one or more reaction conditions such as the catalyst structure, the ratio of the catalyst to the epoxyalkane, the reaction temperature, the carbon dioxide pressure and the reaction time, and the possibility of composition and application of the polycarbonate is widened.
Owner:烯欧途(杭州)新材料科技有限公司
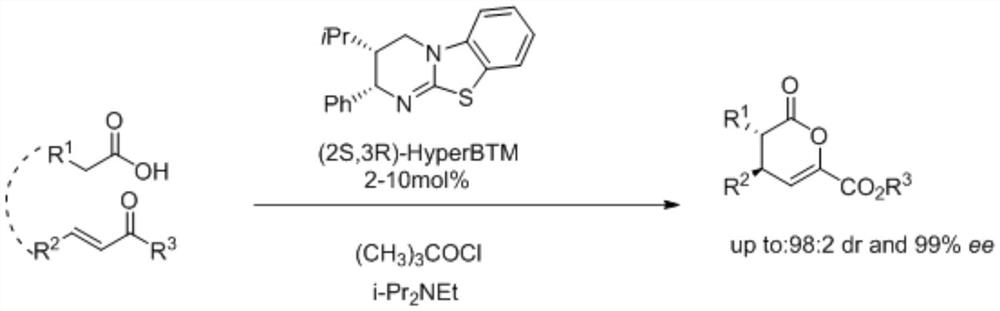
Method for Synthesizing Axial Chiral Biaryl Compounds Using Tertiary Amine Organic Small Molecules
InactiveCN106146306BAtomic economyHigh yieldOrganic compound preparationCarboxylic acid esters preparationGreen environmentBaylis–Hillman reaction
The invention discloses a method for synthesizing axial chiral biaryl compounds by utilizing tertiary amine small organic molecules. According to the method, similar Mortia-Baylis-Hillman reactions in molecules are utilized, and chiral prosthetic groups are used as inducing groups, so that axial chiral biaryl compounds are asymmetrically constructed in one step. The method for synthesizing the axial chiral biaryl compounds has the advantages of environmental friendliness, no use of expensive metal catalysts, atom economy and easiness in modification of substrates, the yield of the biaryl compounds as products can reach 80% to 97%, and a De value reaches 70% to 80%. The invention provides a green environment-friendly way for various preparations of the axial chiral biaryl compounds.
Owner:SHAANXI NORMAL UNIV
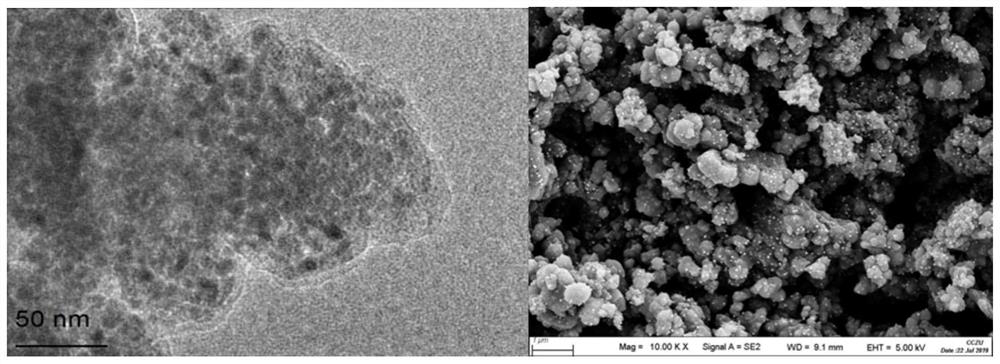
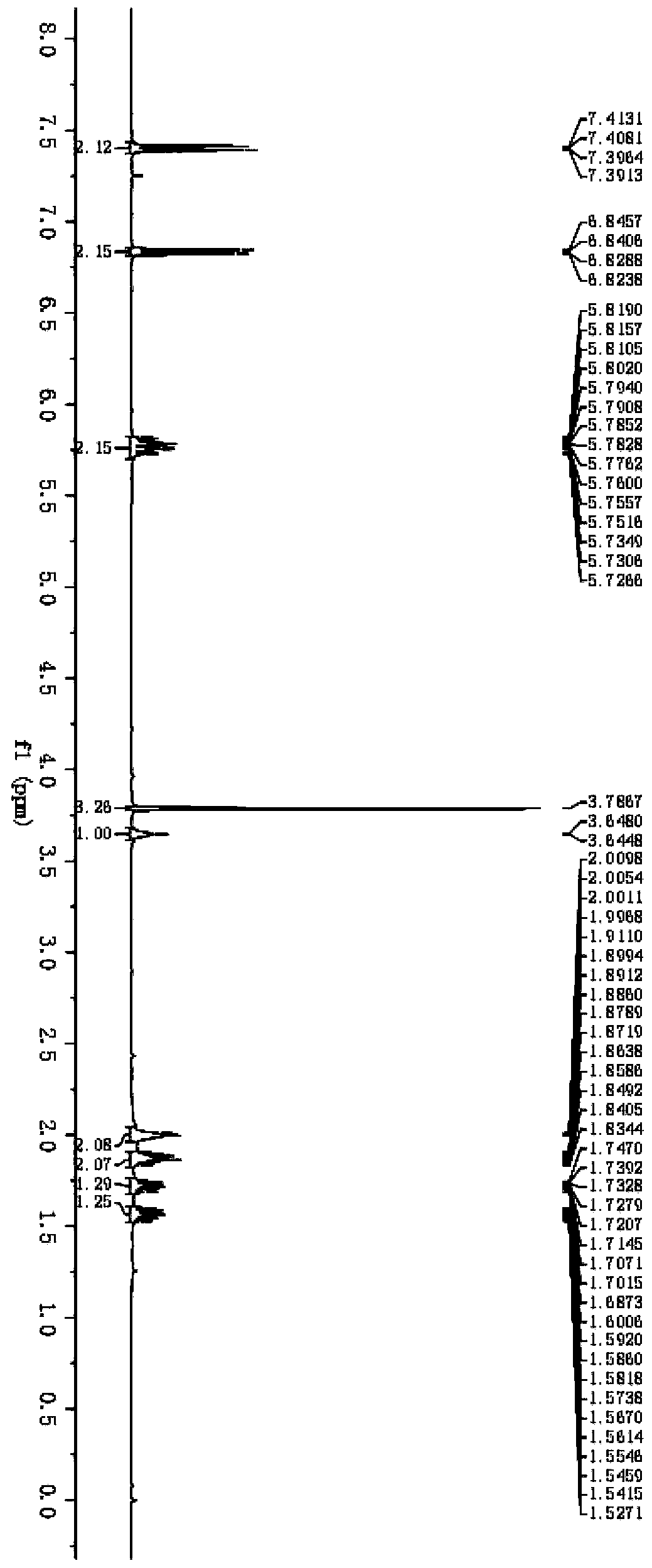
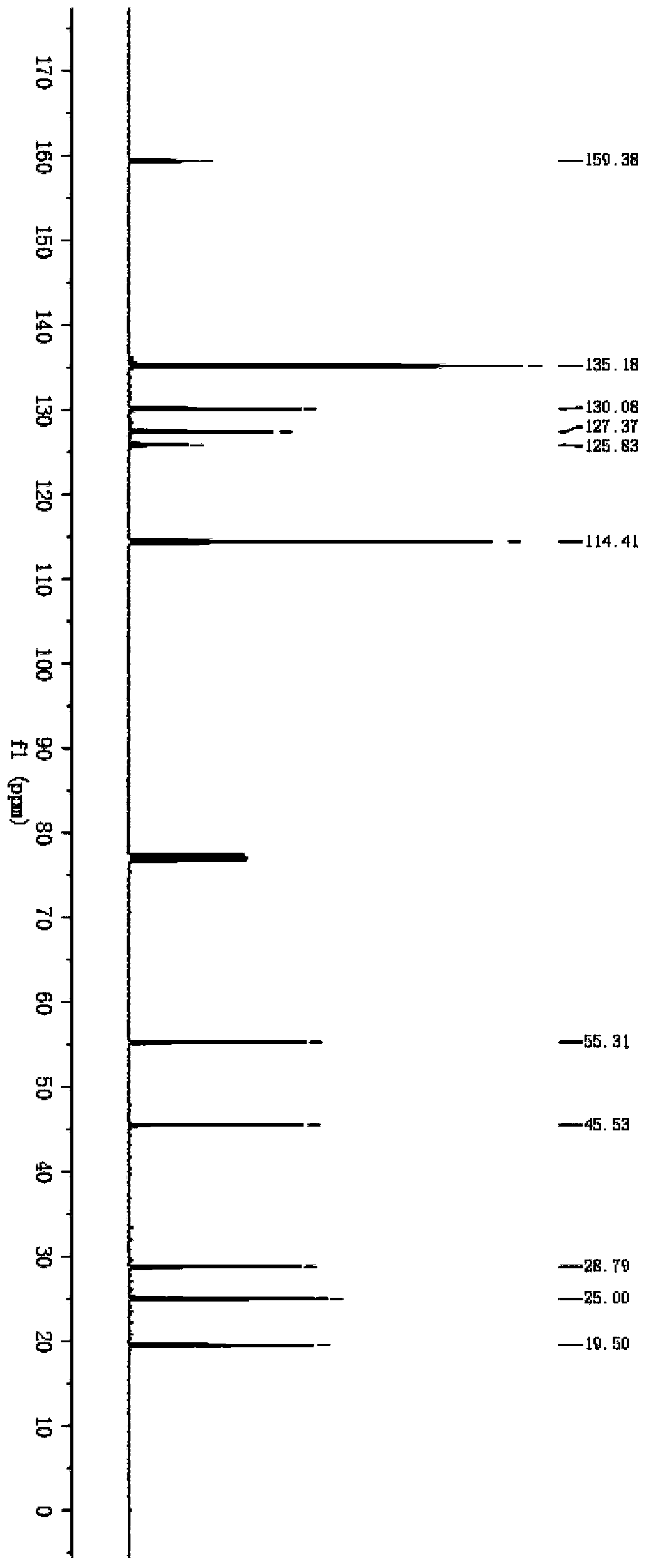
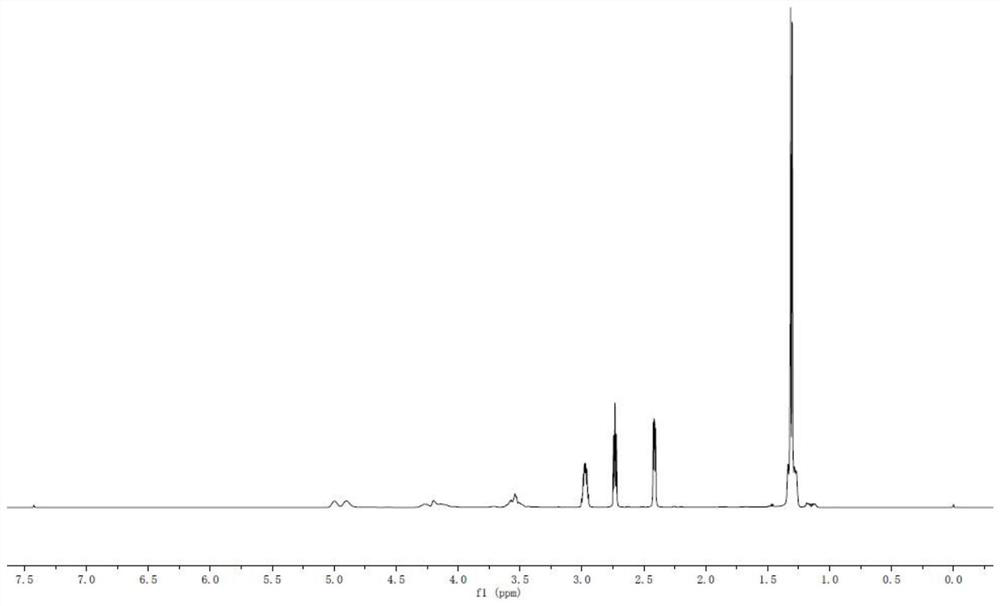
2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof
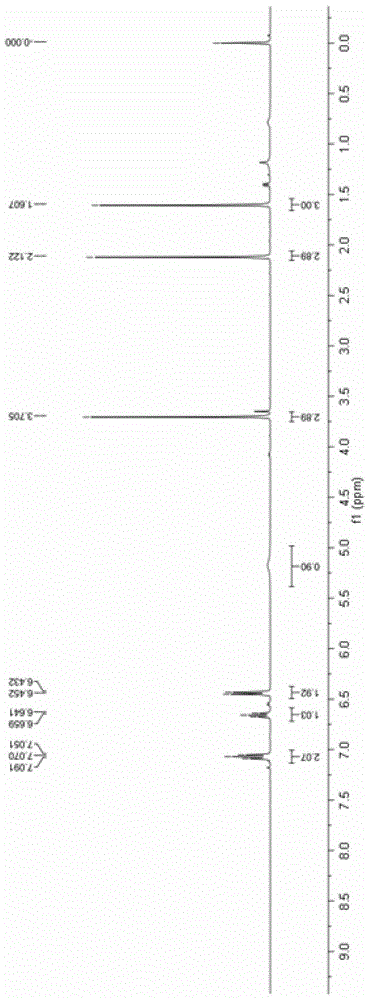
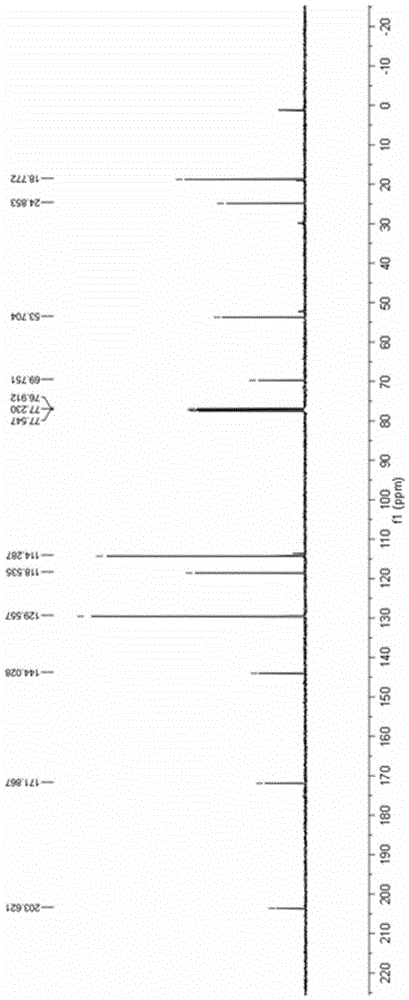
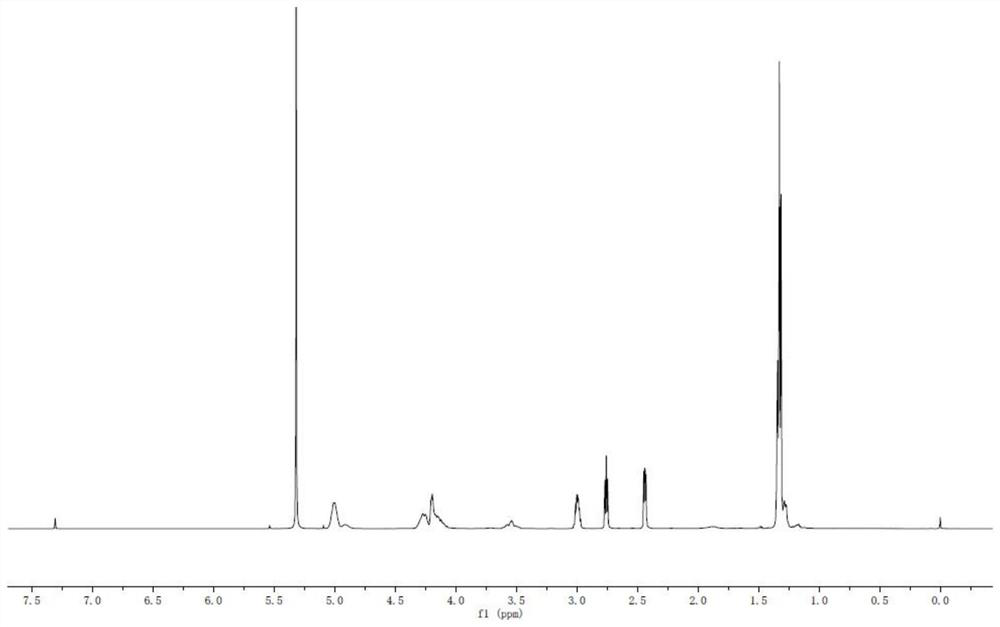
ActiveCN111116510AEasy to operateAtomic economyOrganic chemistryPerylene derivativesChemoselectivity
The invention discloses a 2-substituted methylene dihydrobenzo[d]thiazole derivative and a synthesis method thereof. According to the method, an N-alkyl / N-aryl thioamide derivative as shown in a formula (1) is used as a raw material and undergoes intramolecular alkene-thiol interconversion and then further undergoes C-S coupling dehydrogenation coupling reaction in series in an organic solvent inthe presence of an oxidant and a catalyst under mild reaction conditions so as to obtain the 2-substituted methylene dihydrobenzo[d]thiazole derivative. The synthesis method of the 2-substituted methylene dihydrobenzo[d]thiazole derivative has the advantages of mild conditions, economic atomic steps, high yield and the like through condition control, has high chemical selectivity and regioselectivity, and has wide application prospects. The invention also discloses application of the 2-substituted methylene dihydrobenzo[d]thiazole derivative in medicine and material science.
Owner:EAST CHINA NORMAL UNIVERSITY
Catalyst for synthesizing higher branched alcohols, preparation method and application
ActiveCN107983356BEasy to separateReduce manufacturing costOrganic compound preparationHeterogenous catalyst chemical elementsPtru catalystCerium
The invention discloses a catalyst for synthesizing a higher branched chain alcohol. The catalyst for synthesizing the higher branched chain alcohol comprises MgO, CuO, M1 and M2, the weight percentage of MgO to CuO to M1 to M2 is 100:(3-10):(5-20):(1-5), the M1 is one of silicon oxide, aluminum oxide, titanium oxide, cerium oxide or zirconium oxide, and the M2 is one of zinc oxide, ferric oxide,nickel oxide, chromium oxide and lanthanum oxide. The catalyst has the advantages of high activity and high selectivity and high stability.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
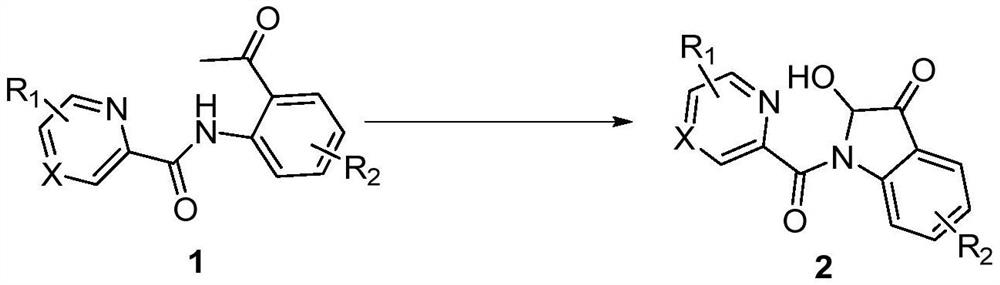
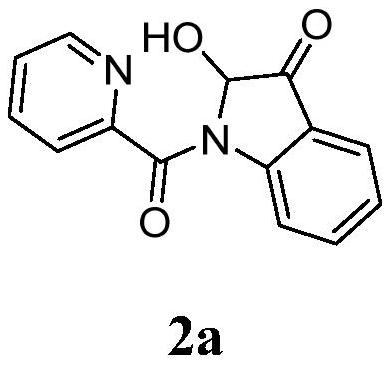
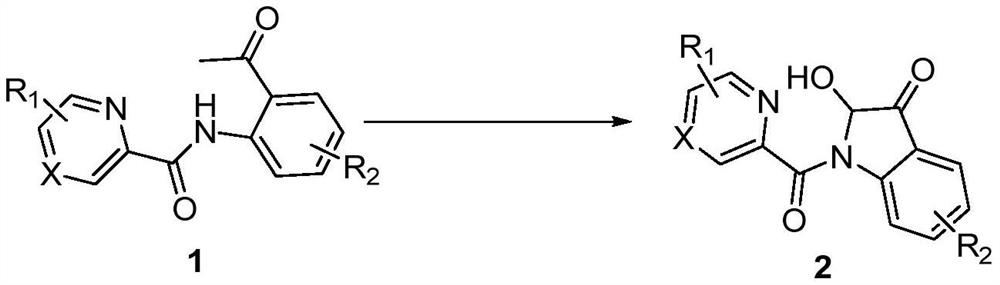
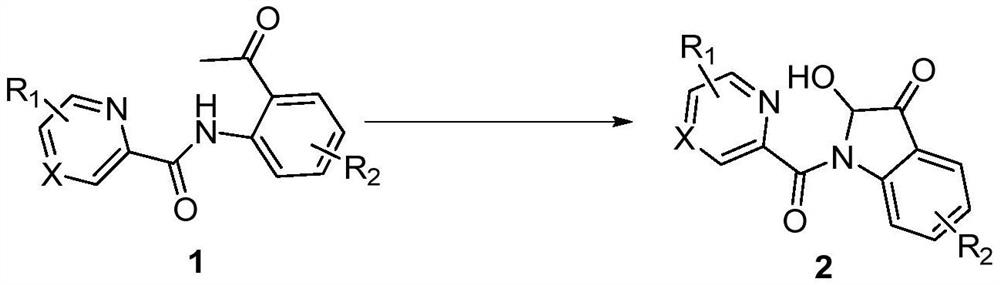
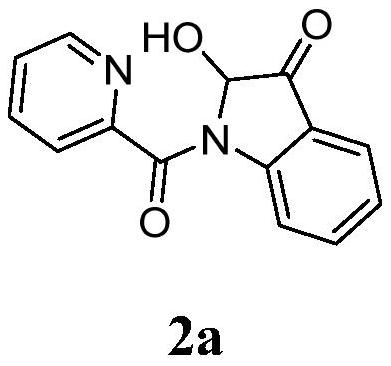
A kind of synthetic method of 2-hydroxyl-indol-3-ketone compound
The invention discloses a synthesis method of 2-hydroxy-indole-3-ketone compounds, using N-(2-acetylphenyl) pyridinoline amide and its derivatives as raw materials, copper iodide and other copper salts As a catalyst, with acetic acid etc. as additives, react under oxygen atmosphere at 80-110°C to obtain 2-hydroxy-indole-3-ketone compounds. The method of the present invention has the characteristics of good substrate applicability, atom economy, easy operation, avoiding the preparation of substrates, cheap reagents, cost saving, mild reaction conditions, one-step synthesis and high yield, and is 2-hydroxy-indole The synthesis of ‑3‑ketones offers new strategies.
Owner:YANAN UNIV
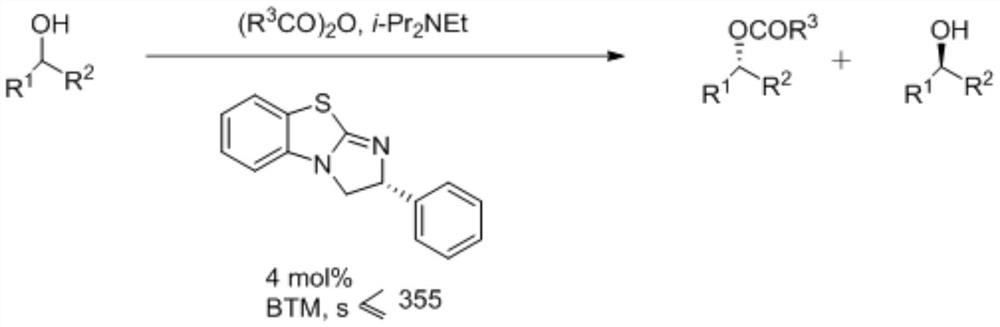
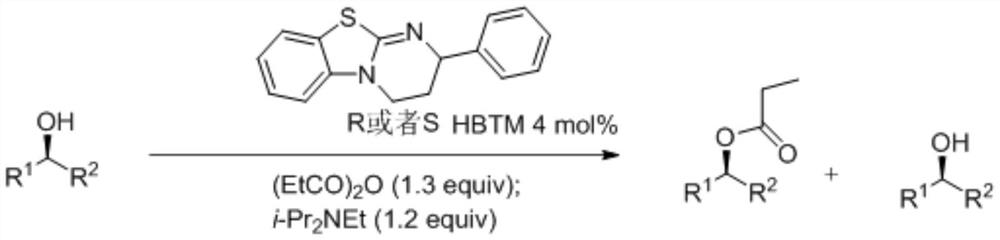
A kind of synthetic method of isothiourea catalyst
ActiveCN109956960BAtomic economyReduce pollutionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystThiourea
Owner:SHANGHAI UNIV OF ENG SCI
Synthesis method of 2-hydroxy-indole-3-ketone compound
The invention discloses a synthesis method of a 2-hydroxy-indole-3-ketone compound, which comprises the steps of by taking N-(2-acetylphenyl) pyridineamide and a derivative thereof as raw materials, cuprous iodide and other copper salts as catalysts and acetic acid and the like as additives, reacting at the temperature of 80-110 DEG C in an oxygen atmosphere to obtain the 2-hydroxy-indole-3-ketone compound. The method has the characteristics of good substrate applicability, atom economy, easiness in operation, avoidance of substrate preparation, low reagent cost, cost saving, mild reaction conditions, one-step synthesis, high yield and the like, and a new strategy is provided for synthesis of the 2-hydroxy-indole-3-ketone compound.
Owner:YANAN UNIV
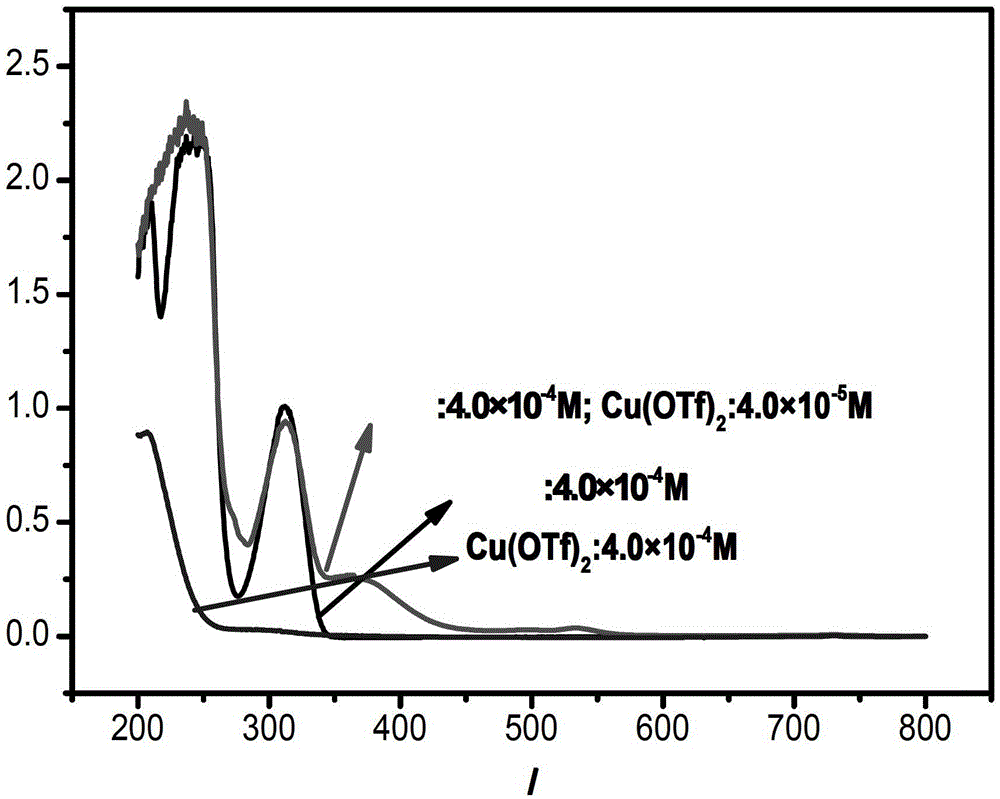
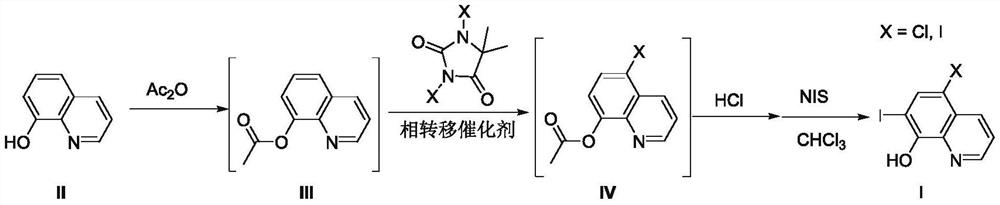
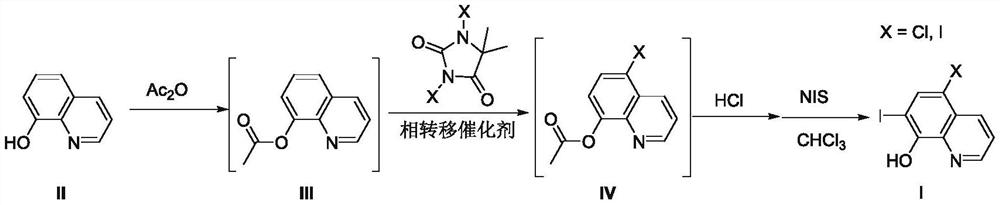
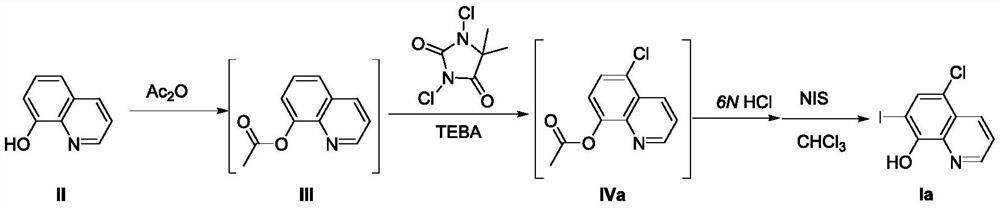
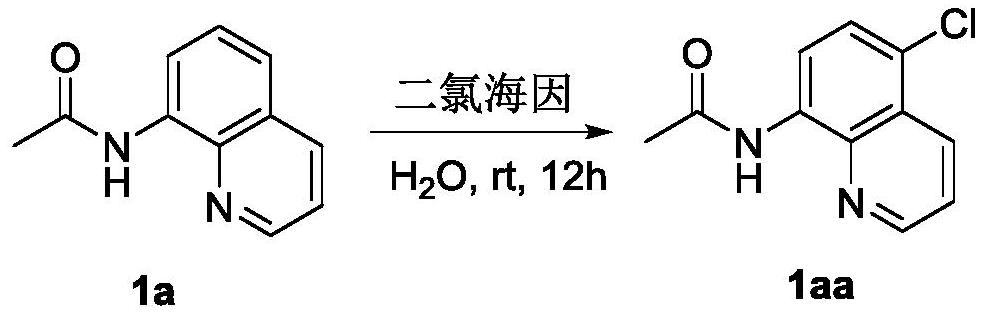
A kind of preparation method of 8-amide-5-haloquinoline derivatives
ActiveCN111320579BAtomic economyHigh yieldOrganic chemistryBiochemical engineeringReaction temperature
The invention discloses a preparation method of 8-amidoquinoline derivatives (compound II) and dihalohydantoin (compound III) at a certain reaction temperature. ) as a raw material, in the presence of a phase transfer catalyst, stirred and reacted for a certain period of time in a reaction solvent; R 1 It is one of C1~C6 alkyl, cycloalkyl and aromatic groups, R 2 or R 3 is H, F, Cl, Br, CH 3 、CF 3 , OCH 3 , NO 2 , one of CN; after the reaction is finished, the 8-amide-5-halogenated quinoline derivative (compound I) is obtained through aftertreatment. The method of the present invention has the characteristics of environmental protection, atom economy, high yield, simple operation, no need to use metals, easy amplification, etc., promotes the development of halogenated quinoline derivatives, and provides a powerful tool for the development of halogenated quinoline derivatives drugs protection.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
A kind of method for preparing compound zolpidem
ActiveCN103360387BHigh yieldThe reaction conditions are mild and controllableOrganic chemistryChemical synthesisState of art
The invention belongs to the field of chemical synthesis, and relates to a method for preparing a compound zolpidem. The invention provides an effective new method for preparing zolpidem by a trimaceral serially reaction. The method for preparing the zolpidem provided by the invention has the advantages that the reaction step is short, the condition is moderate, atom economy and environmentally friendly are achieved, the yield is high, the cost is low, industrialized production is applicable, and the defects in the prior art that the synthetic route is long and the cost is high are overcome.
Owner:JIANGSU HANSOH PHARMA CO LTD
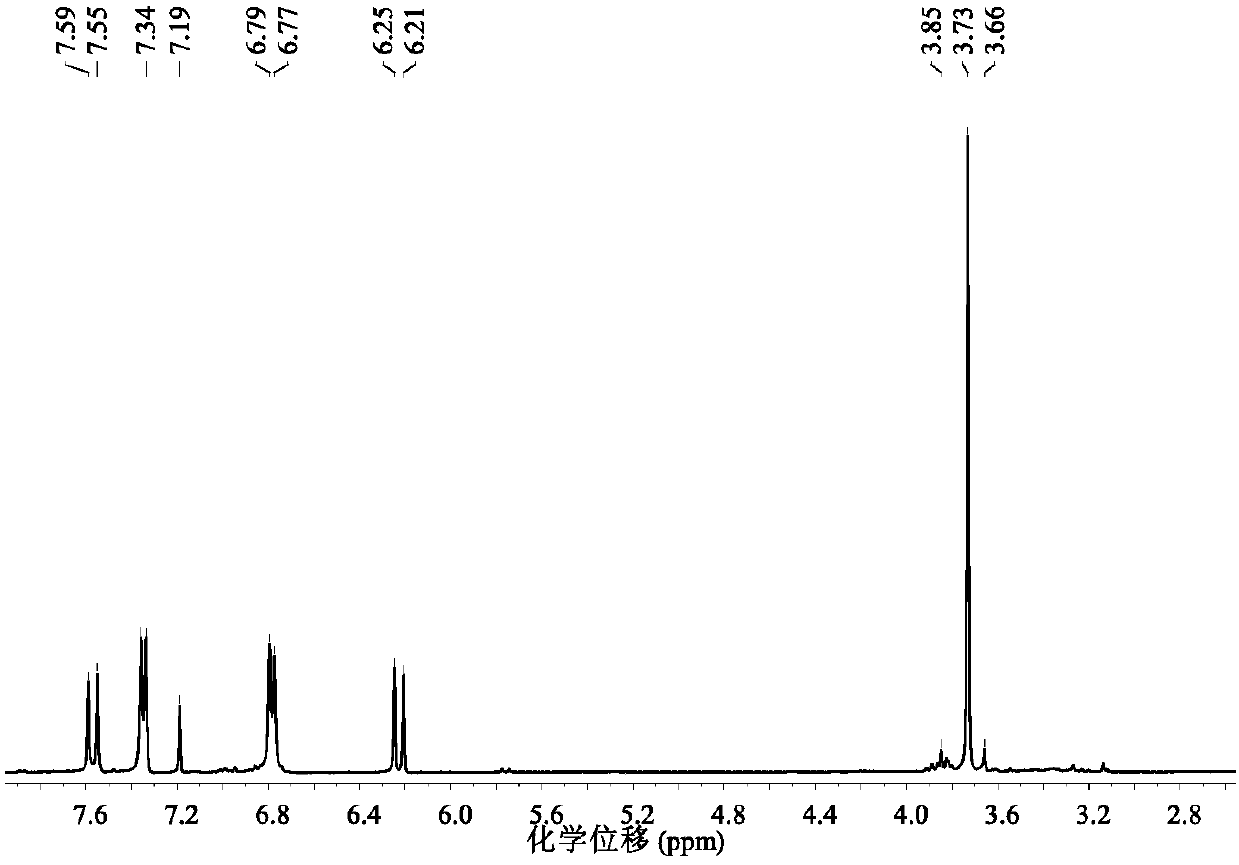
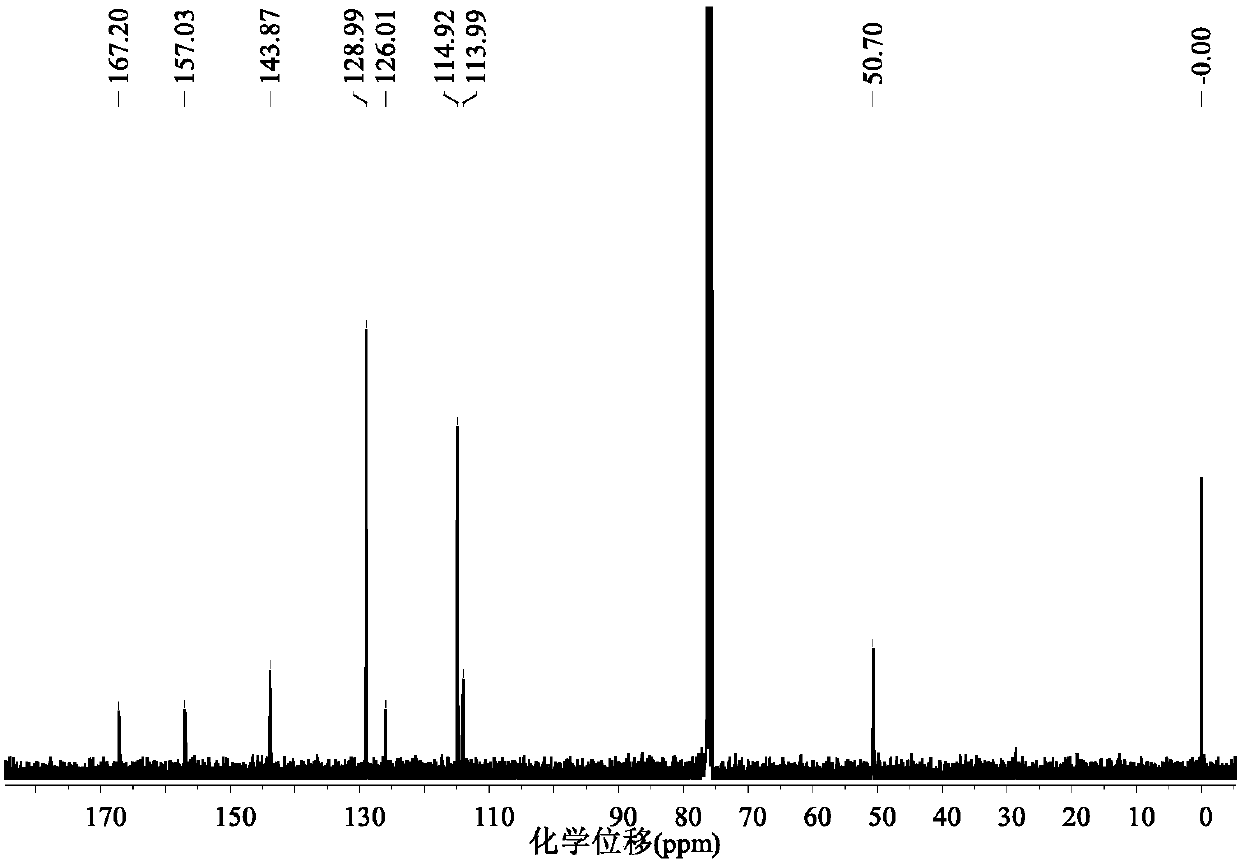
A kind of visible light catalytic method for synthesizing indoloquinoline derivatives
ActiveCN103910723BSynthetic reaction conditions are mildEasy to operateOrganic chemistryPhotosensitizerCoupling
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Catalyst for alcohol-alcohol condensation reaction, preparation method and application thereof
ActiveCN107983328BAlleviate the crisis of overcapacityRealize comprehensive utilizationHeterogenous catalyst chemical elementsPreparation by OH group eliminationPtru catalystAlcohol
The invention discloses a catalyst alcohol alcohol condensation reaction. The catalyst comprises a mixed metallic oxide of M1, Mg, Al and M2, and the molar ratio of catalyst elements is as follows: M1: Mg: Al: M2 is equal to (1-5): (70-80): (15-25): (1-5). M1 is one or more in copper, nickel, cobalt or zinc, and M2 is one in iron, cerium, zirconium or lanthanum. The catalyst has the advantages ofhigh activity and good selectivity and stability.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
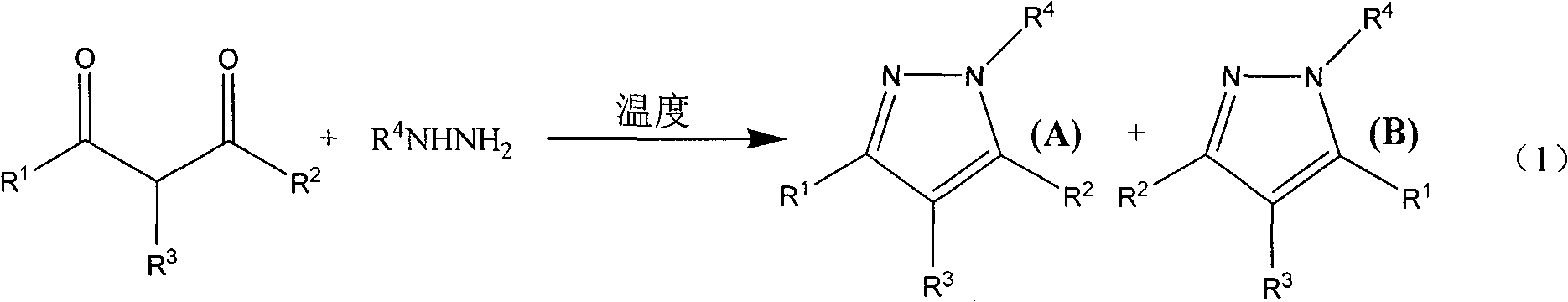
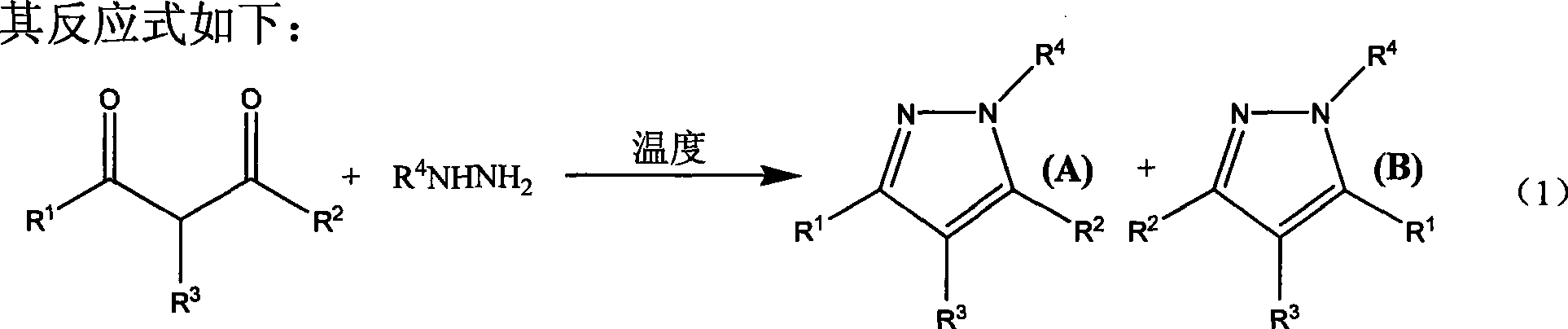
Method for synthesizing pyrazole heterocycle without catalyst or solvent
The invention relates to a method for synthesizing pyrromonazole heterocycle, aiming at providing a synthetic method which causes dicarbonyl compound and hydrazine compound to condense to obtain the pyrromonazole heterocycle under the condition of having no catalyst and no solvent. The method includes the steps: the dicarbonyl compound and the hydrazine compound are mixed together and stirred forreaction at the temperature of 0-100 DEG C; after the reaction, the mixture product is separated by column chromatography, and the pure product of pyrromonazole heterocycle is obtained. The product prepared by the invention is a type of important bioactivator and plays very important role in the synthesis and application of the medicine; the synthetic method has mild conditions, better stereoselectivity of the products, and higher productive rate; as no catalyst and no solvent are available in the course of reaction, the method is economical and environment-friendly; the reaction materials are easy to obtain and operate, thereby being suitable for laboratory preparation and industrial scale production.
Owner:ZHEJIANG UNIV
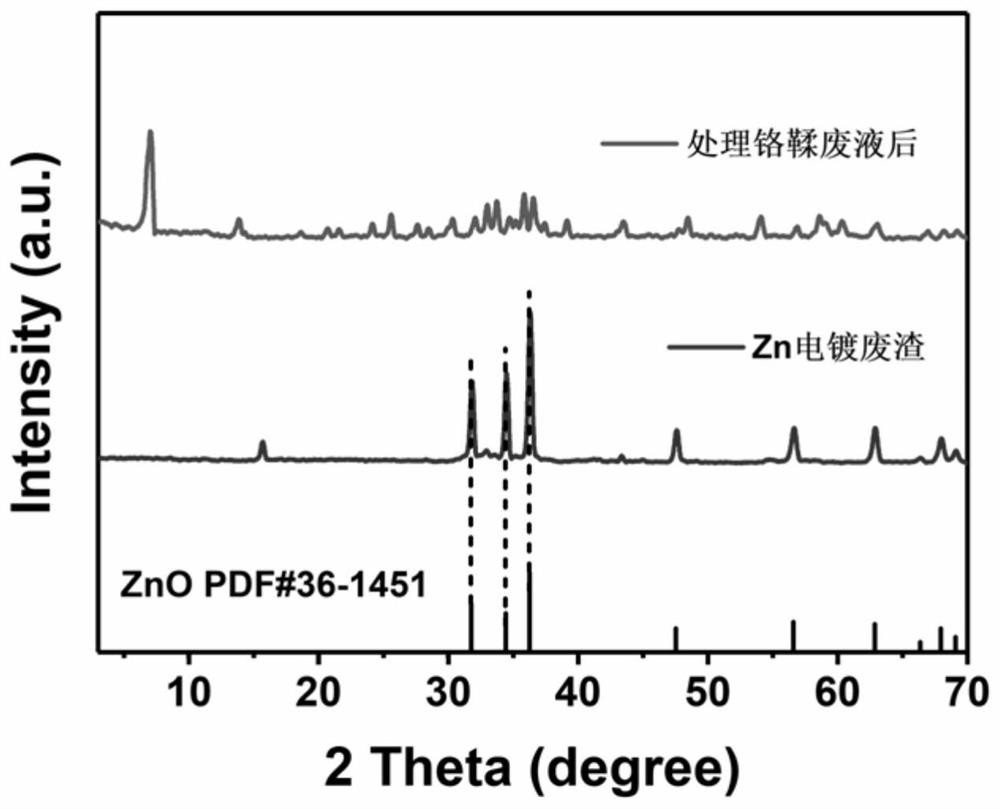
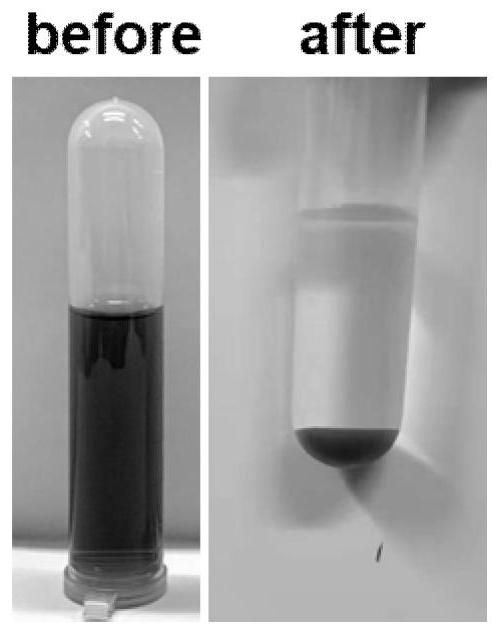
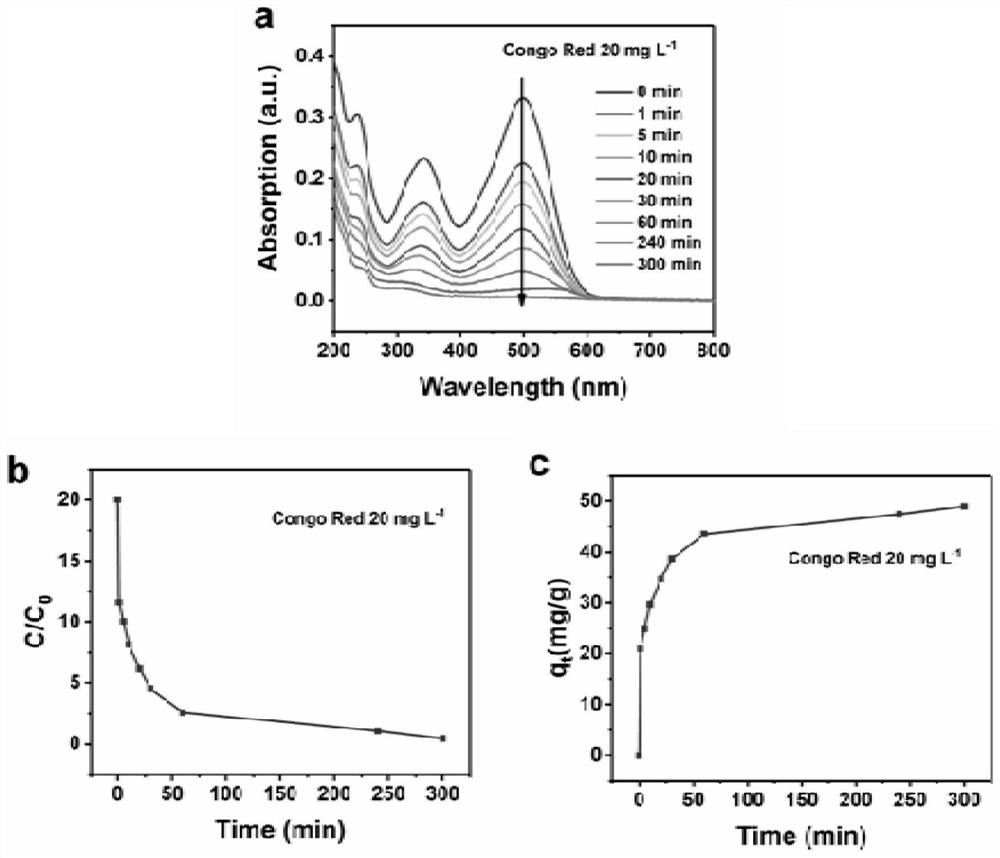
Method for co-treating electroplating waste residues and chromium-containing heavy metal ion waste liquid
ActiveCN113955838AAchieve reusePromote dissolutionOther chemical processesWater contaminantsCongo redOrganic dye
The invention discloses a method for co-treating electroplating waste residues and chromium-containing heavy metal ion waste liquid. According to the method, the electroplating waste residues of an electroplating factory are used as a mineralizer of a chrome tanning waste liquid of a tanning factory, oxides and hydroxides in the electroplating waste residues adsorb chromium ions and are mineralized and converted into chromium-containing hydrotalcite, the obtained chromium-containing hydrotalcite has an ultra-stable mineralization effect, the chromium ions are not prone to being dissolved out, and secondary pollution cannot be caused. By means of the method, the environmental pollution problem caused by the chrome tanning waste liquid can be effectively solved, recycling of the electroplating waste residues is achieved, the filtered supernate can be used for the follow-up leather pickling procedure, the mineralized solid product can effectively adsorb organic dyes such as Congo red and Evans blue, and the green route of treating waste with waste is achieved. The method is mild in reaction condition, simple and convenient to operate, simple in process, free of alkali, economical in atom, low in cost and capable of being applied to electroplate factories and leather factories on a large scale.
Owner:BEIJING UNIV OF CHEM TECH
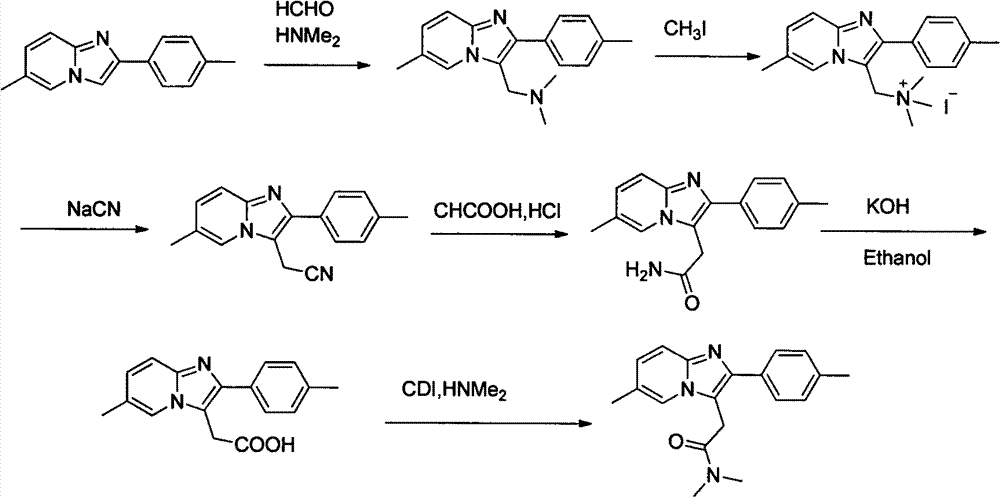
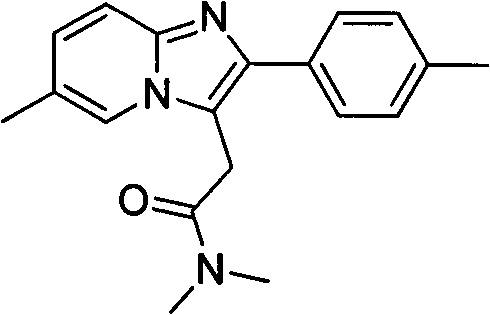
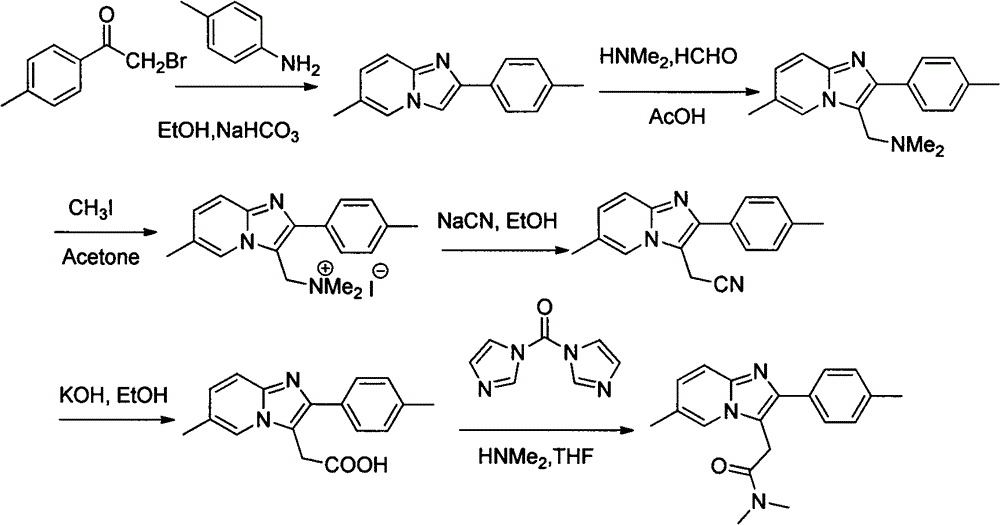
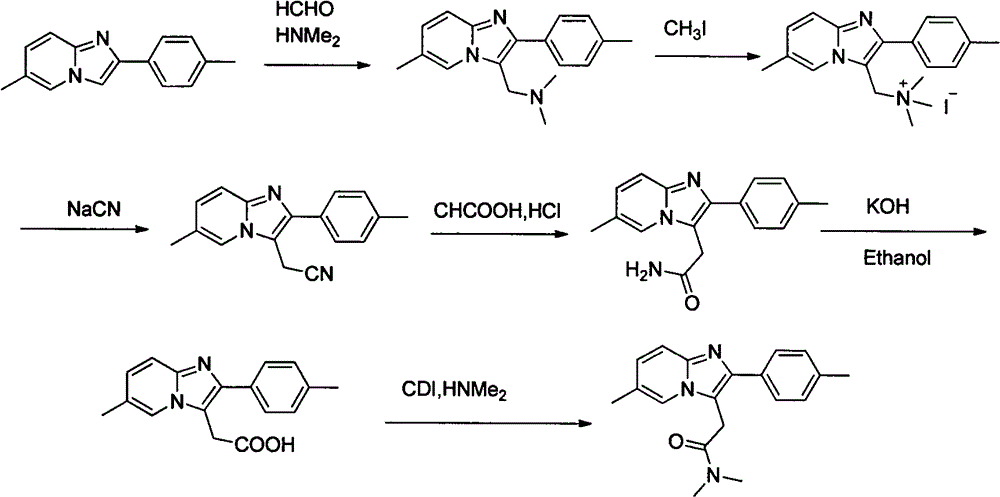
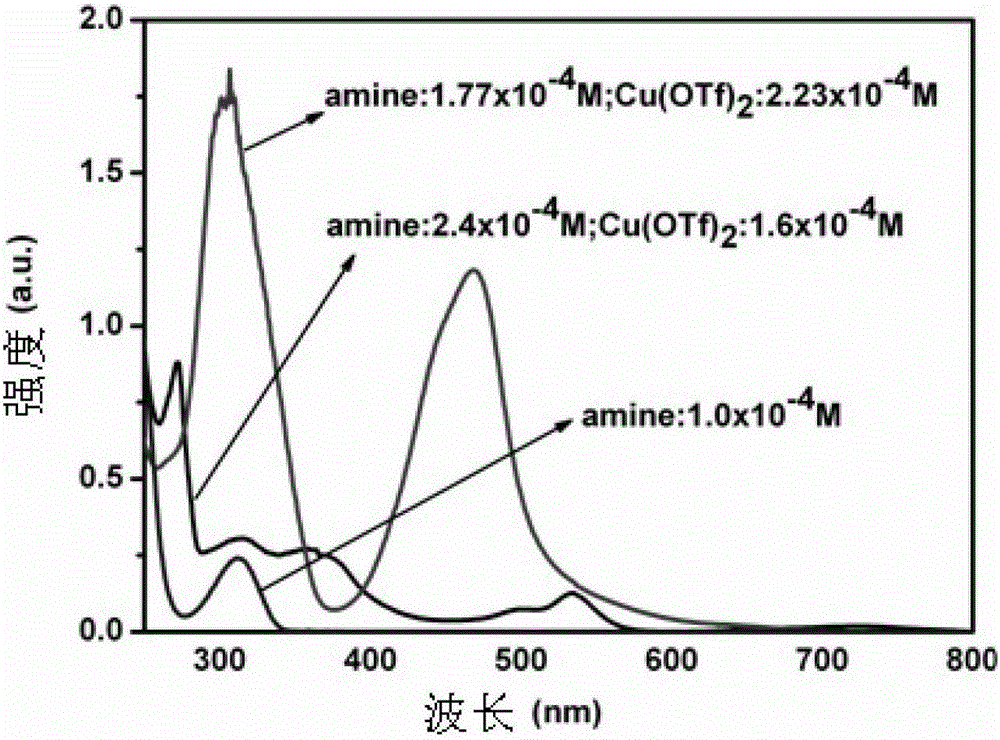
A 3-iminoimidazo[1,2-a]pyridine compound
ActiveCN109734713BEasy to operateMild conditionsOrganic chemistryAir atmosphereCitric Acid Monohydrate
The invention discloses a visible light-catalyzed oxidative cross-coupling reaction of N-aryl glycine ester and imidazo[1,2-a]pyridine compound to prepare 3-imino imidazo[1,2-a]pyridine compound. In the method of the present invention, eosin Y is a photosensitizer, citric acid is an additive, and N-aryl glycine ester and imidazo[1,2-a]pyridine compound are directly dehydrogenated after being irradiated with visible light at room temperature in an organic solvent Cross-coupling yields 3‑iminoimidazo[1,2‑a]pyridine compounds. The method of the present invention uses eosin Y as a photosensitizer, citric acid monohydrate as an additive, and ethanol as a solvent, and utilizes visible light catalysis to effectively prepare a 3-iminoimidazo[1,2-a]pyridine in an air atmosphere at room temperature compound. The method of the invention has the advantages of simple and convenient operation, mild reaction conditions, good selectivity and atom economy.
Owner:EAST CHINA UNIV OF TECH
Preparation method of molybdenum oxide catalyst with hydrotalcite-like structure and application thereof in synthesis of methacrylic acid from isobutyraldehyde
InactiveCN113398917AProcess stabilityImprove oxidation capacityOrganic compound preparationCarboxylic compound preparationAluminium saltsCarbonyl group
Owner:RUNTAI CHEM CO LTD
A method for synthesizing allyl sulfide by direct functionalization of c-h bond
ActiveCN108299261BEfficient synthesisMild reaction conditionsPhysical/chemical process catalystsSulfide preparationSulfide compoundPolymer chemistry
The invention discloses a method for synthesizing allyl sulfide through direct functionalization of C-H bonds. The method is to add olefin compounds and thiol compounds with allyl C-H bonds in a system containing a photocatalyst and a solvent, and use visible light irradiation; the photocatalyst is a quantum dot / rod. In the present invention, under visible light irradiation, C-S bonds are directly constructed through direct functionalization of C-H bonds, thereby realizing the synthesis of allyl sulfide. The present invention uses quantum dots / rods as photocatalysts, has mild reaction conditions, does not require the participation of free radical initiators and oxidants, does not require pre-activation of the substrate, is simple to operate, and is atom-economical.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
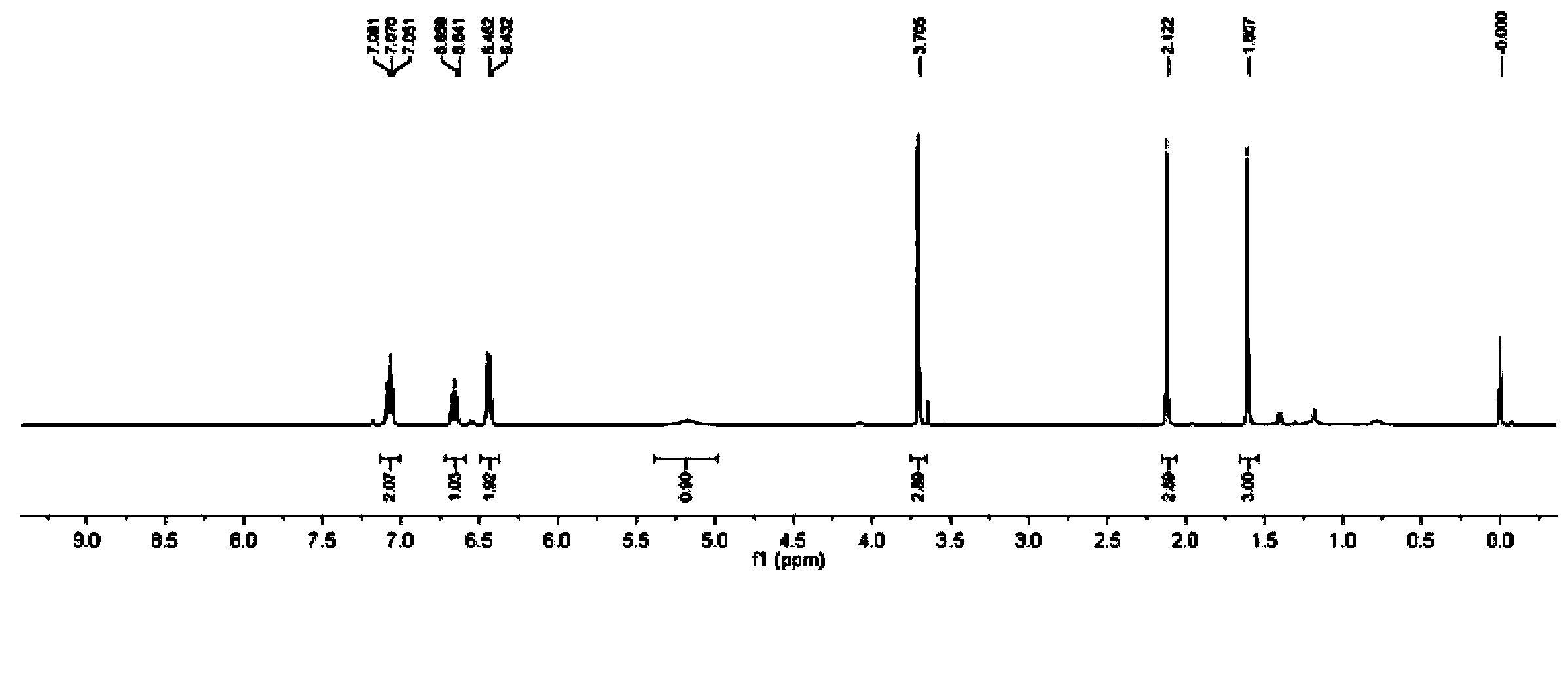
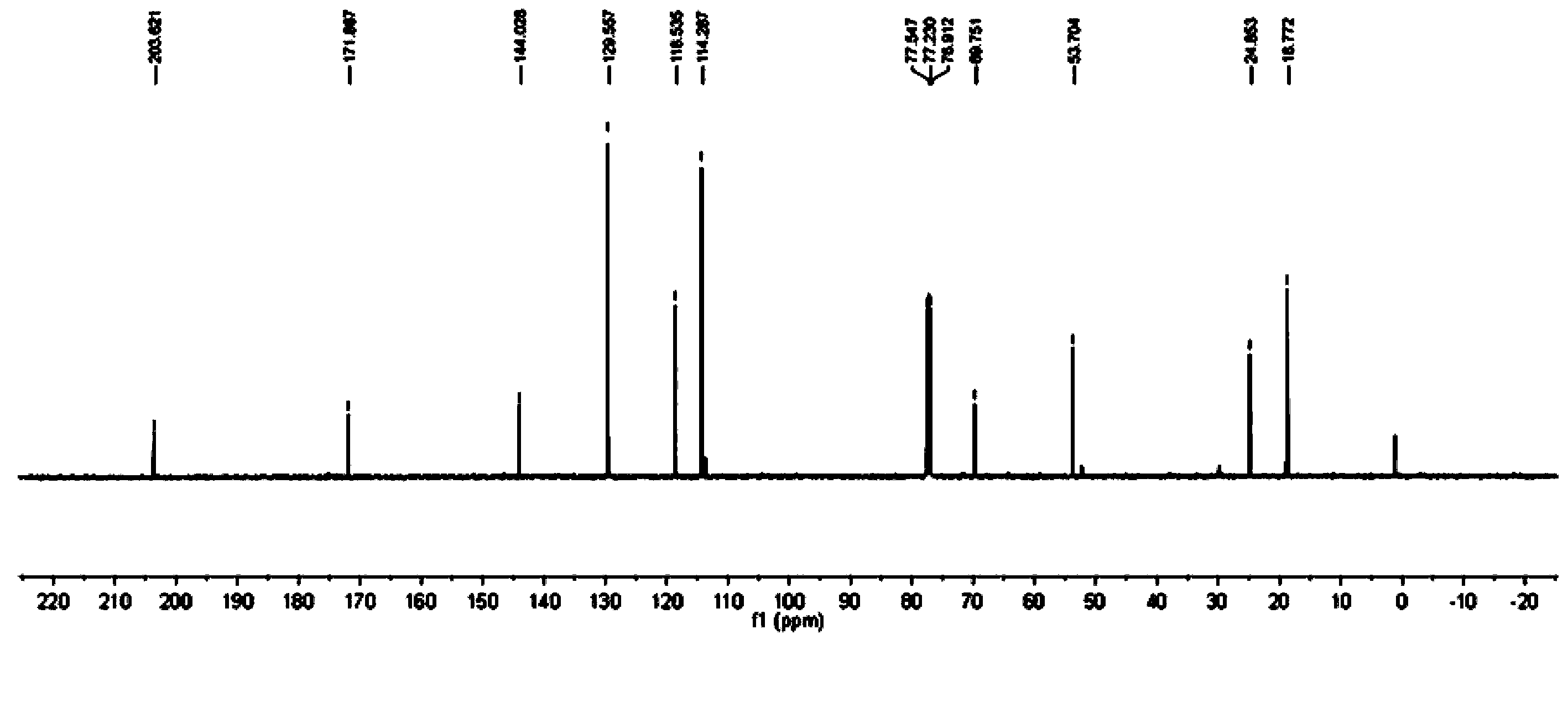

![3-imidazole[1,2-a]pyridine compound 3-imidazole[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/81ea2cf8-e9b4-447f-9c38-f6adc0c1509a/FDA0001988810870000011.png)
![3-imidazole[1,2-a]pyridine compound 3-imidazole[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/81ea2cf8-e9b4-447f-9c38-f6adc0c1509a/FDA0001988810870000012.png)
![3-imidazole[1,2-a]pyridine compound 3-imidazole[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/81ea2cf8-e9b4-447f-9c38-f6adc0c1509a/BDA0001988810880000011.png)


![2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof 2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof](https://images-eureka.patsnap.com/patent_img/4b19602c-906b-48b2-a5d7-ed5358bd5fb3/1.png)
![2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof 2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof](https://images-eureka.patsnap.com/patent_img/4b19602c-906b-48b2-a5d7-ed5358bd5fb3/2.png)
![2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof 2-substituted methylene dihydrobenzo[d]thiazole derivative and synthesis method and application thereof](https://images-eureka.patsnap.com/patent_img/4b19602c-906b-48b2-a5d7-ed5358bd5fb3/3.png)


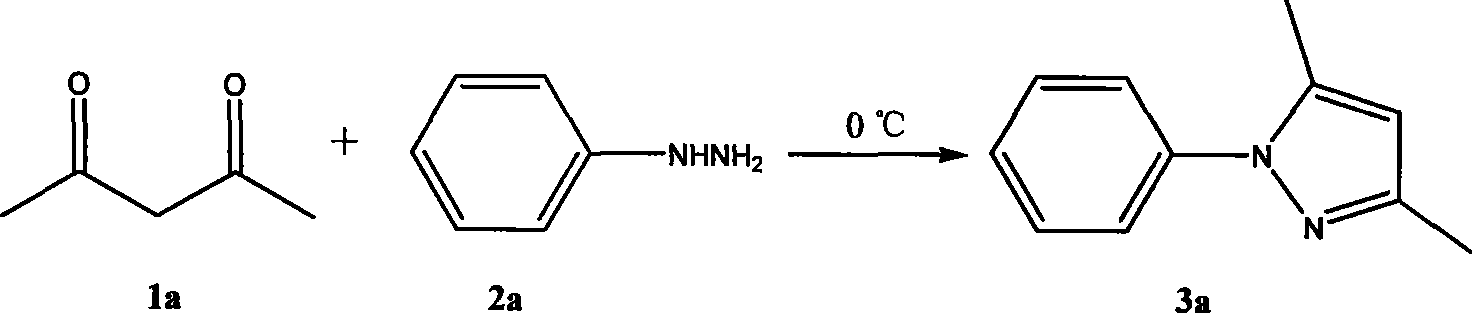
![A 3-iminoimidazo[1,2-a]pyridine compound A 3-iminoimidazo[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/c3c6b9f4-f29f-49c8-acc1-77afcec404e0/BDA0001988810880000011.png)
![A 3-iminoimidazo[1,2-a]pyridine compound A 3-iminoimidazo[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/c3c6b9f4-f29f-49c8-acc1-77afcec404e0/BDA0001988810880000021.png)
![A 3-iminoimidazo[1,2-a]pyridine compound A 3-iminoimidazo[1,2-a]pyridine compound](https://images-eureka.patsnap.com/patent_img/c3c6b9f4-f29f-49c8-acc1-77afcec404e0/BDA0001988810880000031.png)