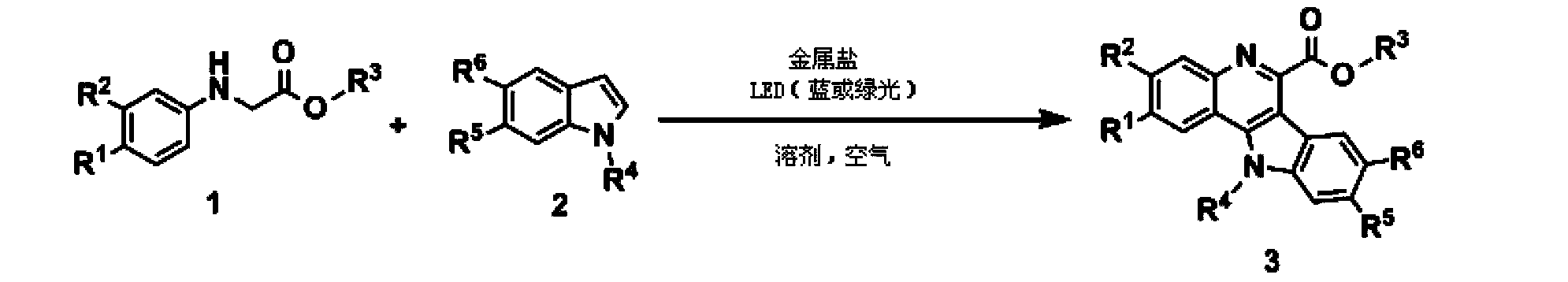
Method for synthesizing quindoline derivative by visible light catalysis
A technology for indoloquinolines and indole derivatives is applied in the field of catalytic synthesis and achieves the effects of mild reaction conditions, simple operation and atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
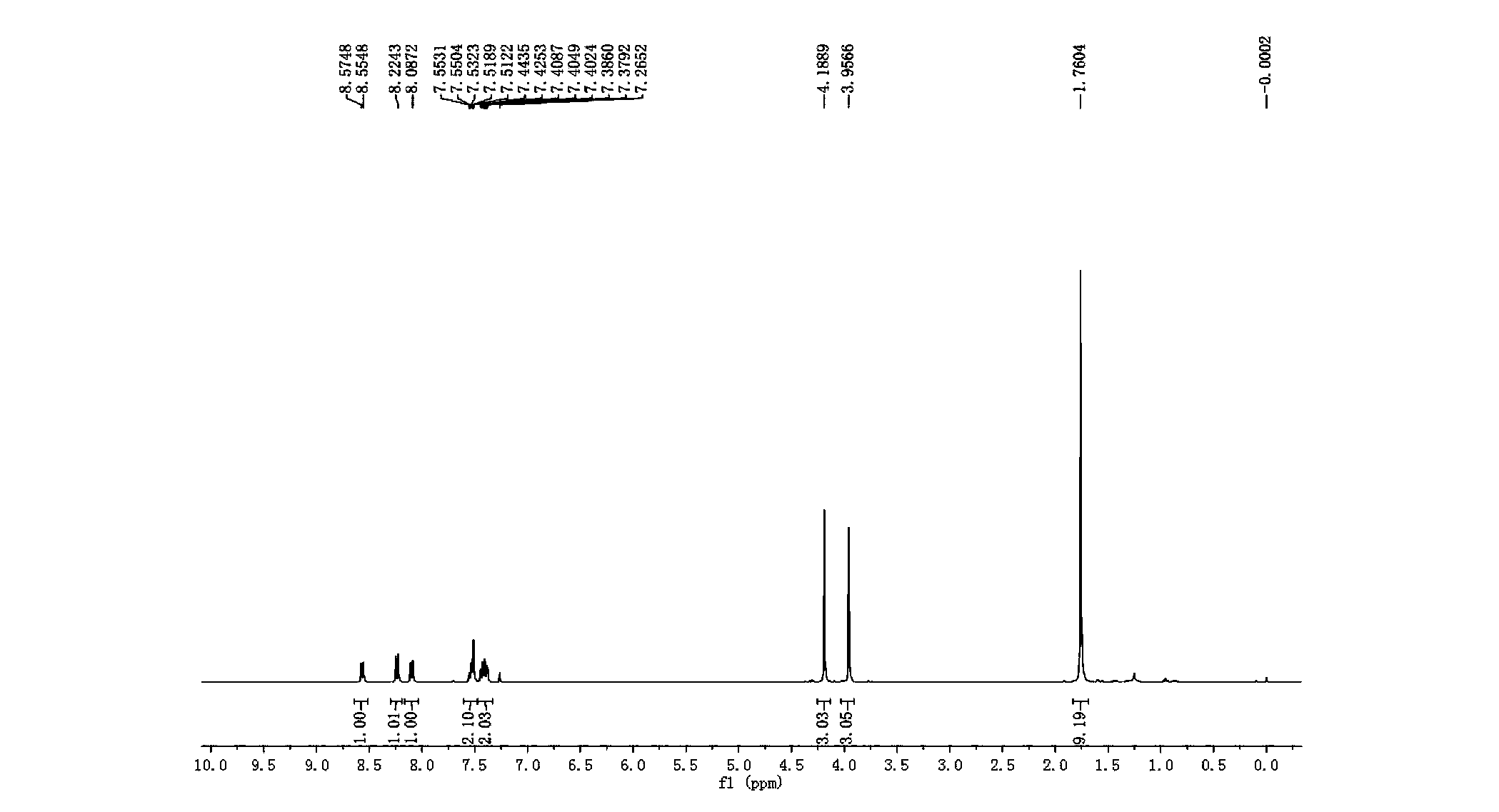
[0041] Add 0.1mmol 2-(4-methoxyanilino)methyl acetate and 0.02mmol copper trifluoromethanesulfonate to a 10mL reaction test tube, use 3mL dichloromethane as the reaction solvent, and add 0.2mmol1H-indole-1 under stirring -Tert-butyl carboxylate, after using LED (blue light) to illuminate the reaction solution for 10 h, the reaction solvent was spin-dried, and then separated by a column, and the yield of the obtained product was 10%. H NMR, C NMR, and mass spectrometry identified the product as tert-butyl 2-methoxy-6-methoxy-11H-indole[3,2-c]quinoline-11H-carboxylate.
[0042] figure 1 Be the general reaction formula of the present invention.
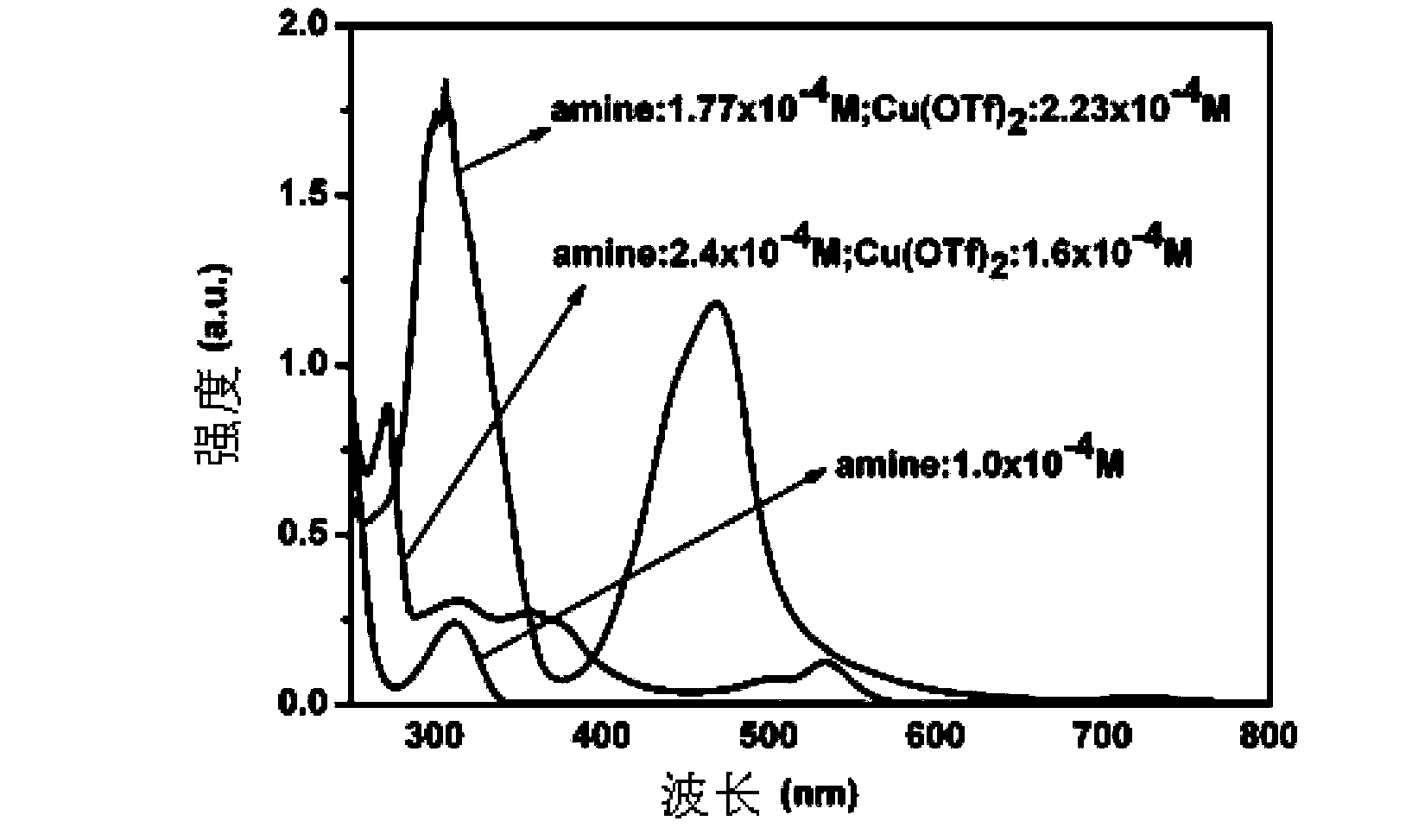
[0043] figure 2 UV-Vis absorption spectra of secondary amine 2-(4-methoxyanilino) ethyl acetate and copper trifluoromethanesulfonate at different concentrations in acetonitrile solvent. The secondary amine 2-(4-methoxyanilino)ethyl acetate has no absorption in the visible light region (as shown in the figure, the concentration of the...
Embodiment 2
[0046] Same as Example 1, the difference is that LED (blue light) is used to illuminate the reaction solution for 20 hours, and the yield of the product obtained is 25%.
Embodiment 3
[0048] Same as Example 1, the difference is that after the reaction solution is illuminated by LED (blue light) for 30 hours, the yield of the product obtained is 28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com