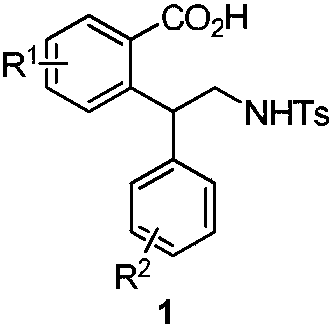
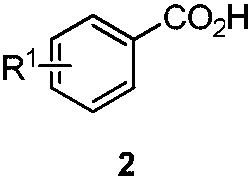
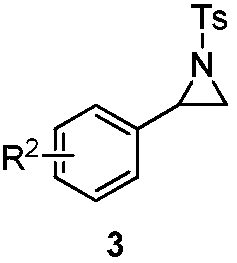
Polysubstituted benzoic acid and a synthesis method thereof
A synthesis method and technology of benzoic acid, applied in chemical instruments and methods, preparation of sulfonic acid amide, preparation of organic compounds, etc., can solve problems such as increased operation complexity and unfavorable product purification, and achieve cheap preparation of raw materials and high yield of target products The effect of high efficiency and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] In a 25mL reaction tube, m-toluic acid 2a (0.2mmol), palladium acetate (10mol%), aziridine 3a (0.4mmol), potassium acetate (0.2mmol), N-acetylglycine ( 20mol%) and 0.5mL of hexafluoroisopropanol, stirred at 80°C for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) to obtain the target product 1a (58 mg, yield 70%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0035]
[0036] In a 25mL reaction tube, under air, add polysubstituted benzoic acid 1a (0.2mmol), dehydroabietylamine 4 (0.22mmol), DCC (0.24mmol), HOBt (0.24mmol) and 2.0Ml dichloromethane, at room temperature Stir for 24 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) to obtain the target product 5 (110 mg, yield 81%). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0037] Compound 1a NMR assignment:
[0038] Multi-substituted benzoic acid derivatives (1a), pale yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ7.97(s,1H,aromatic CH),7.65(d,2H,aromatic CH),7.38(m,4H,aromatic CH),7.24(m,5H,aromatic CH),4.53(m,2H,aromatic CHand NH),3.95(m,1H,CH 2 ),3.70(m,1H,CH 2 ),2,41(s,6H,CH 3 ). 13 C{ 1 H) NMR (100MHz, CDCl 3 ), 50.6 (CH 2 ), 49.2(CH), 21.2and 21.1(CH 3 ). 23 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com