Polysubstituted beta-lactam compound and preparation method thereof
A technology for lactams and compounds, which is applied in the field of multi-substituted β-lactam compounds and their preparation, can solve problems such as complex starting materials, and achieve the effects of simple raw materials and reagents, good functional group diversity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
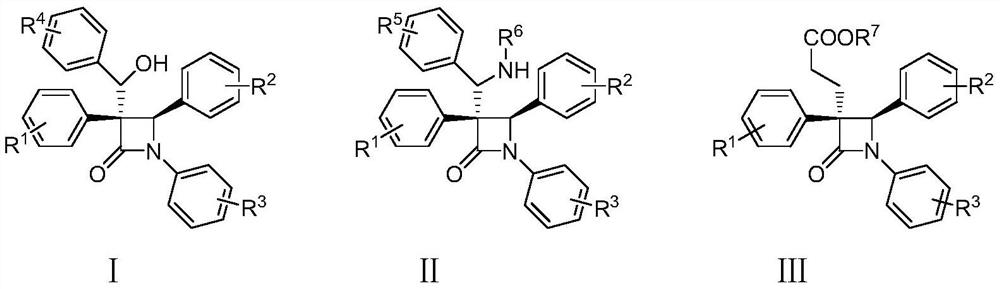
[0046] A multi-substituted β-lactam compound has the structures shown in the following formulas I-1 and I-2, and the reaction route is as follows:
[0047]
[0048] The specific preparation method is as follows:
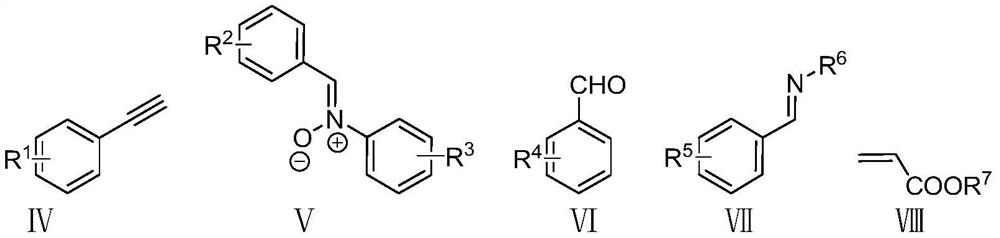
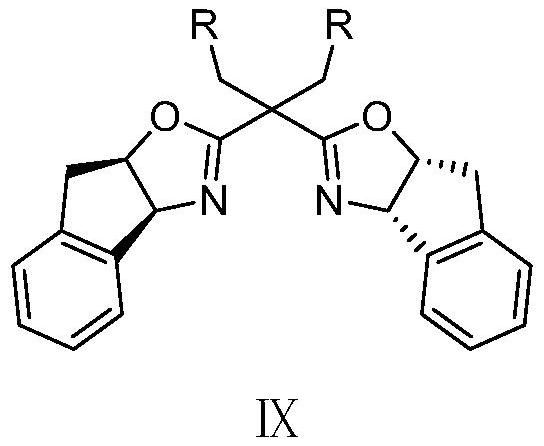
[0049] N 2 Under atmosphere, add 26.4mg (0.2mmol) compound Ⅳ-1, 58.8mg (0.3mmol) compound Ⅴ-1, 45.3mg (0.3mmol) compound Ⅵ-1, 7.5mg (0.02mmol) six Copper(I) tetraethylcyanide fluorophosphate, 13.8mg (0.02mmol) chiral ligand L, 27.6mg (0.2mmol) potassium carbonate, and finally 1mL of dry acetonitrile was added, and the resulting mixture was stirred and reacted at 0°C for 18 hours; the resulting reaction After the mixture was raised to room temperature, it was filtered with diatomaceous earth, and the filtrate was evaporated to remove the solvent to obtain a crude product, which was separated by silica gel column chromatography, and the eluent was a mixed solution of ethyl acetate and petroleum ether. The volume ratio is 2:1~5:1, and a pair of diastereoisomers, na...
Embodiment 2
[0063] A multi-substituted β-lactam compound has the structures shown in the following formulas I-3 and I-4, and the reaction route is as follows:
[0064]
[0065] The specific preparation method is as follows:
[0066] N 2 Under atmosphere, add 26.4mg (0.2mmol) compound IV-1, 69.5mg (0.3mmol) compound V-2, 45.3mg (0.3mmol) compound VI-1, 7.5mg (0.02mmol) six Copper(I) tetraethylcyanide fluorophosphate, 13.8mg (0.02mmol) chiral ligand L, 27.6mg (0.2mmol) potassium carbonate, and finally 1mL of dry acetonitrile was added, and the resulting mixture was stirred and reacted at 0°C for 18 hours; the resulting reaction After the mixture was raised to room temperature, it was filtered with diatomaceous earth, and the filtrate was evaporated to remove the solvent to obtain a crude product, which was separated by silica gel column chromatography, and the eluent was a mixed solution of ethyl acetate and petroleum ether. The volume ratio was 2:1-5:1, and a pair of diastereoisomers,...
Embodiment 3
[0075] A multi-substituted β-lactam compound has the structures shown in the following formulas I-5 and I-6, and the reaction route is as follows:
[0076]
[0077] The specific preparation method is as follows:
[0078] N 2 Under atmosphere, add 26.4mg (0.2mmol) compound IV-1, 63.4mg (0.3mmol) compound V-3, 45.3mg (0.3mmol) compound VI-1, 7.5mg (0.02mmol) six Copper(I) tetraethylcyanide fluorophosphate, 13.8mg (0.02mmol) chiral ligand L, 27.6mg (0.2mmol) potassium carbonate, and finally 1mL of dry acetonitrile was added, and the resulting mixture was stirred and reacted at 0°C for 18 hours; the resulting reaction After the mixture was raised to room temperature, it was filtered with diatomaceous earth, and the filtrate was evaporated to remove the solvent to obtain a crude product, which was separated by silica gel column chromatography, and the eluent was a mixed solution of ethyl acetate and petroleum ether. The volume ratio was 2:1-5:1, and a pair of diastereoisomers,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com